Abstract
The coagulopathy of liver disease in pediatric patients presents an unusual set of challenges. Little pediatric data have been published, so this review is based largely on adult studies. There is a precarious balance between deficiencies of clotting factors and anticoagulation factors in liver disease that result in abnormal prothrombin time (PT) and activated partial thromboplastin time (aPTT) tests that would suggest a bleeding tendency, yet the patients can form a clot and are at risk of thromboembolic disease. Attention has centered on thromboelastography and thrombin-generation assays to clarify the patient's ability to control bleeding, but these tests are not routinely available to many treating physicians.
Introduction
Treatment options for hepatic coagulopathy patients include fresh-frozen plasma (FFP), cryoprecipitate, platelet transfusions, vitamin K replacement, antifibrinolytics, prothrombin complex concentrates (PCCs), and recombinant factor VIIa (rFVIIa). FFP is the core therapy but has many drawbacks, including dubious efficacy, transfusion-related acute lung injury (TRALI), volume overload, allergic reactions, and a theoretical risk of transfusion-associated infections. Platelet transfusions may also have many of the same risks. rFVIIa and PCCs can correct the prothrombin time (PT) when FFP will not and also avoids volume overload, but there are concerns about efficacy, thrombosis risk, and cost.
The general consensus is that the platelet count should be > 50 000 to control active bleeding before major surgery or invasive procedures with a high risk of bleeding. Vitamin K replacement should be given if the PT is abnormal. PCCs and rFVIIa should be reserved for when all standard therapy has failed and the potential benefits outweigh the risks of its use.
Pediatric patients with coagulopathy of liver disease present a complex set of problems that include both bleeding and thrombosis risks that the pediatric hematologist is called on to evaluate and treat. Standard testing with routine coagulation assays for these patients does not fully guide the practitioner in the choice of appropriate management. There is a significant lack of pediatric studies to guide clinical decision making, so the majority of this discussion on management of hepatic coagulopathy is based on adult studies and guidelines.
Causes of pediatric liver failure
Differences between the leading causes of liver failure in adults compared with children (Table 1) make it difficult to apply data on the coagulopathy of liver failure derived from adults to the process found in children. There are 10 diseases that cause 95% of all cases of cholestasis in pediatrics, which are significantly different than the diseases seen in adults.1,2 The causes of liver disease in pediatrics vary with the patient's age. Biliary atresia and idiopathic neonatal hepatitis cause the majority of cases in the neonatal period and account for 60% of all pediatric liver disease.3 Cholestasis can be a complication from as little as 2 weeks of total parenteral nutrition and is seen in almost half of infants whose birth weight is < 1000 g.4 Viral hepatitis varies in the etiologic agents seen in neonates compared with older children or adolescents.1,5 Congestive hepatic failure is seen in pediatric congenital heart disease that causes failure of production of clotting factors and is associated with both bleeding and thrombotic complications.6 Liver failure is divided into acute liver failure, with most cases resolving in 3 months, and chronic liver failure that does not resolve in > 6 months. The general impression is that acute liver failure has less coagulopathy unless there is fulminant hepatic failure compared with chronic, cholestatic liver failure and cirrhosis.7
Diseases causing liver failure in pediatric patients
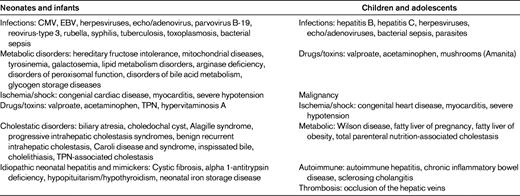
(Used with permission from D'Agata et al.1 )
Pathophysiology of the coagulopathy of liver disease
The liver plays a central role in the hemostatic system because it produces the majority of the coagulation factors, anticoagulation factors, and fibrinolytic proteins found in plasma. The hemostatic changes of liver disease have been extensively reviewed elsewhere,7,10,11 and may include multiple changes such as thrombocytopenia secondary to decreased levels of thrombopoietin, increased splenic sequestration of platelets secondary to portal hypertension and splenomegaly, decreases in levels of both coagulation and anticoagulation proteins (antithrombin, protein C, and protein S), vitamin K deficiency (compromising production of FII, FVII, FIX, and FX plus proteins C and S), decreased levels of fibrinolytic proteins, and increased plasma levels of FVIII and VWF. Platelet function defects are also seen, but are of unclear significance.12,13 Elevated levels of markers of platelet activation, thrombin and fibrin generation, and fibrinolysis are present, but can be due to hyperfibrinolysis, low-grade disseminated intravascular coagulation, or decreased hepatic clearance.12 Other conditions play a role in the bleeding tendency of patients with decompensated chronic liver disease, including hemodynamic alterations due to portal hypertension or endothelial dysfunction and the development of endogenous heparin-like substances due to bacterial infections and renal failure.11
Thrombosis risk in patients with liver disease
Patients with hepatic coagulopathy have been thought to be “auto-anticoagulated” because of their prolonged PT, but several studies show that between 0.5% and 1.9% of patients with liver disease develop deep vein thrombosis and pulmonary emboli, higher rates than observed in the general population.14–16 In a retrospective study of 190 adult chronic liver disease patients with cirrhosis, Dabbagh et al showed that 6.3% of the patient group had an in-hospital venous thromboembolism diagnosis, either a deep venous thrombus or a pulmonary embolism, over the 7 years of the study. There was no protective effect seen from an elevated international normalized ratio (INR), with half of the cases of venous thromboembolism occurring in patients with an INR > 1.6.17
Hypercoagulable states have now been implicated in increased rates of portal vein thrombosis, particularly the prothrombin G20210A mutation in patients with cirrhosis.18 Intrahepatic thrombus formation has been seen in the setting of both cirrhosis and acute liver failure. The intrahepatic microthrombi are thought to play a role in the progression of fibrosis and to result in local ischemia.19 Patients with FV Leiden have been shown to have a faster progression of hepatitis.20 Tripodi et al have shown that the hypercoagulability of plasma from patients with cirrhosis appears to result from increased levels of FVIII and decreased levels of protein C.21 Thrombomodulin does not down-regulate thrombin generation in plasma from chronic liver disease patients as effectively as it does in plasma from healthy subjects, contributing to the risk of thrombosis in liver disease11,22
Concept of rebalanced hemostasis in hepatic coagulopathy
Lisman et al and Tripodi et al have published extensively on the important concept that there is a balance between decreased levels of coagulation proteins and anticoagulation proteins in patients with liver disease that results in their being able to form thrombin and manifest thrombosis, even in the setting of abnormal laboratory tests of general hemostasis such as prolongation of the PT and activated partial thromboplastin time (aPTT).10,23–25 Defects in platelet number and function, procoagulant function, and regulation of fibrinolysis appear to be balanced by substantially elevated levels of FVIII and VW and reduced levels of ADAMTS13 in cirrhosis, which may result in enhanced thrombus formation by decreased proteolysis of VWF, and deficiencies of the natural anticoagulants protein C, protein S, and antithrombin in liver failure. Decreased levels of antifibrinolytics (eg, antiplasmin and thrombin-activatable fibrinolysis inhibitors) may also change the balance of coagulation and anticoagulation in patients with hepatic coagulopathy and reduce the risk of bleeding.26 Lisman postulates that in the “average” patient with liver disease, overall hemostasis is rebalanced due to the alterations in both the pro- and antihemostatic processes, but that this is probably less stable than in the healthy individual due to the reduced plasma levels of most of these proteins, making the individual hepatic coagulopathy patient able to flip into a hypo- or hypercoagulable state, depending on complications such as infections and renal failure.24 Prophylactic treatment with antibiotics in cirrhosis patients has been shown to decrease bleeding risk, presumably by helping to maintain the balance between the decreased levels of pro- and anticoagulation factors, but also possibly by improvement of hemodynamics.27
Standard tests of coagulation are not predictive of bleeding in patients with liver disease
The longstanding dogma that patients with liver disease have a hemostasis-related bleeding tendency is no longer supported by data from either clinical or laboratory studies.24,28,29 Platelet procoagulant activity as assessed in thrombin-generation assays in platelet-rich plasma is fully preserved in patients with cirrhosis.12 Clinical data on the most relevant bleeding problem in adult patients with cirrhosis, bleeding from esophageal varices, show that it is a consequence of local vascular abnormalities and increased splanchnic blood pressure, and the role of abnormal hemostasis is questionable.30 Known bleeding complications seen in liver disease, including bruising, purpura, epistaxis, gingival bleeding, menorrhagia, and bleeding associated with invasive procedures may be related to defects in the levels of hemostatic proteins. However, elevated venous pressure may contribute to bleeding problems that appear initially to be related to hemostatic problems, and FFP infusions may instigate or exaggerate this problem.24 Bacterial infections are common in patients with cirrhosis and are associated with increased bleeding risk.31 Renal failure complicates hepatic failure and uremia is associated with disturbed platelet-vessel wall interaction. It has been shown that renal function is a predictor of intraoperative blood loss and transfusion requirements in liver transplantation.32
It has become such an accepted protocol to check a “clotting screen” before any invasive procedure in a patient with liver disease and to normalize the INR and platelet count before the procedure, that the surgeon or anesthesia service will not accept a patient with an abnormal PT, aPTT, or platelet count for any kind of procedure. Studies as far back as Ewe in 1981 showed that bleeding after liver biopsy was not correlated with the PT, platelet count, whole blood clot time, length of biopsy cylinder, or liver histopathology.33 Further studies have shown that the PT, aPTT, and bleeding time were poorly correlated with the actual duration of bleeding and the amount of blood loss.28,34–37 Abnormal bleeding with liver biopsy cannot be predicted with certainty using present standard coagulation studies, which only look at the coagulation factor part of the “rebalanced” hemostatic system in liver disease. There are no current tests that will allow the practitioner to confidently predict that a patient is not likely to have serious bleeding despite an abnormal bleeding test.29
Thromboelastography (TEG) is a global test of hemostasis that measures the torque exerted on a pin immersed in whole blood in the middle of a rotating cylindrical vessel. This allows measurement of platelet interaction with fibrin and subsequent weakening of these interactions as fibrinolysis occurs. This method monitors more than the function of soluble plasma coagulation factors and is now being used in cardiac surgery and intensive care units to predict bleeding.38,39 TEG also has application in identifying the appropriate blood product needed in the perioperative period for liver transplantation.40 Blood clot formation measured by TEG in patients with cirrhosis is generally preserved.41,42 A recent publication by Stravitz et al looked at 51 patients with acute liver injury or failure in whom bleeding complications are usually rare. The patients' mean INR was 3.4 with a range of 1.5-9.6, TEG was done on all of the patients. The mean TEG values were normal and 5 individual TEG parameters were normal in 32 of 51 patients (63%), suggesting that the dynamics of clot formation were generally well preserved.43 It is important to remember that TEG is not sensitive to VWF levels or function.44
Thrombin-generation tests—both the measurement of thrombin generation with and without added thrombomodulin and the method that uses a snake-venom extract to act as a surrogate activator of protein C (Protac; Pentapharm)—have also been used in studying the ability of the coagulation system to form clots. These tests have shown a procoagulant imbalance that increases with the severity of cirrhosis as assessed by the Child-Pugh score, helping to offset the bleeding tendency associated with the deficiency of clotting factors in liver disease.11,21 The thrombin-generation assays may be more suitable than the PT and aPTT for determining the ability to form a thrombus in liver disease. There has been one abstract looking at use of TEG to predict thrombin generation using a total thrombus generation calculation from the first derivative of the TEG waveform and comparing it with a direct measurement of thrombin generation; significant correlation between the 2 measurements was found.45
The role of platelet count, PT, aPTT, and fibrinogen as basic laboratory tests to assess the status of the liver disease patient's coagulation system must be reconsidered. These tests give an estimate of the synthetic function of the liver in producing coagulation proteins and are valuable, particularly the PT, in scoring systems such as the Childs-Pugh score for prognosis in chronic liver disease and the MELD and PELD score, which are used for ranking of adult and pediatric patients for liver transplantation.46–49 However, they are not able to accurately reflect the risk of bleeding in these patients.
Approaches to the treatment of bleeding in liver disease
There are few data regarding the risk of bleeding with invasive procedures in the setting of acute liver failure, and no evidence-based guidelines regarding the goals of correction of the INR to avoid bleeding problems. Multiple strategies for the management of coagulopathy in adult acute liver failure patients have been published, but they are based on limited data (Table 2). At present, the general consensus is that the INR needs to be corrected to ≤ 1.5 to control bleeding risk before procedures, although there are no data to support this practice.50 The paradigm of correcting abnormal PT before liver biopsy has been challenged by the recent position paper of the American Association for the Study of Liver Disease (AASLD) stating that the “use of prophylaxis or rescue strategy such as plasma, fibrinolysis inhibitors, or recombinant factors should be considered in specific situations, although their effectiveness remain to be established.”51
Management of bleeding diathesis in adult liver failure
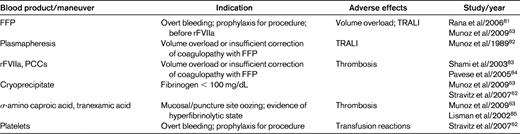
(Used with permission from Stravitz et al.50 )
FFP
Although controlling or preventing significant bleeding provides a clinical rationale for treating coagulopathy with FFP, normalization of the PT/INR without causing volume overload is difficult. Youssef et al reported on FFP transfusions in 100 adult patients with liver disease, and found that it was difficult to correct abnormalities unless large volumes of FFP (6 units) were infused and the correction was short-lived.52 The prophylactic administration of FFP has not been shown to decrease the risk of bleeding or to improve outcome.53 Auzinger et al showed that percutaneous tracheostomy in patients with liver disease and resistant coagulopathy in whom the INR could not be lowered to < 1.6 or the platelets increased to > 50 000 cells/dL was not accompanied by a higher bleeding rate than in patients in whom the INR and/or platelet count could be corrected before the procedure.54 Holland and Brooks showed that patients with minimally prolonged INRs (< 1.6) improved with treatment of the underlying disorder alone, and that adding FFP to the treatment failed to change the decrease in the INR over time. They also observed that only 50% of patients with an INR ≥ 1.7 showed a significant decrease in INR with FFP therapy. Patients with acute trauma, those being treated intraoperatively, those with disseminated intravascular coagulation, or those receiving prothrombin complex concentrates were excluded.55
Plasma transfusions bring with them multiple risks, including TRALI, fluid overload, allergic reactions, and possible transmission of prion disease. Historically, FFP has been the most implicated blood component in TRALI, but transfusion of all types of blood components have been implicated, even those with < 50 mL of plasma in the transfused material.56 TRALI patients usually recover within 96 hours of onset, but the case fatality rate is 5%-10%.57 Allergic reactions to FFP are common, with a frequency of 1%-3% of all transfusions, and can be a cause of frequent morbidity and a potential risk of mortality in multiply transfused patients.58 Infectious risks with known agents such as HIV, hepatitis B, and hepatitis C are low, but the rate of prion transmission via transfusion of blood products, while probably low, remains unknown.56,58,59
Significant concern has been raised about increased vascular volume and fluid overload after FFP infusions causing increased bleeding.24 In a randomized trial comparing a restrictive transfusion policy (that maintained a low central venous pressure) with liberal transfusion during liver transplantation, Feng et al showed that the restrictive policy led to a significant reduction in intraoperative blood loss and transfusion requirements.60 There is very little evidence supporting appropriate dosing of FFP in either pediatric or adult patients. The studies by Holland and Brooks in adults suggest that a dose of 21 mL/kg of body weight is needed to achieve a target INR of 1.7 from an initial INR of 3, but that it takes 50 mL/kg to achieve an INR of 1.3 from a starting INR of 3.55 Current guidelines for FFP dosing in pediatric patients cited in a review by Morley recommend 15 mL/kg of body weight, with a range of dosing of 10-20 mL/kg of body weight,61 which is in agreement with adult guidelines.62 A follow-up PT is needed after the infusion of FFP to ensure that the desired correction of the INR has been achieved.
Fibrinogen replacement
There is general agreement between existing adult and pediatric guidelines that fibrinogen should be replaced if it is < 100 g/dL.61–63 Dosing of cryoprecipitate for pediatric use is typically 4-5 mL/kg of body weight, with adult dosing being 1 unit (10-20 mL)/10 kg of body weight.61,62 A fibrinogen concentrate (Haemocomplettan P/RiaSTAP, CSL Behring) is being marketed for the treatment of congenital hypofibrinogenemia, and has been used in some European countries for the management of acquired hypofibrinogenemia. Little research is available to guide its use in acquired coagulopathy, and it cannot be recommended at present for routine treatment, except for its labeled indication to treat congenital hypofibrinogenemia.61
Platelet transfusions
Keeping the platelet count > 50 000/dL has been suggested as an acceptable threshold before an invasive procedure with high risk of bleeding or for the treatment of overt bleeding.50,62,63 The response to the platelet transfusion may be markedly less than the expected 8000-10 000 cells/mL per unit of random donor platelets transfused in the average adult because of the enlarged, congested spleen and possible disseminated intravascular coagulation seen in liver disease.7
Vitamin K replacement
There is general agreement in the adult guidelines that replacement of vitamin K should be considered in all patients with liver failure because > 25% may have vitamin K deficiency as a contributing factor. A dose of 10 mg of vitamin K administered parenterally is recommended for adults.64 Pediatric guidelines on acquired coagulopathy recommend that any patient suspected of having vitamin K deficiency should receive supplementation, but that if there is no response, repeated administration of large doses should be avoided61 Pediatric dosing for vitamin K deficiency is 30 μg/kg given intravenously, subcutaneously, or orally, based on guidelines for reversal of warfarin.65 Parenteral administration of phytonadione can be accompanied by hypersensitivity reactions, anaphylaxis, and/or shock, and therefore published guidelines should be followed closely.
rFVIIa and prothrombin complex concentrates
Most of the rFVIIa (NovoSeven; Novo Nordisk) used now is off-label, which includes its use in the setting of hepatic coagulopathy. There has been significant heterogeneity of dosing schedules used in this setting, which makes the data difficult to interpret. The approved dose for inhibitor therapy in hemophilia is 90 μg/kg/dose every 2 hours as needed, and for congenital FVII deficiency is 15-30 μg/kg/dose every 4-6 hours until hemostasis is obtained.66 Clinical trials of rFVIIa in the treatment of active bleeding in liver transplantation have used doses ranging from 20-120 μg/kg/dose on varying schedules.67–70
The clinical efficacy of rFVIIa in the treatment of cirrhotic patients with variceal bleeding has been studied in 2 randomized, double-blinded trials by Bosch et al, which showed no overall effect of rFVIIa versus placebo and no significant differences in reported mortality, incidence of adverse events, or treatment failure.71,72 In the earlier trial, post hoc analysis of rFVIIa in patients with moderate to severe cirrhosis (Child-Pugh class B and C) and variceal bleeding showed a significant decrease in the number of patients in whom this failed to control the bleeding.72 There have been 4 randomized, controlled trials of prophylactic rFVIIa used during orthotopic liver transplantation. These studies are heterogeneous in design, primary outcomes, and doses of rFVIIa used. None showed any difference in mortality or thromboembolic adverse events (TAEs), and only one study showed a benefit of rFVIIa on decreasing RBC transfusion requirements.66–70
Whereas rFVIIa has rarely been associated with TAEs when used for labeled indications, there has been concern with increased rates of TAEs with off-label use. A meta-analysis by Hsia et al of TAE rates across 22 clinical trials for various indications revealed a statistically significant increase in arterial thromboembolism, with a 4.5% rate in the rFVIIa-treated patients and a 2% rate with placebo.73 Birchall et al found an overall risk of TAEs of 8% in the rFVIIa-treated patients in the 17 studies included in their review, compared with a 5% TAE rate for patients treated with placebo.74
Consensus recommendations for the off-label use of rFVIIa were published by a multidisciplinary panel convened jointly by the Society for the Advancement of Blood Management and the University Health System Consortium in 2005. Rescue therapy was deemed to be appropriate for adult patients with uncontrolled bleeding in the setting of cardiac, aortic, hepatic, or orthopedic surgery if they failed to control bleeding with significant clotting factor replacement, which was defined as 20 mL/kg or 6 units of FFP, 6 units of platelets twice for platelet counts < 50 000 cells/mL and/or 10 bags of cryoprecipitate when the fibrinogen was low.75 Logan and Goodnough recommended that practicing hematologists exercise restraint in the use of rFVIIa in the off-label setting.66
aPCCs contain FII, FVII, FIX and FX plus protein C, protein S, and traces of antithrombin, heparin, and vitronectin. These are pooled plasma products that have viral inactivation methods applied during their manufacture. They have been used in the setting of severe liver disease to control bleeding and in preparation for elective surgery carrying the risk of bleeding. They have also been used when the risk of circulatory overload limits the use of FFP.76 Lorenz et al reported on use of PCCs in treating 22 adults with severe liver disease who required rapid hemostatic correction for control of bleeding or before urgent surgery or invasive procedures. Clinical efficacy of a median dose of 25.7 units/kg was rated as very good in 76% of patients, and there were no adverse events reported.77 The Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party reported their recommendations for the use of PCCs in 2009, and stated that there are no randomized, controlled trials providing clear evidence on the use of PCCs, only observational and retrospective studies on which to make recommendations. They considered liver disease with acquired deficiencies of factors in the prothrombin complex to be an indication for the use of PCCs as a second-choice alternative to FFP because the risk of thrombosis was higher with the PCCs. They did recommend a first dose bolus of 20-25 units/kg and that further dosing would need to be adjusted based on the PT and INR response to the initial bolus.76 Many of the same concerns that are present with rFVIIa would seem to apply to the use of PCCs in liver coagulopathy. Use of antithrombin concentrates was also reviewed by the Italian group, and in patients with acquired antithrombin deficiency, it was felt that there were too few studies available and that it was not indicated due to the lack of proof of clinical efficacy in the setting of acute or chronic liver disease.76
Antifibrinolytics
Antifibrinolytics such as aprotinin and tranexamic acid have been shown to reduce blood loss during liver transplantation, but aprotinin was taken off the market in 2007 due to an increased mortality risk compared with epsilon-aminocaproic acid and tranexamic acid in patients undergoing coronary artery bypass grafting. One controlled trial from 1976 has been published on the use of tranexamic acid outside of surgical procedures in liver disease patients.78 That trial showed that tranexamic acid therapy versus a placebo resulted in a significant reduction in the rate of surgical intervention to control upper gastrointestinal bleeding. There also appeared to be a reduction in the transfusion rate after the first 3 days of hospitalization. There was no difference in mortality rates between the 2 arms of the study.78 Two controlled trials of tranexamic acid therapy during orthotopic liver transplantation have been performed,79,80 with the first comparing it with epsilon-aminocaproic acid and placebo.79,80 Administration of peripheral RBCs was significantly reduced during orthotopic liver transplantation, but not with epsilon-aminocaproic acid. There were no differences in transfusion requirements after transplantation, thromboembolic events, reoperations, or mortality between the 3 groups.79 In the second study, small-dose tranexamic acid was given by continuous infusion versus a placebo during liver transplantation surgery. Fibrinolysis was decreased in the tranexamic acid group, with only 3 of 16 patients requiring epsilon-aminocaproic acid rescue for fibrinolysis, whereas 9 of the 16 control patients required rescue therapy with epsilon-aminocaproic acid. There was no difference in the transfusion requirements during surgery and for the first 24 hours after surgery in the study.80 Stravitz considers it reasonable to add epsilon-aminocaproic acid to the treatment of acute liver failure in the setting of severe hypofibrinogenemia and possible hyperfibrinolysis, but Morley recommends in pediatric practice to restrict antifibrinolytic therapy to use in specific settings such as prevention of bleeding after cardiac or orthopedic surgery, severe menorrhagia, or severe epistaxis or bleeding after dental surgery.50,61
My approach to treating bleeding in pediatric patients with liver disease, based on published data on the differences between pediatric and adult patients and pathologic conditions, is summarized in Table 3.
Disclosures
Conflict-of-interest disclosure: The author has consulted for, received honoraria from, and is on advisory committees for Novo Nordisk and Bayer. Off-label drug use: rFVIIa and PCCs used in hepatic coagulopathy management.
Correspondence
Brian M. Wicklund, MDCM, MPH, Department of Hematology/Oncology, Children's Mercy Hospital, 2401 Gilham Rd, Kansas City, MO 64108; Phone: (816) 234-3265; Fax: (816) 855-1700; e-mail: bmwicklund@cmh.edu.

