Abstract
The diagnosis and management of heparin-induced thrombocytopenia (HIT) in pediatric patients poses significant challenges. The cardinal findings in HIT, thrombocytopenia and thrombosis with heparin exposure, are seen commonly in critically ill children, but are most often secondary to etiologies other than HIT. However, without prompt diagnosis, discontinuation of heparin, and treatment with an alternative anticoagulant such as a direct thrombin inhibitor (DTI), HIT can result in life- and limb-threatening thrombotic complications. Conversely, DTIs are associated with higher bleeding risks than heparin in adults and their anticoagulant effects are not rapidly reversible; furthermore, the experience with their use in pediatrics is limited. Whereas immunoassays are widely available to aid in diagnosis, they carry a significant false positive rate. Age-dependent differences in the coagulation and immune system may potentially affect manifestations of HIT in children, but have not been extensively examined. In this chapter, diagnostic approaches and management strategies based on a synthesis of the available pediatric studies and adult literature on HIT are discussed.
Introduction
Heparin-induced thrombocytopenia (HIT) is a drug-induced, immune-mediated form of thrombocytopenia with a potential for serious thrombotic complications such as HIT with thrombosis (HIT-T).1,2 The diagnosis relies on a combination of clinical findings and laboratory testing. The management in children is especially challenging due to the paucity of studies on the epidemiology, clinical presentation, and treatment of HIT in this population. HIT results from the formation of an autoantibody directed against the heparin-platelet factor-4 (PF4) complex. Diagnostic testing includes immunoassays such as the ELISA for anti-PF4/heparin antibodies, which is highly sensitive, but has limited specificity, and functional assays such as the 14C-serotonin release assay, which is highly sensitive and specific, but not routinely available in most hospitals. It remains unclear whether the incidence of antibody formation and disease manifestations are the same in children as in adults. Clinical scoring systems to assess pretest probability of HIT improve the accuracy of the HIT diagnosis, but have not been validated in children. Whereas the clinical course in children appears to be similar to that in adults, relatively little has been published, and management is largely based on extrapolation from adult studies. Alternatives to heparin, such as direct thrombin inhibitors (DTIs) and Xa inhibitors, are widely used for the management of HIT in adults; however, the pediatric experience with these agents is sparse. We review herein the available pediatric data, and discuss the similarities and potential differences in pathophysiology, epidemiology, and management of HIT between children and adults.
Pathophysiology
In the majority of HIT patients, antibodies to a complex of heparin and PF4 can be demonstrated. The antigens recognized on PF4 are formed upon heparin binding. In the minority of patients, antibodies to other proteins (eg, NAP-2 and IL-8) have been identified. Pathogenic HIT antibodies are primarily IgG. Although IgM and IgA antibodies can occur, these do not appear to cause HIT in most instances. Whereas most drug-dependent forms of thrombocytopenia result from antibody-mediated clearance of platelets, the consumptive thrombocytopenia in HIT is due to activation of platelets by the IgG-heparin/PF4 complex when it binds to platelet FcRIIA surface receptors.
Unlike other drug-dependent forms of immune thrombocytopenia, HIT typically results in moderate thrombocytopenia; severe thrombocytopenia and bleeding are rare. Paradoxically, HIT increases the risk for both arterial and venous thrombosis. Several mechanisms have been proposed to explain the prothrombotic state associated with HIT. HIT antibodies promote platelet activation and procoagulant microparticle formation. HIT antibodies also bind to surface heparin PF4 complexes on endothelial cells, monocytes, and neutrophils, resulting in vascular inflammation/injury, neutrophil activation, and tissue factor expression by monocytes. These mechanisms may explain the high risk of thrombosis that persists after resolution of thrombocytopenia.
HIT: how do children and adults differ?
Several age-dependent differences in the coagulation and immune systems may potentially influence HIT antibody formation. The development of HIT is dependent on the stoichiometric balance of heparin and PF4 complexes. However, PF4 levels vary with age, with infants having the lowest levels.3 In addition, the binding of heparin to other proteins such as vitronectin and antithrombin differ between children and adults, which may also affect the stoichiometry of heparin-PF4 complex formation.3,4 Furthermore, there are age-dependent differences in the immune system. Antibody responses to certain antigenic stimuli are decreased in infants compared with adults; however, it is unclear whether there are age-dependent differences in the capacity to form HIT antibodies. Lastly, age-dependent mechanisms may contribute to a decreased risk of thrombosis in children with HIT. Platelet activation is thought to be central to the development of disease. Platelets from infants and neonates are less reactive to platelet agonists.5 Whether neonatal platelets differ in their response to HIT antibody binding has not been examined. The antithrombotic effect of tissue factor pathway inhibitor (TFPI) may also be enhanced with heparin exposure; TFPI increases with unfractionated heparin (UFH), and these increases are prolonged in children compared with adults.6 It remains to be determined whether these age-dependent biological differences influence the incidence and clinical course of HIT in pediatric patients.
Frequency of HIT in children
The overall number of children who develop HIT is significantly fewer than the number of adults. This observation is likely due in part to the markedly decreased baseline risk of thrombosis in children and the smaller number of children exposed to heparin. However, the frequency of antibody formation and HIT in children is not well known. Several studies have examined the prevalence of HIT in selected pediatric populations. In infants and children exposed to UFH during cardiac surgery, the incidence of antibody formation has been reported to be 1.7%-6% on postoperative days 5-10. However, over 50% of children are antibody positive after reexposure to UFH with reoperations. Only 1.3% develop clinical HIT.7 These frequencies are similar to that of adults undergoing cardiac bypass surgery in which 27%-50% of patients develop a positive antibody test and 1%-2% develop clinical HIT. In the intensive care unit setting, the incidence of HIT in children has been reported to be 1.5% in the neonatal unit8 and 2.3% in the pediatric unit.9 It is important to note that in the latter population, the majority of patients diagnosed with HIT (11 of 14) underwent cardiothoracic surgery. In adults, the incidence of HIT is highest in patients undergoing orthopedic surgery (3%-5%), and lower in general medical patients (1%-3%). Whereas the reported incidences of HIT in pediatric populations may be similar to incidences in adults, they may not be directly comparable due to differences in the definition and criteria of HIT diagnosis in these pediatric studies (summarized in Table 1).
Reported incidence of clinical HIT and antibody formation in pediatric patients
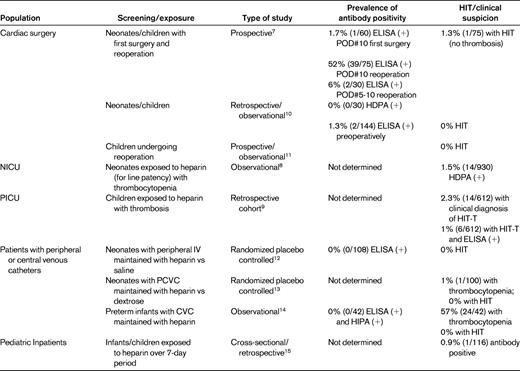
NICU indicates neonatal intensive care unit; PICU, pediatric intensive care unit; PCVC, percutaneous central venous catheter; CVC, central venous catheter.
In adults, the risk of developing HIT is influenced by the patient population (surgical > medical), the type (bovine > porcine) and formulation (UFH > low-molecular-weight heparin [LMWH] >fondaparinux) of heparin, the dose (therapeutic > prophylactic > heparin flushes), the route of administration (intravenous > subcutaneous), the duration of administration (higher with 4 or more days), and gender (female > male). Evidence suggests that some of these characteristics may also hold true in pediatric patients. Three studies in neonates found no cases of HIT or antibody formation associated with the use of low-dose UFH to maintain catheter patency (N = 253 patients).12–14 However, an earlier study demonstrated positive heparin-induced platelet aggregation (HIPA) tests in 41% of infants with thrombocytopenia exposed to heparin (13 of 42 patients).8 All of these infants were receiving heparin to maintain line patency and were not on a typical treatment dose. The reason for these different results is not clear, but may have been due to the different assays used to detect HIT antibodies. In children, the majority of reported cases of pediatric HIT are associated with UFH exposure,15,16 but at least one case of HIT with LMWH has been described.17 Lastly, age also may affect the risk of HIT development. Infants have a reduced capacity for antibody formation, yet HIT is reported frequently in this population. HIT also appears to be more common in adolescents.15,16 The reason for the increased risk in these age groups is not clear but may reflect the increased heparin exposure in these populations.
Clinical manifestations of HIT in pediatrics
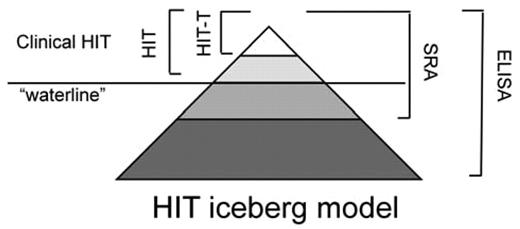
Only a minority of patients who generate HIT antibodies develop clinical manifestations; a relationship depicted in the “iceberg model” of HIT conceived by Warkentin et al18 (Figure 1). It is likely that Warkentin's iceberg model will also serve as a useful paradigm in pediatric patients; however, accurate percentages of patients who develop clinical disease is not well known given the relatively few surveillance studies performed in children. In adults with HIT-associated thrombocytopenia, 30%-60% have thrombosis at the time of clinical diagnosis and venous events are more common than arterial.19 Most thrombotic events reported in pediatric patients appear to be venous rather than arterial; perhaps in part due to a decreased incidence of atherosclerosis in children.15,16 In adults with HIT, gangrene, skin necrosis, and systemic reactions have all been described; these events have also been rarely reported in children. However, the true frequency of these life- and limb-threatening occurrences in children is not well known.
Relationship between clinical HIT and antibody formation. Only a minority of patients with heparin-PF4 antibodies shown by ELISA demonstrate functional antibodies by SRA, and only a fraction of these will develop clinical disease (HIT or HIT-T). The true frequency of antibody formation and an accurate estimate of risk for clinical disease in the pediatric population are not well known.
Relationship between clinical HIT and antibody formation. Only a minority of patients with heparin-PF4 antibodies shown by ELISA demonstrate functional antibodies by SRA, and only a fraction of these will develop clinical disease (HIT or HIT-T). The true frequency of antibody formation and an accurate estimate of risk for clinical disease in the pediatric population are not well known.
Diagnosing HIT in pediatric patients
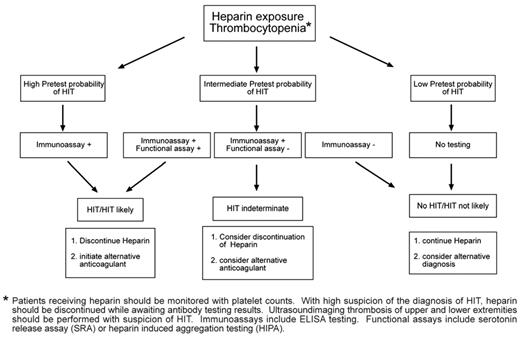
Despite the availability of laboratory tests, the diagnosis of HIT in children, as in adults, remains a clinical diagnosis that is confirmed/supported by diagnostic laboratory testing. HIT should be suspected in any child who develops thrombocytopenia during heparin exposure. Classically, clinical HIT (thrombocytopenia with or without thrombotic sequelae) develops after 5-10 days of heparin exposure. Because thrombotic events can occur before the development of absolute thrombocytopenia in patients with baseline thrombocytosis, a 50% decline in the platelet count during heparin therapy should be a warning that HIT is present. Less commonly, clinical HIT develops within 24 hours of heparin therapy (acute HIT, thought to be associated with preexisting HIT antibodies) or days after heparin is discontinued (delayed HIT). Any time HIT is suspected, discontinuation of heparin should be strongly considered and confirmatory diagnostic testing should be performed. The decision to prescribe an alternative anticoagulant should be based on the clinical suspicion of HIT and the individual bleeding and thrombotic risk of the patient.
Predictive scoring systems
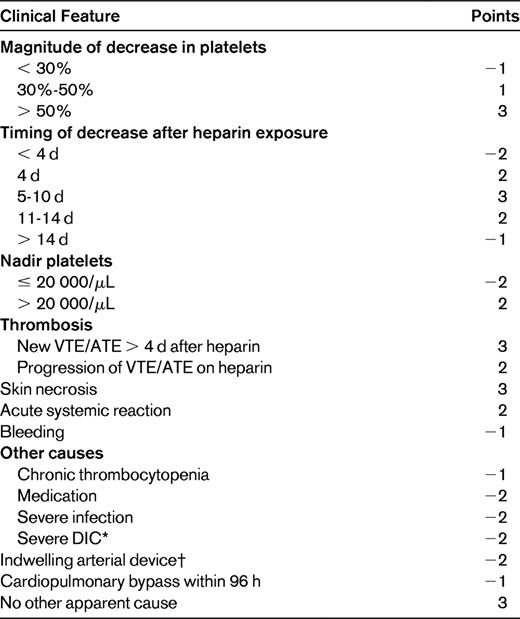
To improve the accuracy of pretest assessment of the likelihood of HIT and to facilitate risk-appropriate management, several clinical prediction models have been developed. Pretest probability assessment is important because the results of diagnostic HIT assays are often delayed, the heparin-PF4 ELISA assay has a significant false positive rate, and alternative anticoagulants have a higher bleeding risk. The “4T score” pretest probability model developed by Warkentin et al is highly sensitive (with a sensitivity of 98.4%) and relies on the clinical characteristics of the degree and Timing of Thrombocytopenia in relation to heparin exposure, the presence of new Thrombosis, and the exclusion of oTher etiologies of thrombocytopenia (Table 2). Thrombocytopenia usually develops after 5-10 days of heparin exposure and moderate thrombocytopenia is typical. Early thrombocytopenia (within 24 hours) in the absence of recent heparin exposure (within 1-3 months), delayed thrombocytopenia, severe thrombocytopenia, or reductions < 30% make HIT less likely. Platelet nadirs in reported cases of HIT in children fall within the 5- to 10-day window, although cases with later onset have been reported. Severe thrombocytopenia in children with HIT also occurs, but, similar to the case in adults, moderate thrombocytopenia is more common. The presence or absence of clinical manifestations (thrombosis, local skin reactions, etc) and the presence of other potential causes of thrombocytopenia also influence the likelihood of HIT and also occur in children. A low 4T score (0-3) has been found to be associated with a low incidence of HIT antibody test positivity (1.6%) among hospitalized patients at 2 centers and critically ill patients in 3 prospective studies conducted in intensive care unit patients.20,21 Whereas the key clinical features of HIT used for diagnosis appear to be similar between adults and children, it is important to note that the 4T scoring system has not been validated in pediatrics.
4T score for pretest probability of HIT
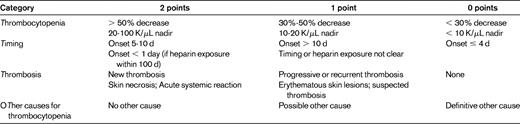
Score of 0-3 indicates low probability; 4-5, intermediate probability; and 6-8, high probability.
More recently, the HIT Expert Probability (HEP) score was developed as pretest clinical scoring system that is based on the 4Ts, but expanded to include other clinical characteristics that influence the likelihood of HIT22 (Table 3). Central to both scoring systems are the timing and magnitude of thrombocytopenia, the presence of new thrombosis, and exclusion of other etiologies of thrombocytopenia. Whereas it is more extensive in detail, the inclusion of additional clinical features of HIT in the HEP scoring system may be useful for clinicians with less extensive experience with HIT diagnosis. A score of 2 was associated with 100% sensitivity but only 60% specificity for the diagnosis of HIT, whereas a score of 5 was 86% sensitive and 88% specific. The use of these clinical scoring systems may improve the accuracy of the diagnosis of HIT, but will require further study in the pediatric population.
Laboratory testing
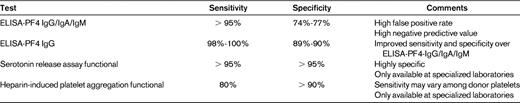
The diagnosis of HIT relies on clinical and supporting laboratory evaluation. ELISA assays of polyclonal antibodies to the PF4/heparin complex are available in most laboratories with a rapid turnaround time. These tests are highly sensitive, but have a significant false positive rate. Therefore, this test is useful in ruling out the diagnosis of HIT with a negative predictive value (> 95%). Immunoassays detecting only IgG antibodies appear to have greater specificity with sensitivity similar to assays measuring IgG/IgA/IgM antibodies; this suggests that IgG antibodies are the major contributors to the pathogenesis of HIT.23,24 Antibody titer as measured by ELISA also appears to be correlated with the likelihood of clinical manifestations in adults.25–27 Patients with a low antibody titer, as measured by an optical density (OD) of 0.4-1.0, have a 5% chance of a positive serotonin release assay (SRA) assay, whereas OD values between 1.0 and 1.4, 1.41 and 1.99, and 2.0 or greater were associated with 30%, 58%, and 92% likelihood of a positive SRA (> 50% activation), respectively.26 It has been suggested that HIT may occur at lower antibody levels in children,9 but the specificity and sensitivity of ELISA testing in the pediatric population has not been formally studied.
The SRA is highly specific and measures platelet activation by the release of radiolabeled 14C-serotonin by HIT antibodies in the presence of pharmacologic concentrations of heparin (0.1 U/mL); platelet activation is inhibited with excessive concentrations of heparin (> 5 U/mL).24 A positive assay is defined as > 20% release, but higher levels of platelet activation are associated with a greater likelihood of thrombotic complications. More than half of patients (51.4%) with at least 80% SRA activation suffered a thrombotic event compared with 58.1% of patients with 90% or more activation. The HIPA test is also a functional test of heparin-dependent antibodies, but its sensitivity may be affected by variations in the reactivity of donor platelets for the assay.28 Both the SRA and the HIPA are more specific than the ELISA, but are only performed in a limited number of specialized laboratories. Furthermore, the results of these functional tests are may take several days, which is problematic when making urgent management decisions. Table 4 summarizes these assays.
Thrombocytopenia and heparin exposure in pediatrics
One of the major challenges in the diagnosis of HIT is the relatively common occurrence of thrombocytopenia in critically ill patients, many of whom are exposed to heparin but few of whom will have HIT. Thrombocytopenia is frequently encountered in pediatric patients on extracorporeal membrane oxygenation (ECMO); the average patient requires 1.3 platelet transfusions per day. UFH is used routinely and thrombosis risk is increased due to the presence of multiple vascular catheters. The mechanism of non-HIT-associated thrombocytopenia in these patients is thought to be due to activation of platelets within circuit tubing and/or the membrane oxygenator. In addition, patients may develop disseminated intravascular coagulation secondary to infection and organ damage. Similarly, left ventricular devices are increasingly being used as a bridge to cardiac transplantation in pediatric patients. Similar to patients on ECMO, heparin is used routinely, thrombocytopenia is common, and thrombosis risk is increased. Another group of patients with frequent heparin exposure and thrombocytopenia are those with congenital heart disease who undergo cardiac bypass. Thrombocytopenia is also common after surgery but usually recovers 3-4 days postoperatively. However, late thrombocytopenia can be seen in 24% of patients who undergo hypothermia during bypass; the thrombocytopenia is not related to HIT.29 Disseminated intravascular coagulation, infection, and certain medications also commonly result in thrombocytopenia. These conditions provide alternative etiologies for thrombocytopenia, and decrease the pretest probability of the diagnosis of HIT. However, it is important to note that these conditions do not preclude HIT, which poses significant challenges for the diagnosis.
Management of HIT
If HIT is suspected, all forms of heparin exposure should be eliminated (UFH and LMWH) and the patient should be evaluated for thrombosis in the upper and lower extremities. Initiation of a nonheparin anticoagulant such as a DTI is recommended if HIT is suspected, even in the absence of thrombosis, because more than 50% of adult patients with HIT will suffer a thrombotic event within 30 days if heparin is simply discontinued and an alternative form of anticoagulation is not used.30 The rate of thrombosis in children with isolated thrombocytopenia and HIT antibodies, however, is not known. Therefore, until these data are available, we recommend that children should be managed similar to adult patients with HIT. Therefore, with high clinical suspicion of HIT and positive antigenic and functional antibody testing, alternative anticoagulation with a DTI should be considered. Initiation of warfarin should be avoided until after recovery of the platelet count is achieved, and concomitant therapy with a DTI should be used to prevent new or recurrent thrombotic events and skin necrosis.31 The duration of anticoagulation for HIT depends upon the presence/absence and type of associated thrombotic events. In patients with isolated thrombocytopenia, anticoagulation with a DTI until platelet recovery, followed by warfarin for up to 1-3 months, has been recommended.1 In the presence of thrombosis, anticoagulation for a duration appropriate for the thrombotic event is generally recommended (deep vein thrombosis at least 3 months, pulmonary embolism 3-6 months). Further investigation to provide an evidence basis for these recommendations is warranted. Platelet transfusions should be avoided because bleeding is rare in HIT and platelet transfusions have been associated with thrombotic events in some patients.32
We recommend that an assessment of the pretest probability for the diagnosis of HIT be made before performing antigenic and functional antibody testing. Because of the significant false-positive rate of ELISA antibodies, we do not recommend testing for low pretest probability of HIT. In the case of a high pretest probability of HIT, empiric management for HIT is suggested while awaiting the results of antibody testing. An intermediate or low pretest probability or a case in which antibody testing is positive present management challenges. A suggested algorithm is shown in Figure 2 and several clinical scenarios are discussed below.
Algorithm for the workup and diagnosis of suspected HIT in pediatrics.
Intermediate/low pretest probability with positive antigenic HIT test
A negative HIT test with a low/intermediate probability likely excludes the diagnosis of HIT. However, a positive HIT test with intermediate/low probability poses diagnostic challenges. In adults, the magnitude of the OD value for the ELISA test should be considered: higher OD values (and thus HIT antibody titers) are associated with a greater likelihood of positive SRA test results and clinical events. Additional testing with SRA or HIPA can increase the accuracy of diagnosis. However, these functional tests are performed only in specialized laboratories and it often takes several days to receive the results. Depending of the risk of thrombosis, bleeding, and the OD of the ELISA, the decision to withhold DTI or even continuation of heparin may be considered. In children, it is worth noting that the positive predictive value of higher ELISA OD values has not been studied; in fact, some investigators suggest that lower antibody titers may be associated with HIT or even thrombosis without thrombocytopenia, although these findings have not been demonstrated in other studies.9,33
History of positive HIT test and cardiac bypass surgery
There is a significant incidence of HIT antibody formation in both adult and pediatric patients undergoing cardiac bypass surgery. It is not uncommon to have patients with a history of HIT antibodies needing repeat bypass surgery. HIT antibodies using the SRA and ELISA are detectable at a median of 50 and 85 days, respectively, after an episode of HIT. However, antibody remains detectable in 35% of adult patients up to 1 year after HIT. In general, avoidance of all heparin products is recommended in patients with a past history of HIT. However, exceptions are patients scheduled to undergo cardiac surgery. In this case, the risk of heparin exposure and recurrent HIT must be weighed against the increased risk of bleeding associated with the use of a DTI for the bypass procedure. In adult patients with recent HIT-T, preoperative plasmapheresis, and intraoperative use of antiplatelet medications such as epoprostenol have been used in conjunction with heparin anticoagulation on bypass when the bleeding risks with a DTI have been considered to be prohibitive. In most adult patients with a more distant history of HIT, use of heparin for the bypass procedure may be the safest course of action, with avoidance of heparin perioperatively. In pediatric patients, several cases of cardiac bypass and ECMO using DTIs for the management of HIT have been reported.
Thrombosis and thrombocytopenia with negative HIT antibodies
Moderate thrombocytopenia is common in critically ill patients. Many have central venous catheters, which are a major risk factor for thrombosis. A negative HIT antibody test in these cases makes the diagnosis of true HIT unlikely. In rare cases, HIT antibodies are directed against antigens other than PF4, such as IL-8 and NAP-2; this may result in a negative HIT ELISA but a positive SRA. However, if clinical suspicion of HIT is high based on the timing of platelet decrease, history of thrombosis, and absence of other etiologies, presumptive treatment of HIT should be considered despite a negative ELISA antibody test. The use of alternative anticoagulants may be warranted in these cases while awaiting the results of the SRA; other clinical scenarios in which suspicion of HIT may be particularly high are recurrent thrombosis and/or thrombocytopenia with heparin reexposure.
Nonheparin anticoagulants in pediatrics
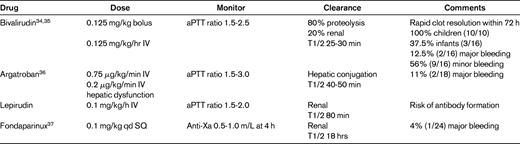
Two classes of nonheparin anticoagulants are available for the treatment of HIT: DTIs and indirect Xa inhibitors. The pharmacokinetics, safety, and efficacy of 2 DTIs have been studied for pediatric use: bivalirudin and argatroban. Both bivalirudin and argatroban have short half-lives. Argatroban is cleared hepatically, whereas bivalirudin is primarily proteolyzed in the plasma. A third DTI, lepirudin, has a longer half-life than bivalirudin and argatroban and is cleared renally. No formal pediatric pharmacokinetic or efficacy/safety studies have been performed with lepirudin. However, all 3 of these agents have been used in the management of HIT in children. These drugs are administered intravenously and monitored by the activated partial thromboplastin time; no reversal agents are available. Fondaparinux, a synthetic pentasaccharide that is an indirect inhibitor of Xa, has also been studied recently in children. Like LMWH, it is administered subcutaneously and its activity can be measured by anti-Xa activity. There may be a role for this agent in extended anticoagulation with the diagnosis of HIT. Danaparoid is a mixture of glycosaminoglycans that is not available in the United States. Currently approved for use in adults is the oral DTI dabigatran, and pediatric trials are in progress. The efficacy of dabigatran in the treatment of HIT has yet to be investigated.
Summary
The diagnosis of HIT in critically ill children is often difficult. Although it is rare, it should be considered with the development of thrombocytopenia with or without thrombosis after heparin exposure. Even though they have not been formally validated in children, pretest scoring systems in conjunction with appropriate antibody testing can aide in accurate diagnosis. Management of indeterminate cases needs to be based on individual assessment of the likelihood of disease and the thrombotic and bleeding risk. Pediatric pharmacokinetic data with nonheparin alternatives such as DTIs and Xa inhibitors are now available to guide dosing for the treatment of HIT. Further studies are needed to improve the diagnosis and management of HIT in pediatric patients.
Disclosures
Conflict-of-interest disclosures: C.M.T. declares no competing financial interests. M.B.S. has received research funding from Bristol Myers Squibb; has consulted for Eisai Inc; and has consulted for, received honoraria from, and is affiliated with the speakers' bureau for Sanofi-Aventis. Off-label drug use: enoxaparin for the treatment of thrombosis in children; lepirudin for the treatment of HIT in children; argatroban for the treatment of HIT in children; and bivalirudin for the treatment of HIT in children.
Correspondence
Clifford M. Takemoto, Division of Pediatric Hematology, The Johns Hopkins University, Ross 1125, 720 Rutland Ave, Baltimore, MD 21205; phone: (410) 955-6132; fax: (410) 955-8208; e-mail: ctakemot@jhmi.edu.