Abstract
Thrombophilia is found in many patients presenting with venous thromboembolism (VTE). However, whether the results of such tests help in the clinical management of such patients has not been determined. Thrombophilia testing in asymptomatic relatives may be useful in families with antithrombin, protein C, or protein S deficiency or homozygosity for factor V Leiden, but is limited to women who intend to become pregnant or who would like to use oral contraceptives. Careful counseling with knowledge of absolute risks helps patients in making an informed decision in which their own preferences can be taken into account. Observational studies show that patients who have had VTE and have thrombophilia are at most at a slightly increased risk for recurrence. In an observational study, the risk of recurrent VTE in patients who had been tested for inherited thrombophilia was not lower than in patients who had not been tested. In the absence of trials comparing routine and prolonged anticoagulant treatment in patients testing positive for thrombophilia, testing for such defects to prolong anticoagulant therapy cannot be justified. Diagnosing antiphospholipid syndrome (APS) in women with recurrent miscarriage usually leads to treatment with aspirin and low-molecular-weight heparin (LMWH), although the evidence to support this treatment is limited. Because testing for thrombophilia serves a limited purpose, this test should not be performed on a routine basis.
Introduction
Some form of thrombophilia (eg, deep vein thrombosis or pulmonary embolism) can be identified in approximately half of patients presenting with venous thromboembolism (VTE). Over the past decades, testing for thrombophilia has increased tremendously for various indications,1 but whether the results of such tests help in the clinical management of patients has not been determined.2,3 This chapter reviews the most commonly tested thrombophilic abnormalities and their associations with VTE and other clinical conditions. The focus is on whether and how testing might help in the clinical management of VTE patients, using absolute risk estimates of VTE and balancing these against the drawbacks of testing.
Definition of thrombophilia
In the 19th century, Virchow's triad included changes in blood coagulability as one of the mechanisms that predispose to thrombosis. These changes in blood coagulability (ie, thrombophilia) indicate the presence of a hypercoagulable state leading to a thrombotic tendency. Important risk factors for VTE were mainly identified in studies composed of families with a high incidence of thrombotic disease. Acquired risk factors also increase coagulability, as is the case in antiphospholipid syndrome (APS). Thrombophilia may therefore be defined as an inherited or acquired abnormality of hemostasis predisposing to thrombosis.
The currently most commonly tested inherited thrombophilias include deficiencies of antithrombin, protein C, or protein S, and the gain-of-function mutations factor V Leiden and prothrombin G20210A, which affect either the procoagulant or the anticoagulant pathways.2 Lupus anticoagulant, anticardiolipin antibodies, and anti–β2-glycoprotein 1 antibodies, all laboratory features of acquired thrombophilic APS, are also usually included in a thrombophilia testing panel.4 Elevated levels of several coagulation factors, including factors VIII, IX, and XI, also increase the risk of VTE.5–7 Although the levels of coagulation are in part determined genetically, factor VIII increases with age and during various inflammatory diseases including VTE, but this is beyond the scope of this chapter.
Some laboratories also include other, less well-established polymorphisms in their thrombophilia panel, for which clinical implications are uncertain. Examples of this are MTHFR 677TT and PAI-1 4G/5G, both of which have a weak association with VTE at most.8
Thrombophilia testing: prevalence and association with various clinical conditions
Table 1 depicts the prevalence of various established thrombophilias in the general population, as well as their relationship with first and recurrent episodes of VTE, arterial thrombosis, and pregnancy complications. These defects are consistently associated with a first episode of VTE, with relative risk increases of 2 to 10.2,9 However, inherited thrombophilias only modestly increase the risk of recurrent episodes.2,10 In addition, the association between thrombophilias and arterial thrombosis or pregnancy complications is not consistent11,12 ; nevertheless, approximately half of all thrombophilia tests are performed in this setting.1 The prevalence of persistent lupus anticoagulant or antibodies against phospholipid in the general population is not well known, because in most population-based studies these were only assessed once.2
Prevalence of thrombophilia and relative risk estimates for various clinical manifestations
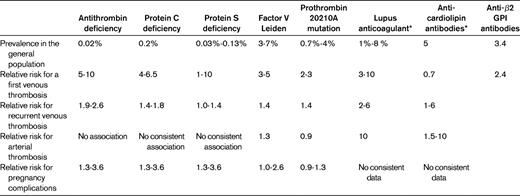
Figures are derived from studies that are reviewed in detail elsewhere.2
*In most studies, the presence of these thrombophilic risk factors was only assessed once.
Testing for thrombophilia to modify the risk of a first VTE
In clinical practice, requests for thrombophilia testing often come from asymptomatic individuals with a family history of VTE, in whom the index patients may have a known specific thrombophilic defect. Having a family history of VTE is a very poor predictor of the presence of thrombophilia.13,14 However, VTE in one or more first-degree relatives increases the risk of VTE by approximately 2-fold, also in the absence of an inherited thrombophilic defect.14 Nevertheless, a potential advantage of testing patients with VTE for thrombophilia may be the identification of asymptomatic family members who test positive so that preventive measures can be taken (and to withhold such measures if relatives have tested negative). An important requisite is that a test result dichotomizes carriers and noncarriers in terms of their risk for a first episode of VTE.
VTE risks have been assessed in several retrospective and prospective family cohort studies with similar design, which are summarized in Table 2 and in a recent review.2 The overall annual incidence of a first VTE in individuals with antithrombin, protein C, or protein S deficiency is ∼ 1.5%, whereas this risk is ∼ 0.5% for carriers of the factor V Leiden or prothrombin 20210A mutation. These estimates roughly correspond to the results of multiplying the baseline risk in the general population with the relative risk estimates listed in Table 1. Obviously, the 2% annual major bleeding risk associated with continuous anticoagulant treatment outweighs the risk of VTE.15 Table 2 also shows that even during high-risk situations such as surgery, immobilization, trauma, pregnancy, the postpartum period, and the use of oral contraceptives, the absolute risk of VTE is generally low, with the exception of women with some genetic defects who use oral contraceptives or are pregnant.
Estimated incidence of a first episode of VTE in carriers of various thrombophilic defects (data apply to individuals with at least one symptomatic first-degree relative)
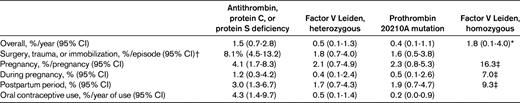
Figures are derived from numerous family studies that are reviewed in detail elsewhere.2
*Based on pooled overall risk of 18 (range, 8-40) and an incidence of 0.1% in noncarriers.
†These risk estimates largely reflect the situation before thrombosis prophylaxis was routine patient care.
‡Data are from family studies, and risk estimates are lower in other settings.
For women who wish to use oral contraceptives and have a positive first-degree relative with VTE and a known thrombophilic defect, one can estimate the effect of avoidance of oral contraceptives on the number of prevented episodes of VTE by thrombophilia testing, or alternatively, using a positive family history without thrombophilia testing. These results are listed in Table 3, where the first column shows the observed incidence of VTE during one year of oral contraceptive use in carriers and noncarriers from thrombophilic families. From the risk difference between carriers and noncarriers (shown in the second column of Table 3), the number of women who need to refrain from oral contraceptive use to prevent one episode of VTE can be calculated (shown in the third column). Table 3 clearly indicates that women with antithrombin, protein C, or protein S deficiency have a high risk of VTE that is provoked by the use of oral contraceptives. However, in these families, women without such deficiencies also have a markedly increased risk of oral contraceptive–related VTE compared with the general population (0.7% vs 0.04% per year of use), reflecting a selection of families with a strong thrombotic tendency in which as-yet-unknown thrombophilic defects have cosegregated. Therefore, although selective avoidance of oral contraceptives prevents VTE episodes in deficient women, a negative thrombophilia test may lead to false reassurance. The risk estimates for the more common and less severe thrombophilias such as factor V Leiden and the prothrombin 20210A mutation lead to a large number of women needing to avoid use of oral contraceptives to avoid one VTE, with a corresponding number needed to test of 666. Women from these families who do not have the mutation have a higher incidence of oral contraceptive–related VTE than women in the general population (0.2% vs 0.04% per year of use).
Estimated number of asymptomatic thrombophilic women or women with a positive family history for VTE who should avoid using oral contraceptives to prevent one VTE, and estimated number needed to test
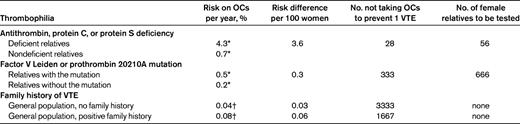
OCs indicates oral contraceptives.
*Based on family studies as outlined in Table 2.
Table 4 indicates the numbers needed to test to initiate prophylactic measurements around pregnancy, again applicable to women from thrombophilic families. For women with antithrombin, protein C, or protein S deficiency, or those who are homozygous for factor V Leiden, the risks of 4% and 16%, respectively, during pregnancy and the postpartum period may outweigh the nuisance of daily subcutaneous low-molecular-weight heparin (LMWH) injections with frequently occurring skin reactions and the very small risk for severe complications of anticoagulant therapy during pregnancy.18–20 However, the optimal dose of LMWH prophylaxis in pregnancy has not been established, and the most often used regimen of low-dose LMWH is certainly not 100% effective.20,21 Therefore, the figures shown in Table 4 underestimate the true number of women who need to use prophylaxis (and be tested before this decision) to avoid pregnancy-related VTE. Whether the absolute risks of pregnancy-related episodes justifies prophylaxis for 8 months during pregnancy or the shorter postpartum period of 6 weeks is a matter of physician and patient preference. The risk of pregnancy-related VTE in women from these families who do not have the inherited thrombophilic defect is ∼ 0.5%, compared with 0.2% in the general population,22 so withholding prophylaxis from women from thrombophilic families who do not have the defect is supported by evidence.
Estimated number of asymptomatic thrombophilic women who should use LMWH prophylaxis during pregnancy and/or the postpartum period to prevent pregnancy-related VTE, and estimated number needed to test
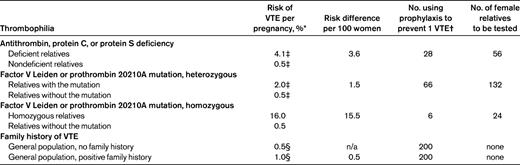
n/a indicates not applicable.
*Antepartum and postpartum combined.
†These estimates apply to women with a positive family history of VTE and assume an unrealistic 100% efficacy of prophylaxis with LMWH.
‡Based on family studies as outlined in Table 2.
Thrombophilia testing for patients with VTE
Thrombophilia testing is most often considered in patients with VTE, particularly if they are young, have recurrent episodes, have thrombosis at unusual sites, or have a positive family history for the disease. However, although such a strategy may lead to an increased yield of testing, the main question is whether a positive test result alters management. VTE tends to recur, with a cumulative incidence of a second episode of approximately 25% in 5 years. Patients with a transient clinical risk factor such as surgery eliciting their first VTE have a very low risk of recurrence.23,24 However, whether the presence of thrombophilia is able to predict recurrence is much less clear, with conflicting results in various studies comparing the prevalences of thrombophilia in patients with recurrent VTE with those in patients without recurrence.2,10
The relative risk of recurrent VTE for carriers of inherited thrombophilia found in most population-based cohorts is estimated to be ∼ 1.5-fold for most defects (Table 1). In a large pooled study of thrombophilic families, we observed a cumulative incidence of VTE recurrences after 10 years of 55% in relatives with a deficiency of antithrombin, protein C, or protein S deficiency compared with 25% in those with the factor V Leiden mutation, the prothrombin 20210A mutation, or high levels of factor VIII.25 For homozygous or double-heterozygous carriers of factor V Leiden and/or the prothrombin 20210A mutation, the estimated risks of recurrence vary widely between studies, with a pooled relative risk estimate of 2.65 (95% confidence interval [95% CI]: 1.18-5.97).26,27 Whether such a risk increase warrants prolongation of the duration of anticoagulation, particularly after provoked VTE, is a matter of debate.28 Furthermore, given the rarity of homozygous or double-heterozygous thrombophilic defects in unselected patients with VTE, the efficiency of testing is obviously very low.3,29 A randomized controlled trial in which testing for thrombophilia in patients with a first episode of VTE is the intervention and recurrent VTE is the outcome is needed. Testing should lead to a predefined strategy to prevent recurrence, for example, with a longer or indefinite duration of anticoagulant therapy. To my knowledge, no such trials have been performed successfully.30
To investigate whether testing for thrombophilia reduces the risk of recurrent VTE in patients after a first episode, for example, by prolonged use of anticoagulation, avoidance of high-risk situations, or intensified prophylaxis in high-risk situations, we selected 197 patients from the MEGA case control study who had had a recurrent event during follow-up.31 We compared the proportion of those patients who had been tested with the proportion of 324 control patients who did not have a recurrence during follow-up, matched for age, sex, year of event, and geographical region. Thrombophilia tests were performed in 35% of cases and in 30% of controls. The overall risk for recurrence was 1.2 (95% CI 0.9-1.8) for tested versus nontested patients, indicating that testing does not reduce the risk of recurrent VTE in patients who have experienced a first episode.
For patients with APS, the issue is more complicated. It is a heterogeneous syndrome, in both a clinical sense and regarding problems in the standardization of laboratory tests. There is no evidence on the optimal treatment duration of consecutive patients with VTE and persistent laboratory criteria for APS, although it is widely recommended to treat such patients for a prolonged period with anticoagulant medication.32 Vitamin K antagonists at a higher than normal international normalized ratio intensity do not decrease the risk of recurrence in patients with well-defined APS.33,34 Even if the prevalence of persistently positive tests were 10%, 10 patients with VTE would need to be tested to identify one patient with APS in whom prolonged anticoagulant treatment should be initiated.
Thrombophilia testing for other clinical indications
Numerous studies have investigated the association between thrombophilia and arterial cardiovascular diseases, and positive and negative studies are equally available.11 There is no evidence that the presence of inherited thrombophilia should lead to different secondary prevention, and testing in this clinical setting is not justified.
Pregnancy complications, such as recurrent miscarriage and fetal death, are among the clinical manifestations of APS.35 Aspirin and LMWH treatment is suggested for women with APS and recurrent miscarriage, although the evidence that this treatment is efficacious is limited.36
The association between inherited thrombophilia and pregnancy complications varies depending on the type of thrombophilia and complication (Table 1).12 However, whether this association is causal is controversial, because many other factors play a role in the risk of pregnancy complications.37,38 Therapeutic options to prevent pregnancy complications in women with thrombophilia include aspirin and LMWH. However there is currently no evidence supporting treatment, because observational research is hampered by poor methodology or inconsistent results.38,39 In women with unexplained recurrent miscarriage, 2 recent randomized controlled trials were unable to demonstrate a beneficial effect of anticoagulant therapy compared with no pharmacological treatment or placebo.40,41 Although the ALIFE study was underpowered for subgroup analyses, an a priori planned analysis in women with inherited thrombophilia showed a relative risk for live birth of 1.31 (95% CI: 0.74-2.33) for the combined intervention compared with placebo, and of 1.22 (95% CI: 0.69-2.16) for aspirin, with corresponding absolute difference in live birth rates of 16.3% (95% CI: − 18.2-50.8) and 11.8% (95% CI: − 21.1-44.6), respectively.40 The possibility that one or both of these interventions might be beneficial in such women warrants further study in adequately powered, controlled trials. With the current evidence, using anticoagulant therapy to improve the prognosis of a pregnancy in women with pregnancy complications must be considered experimental.
Drawbacks of thrombophilia testing
A disadvantage of testing patients with a VTE for thrombophilia is the high costs of testing. Although 2 studies concluded that testing for thrombophilia in some scenarios could indeed be cost-effective, the underlying assumptions from inconsistent observational studies seriously hamper their interpretation.42,43 The psychological impact and consequences of knowing that one is a carrier of a (genetic) thrombophilic defect are considered limited, although a qualitative study described several negative effects of both psychological and social origin.44,45 Furthermore, difficulties in obtaining life or disability insurance are frequently encountered by individuals who are known carriers of thrombophilia, regardless of whether they are symptomatic or asymptomatic.44
Conclusions
Despite the increasing knowledge about the etiology of VTE, testing for thrombophilia serves only a limited purpose and should not be performed on a routine basis. Thrombophilia testing in asymptomatic relatives may be useful in families with antithrombin, protein C, or protein S deficiency, or for siblings of patients who are homozygous for factor V Leiden, and is limited to women who intend to become pregnant or who would like to use oral contraceptives. Careful counseling with knowledge of absolute risks helps patients to making an informed decision in which their own preferences can be taken into account. Observational studies show that patients who have had VTE and have thrombophilia are at most at a slightly increased risk for recurrence. Furthermore, no beneficial effect on the risk of recurrent VTE was observed in patients who had been tested for inherited thrombophilia. In the absence of trials comparing routine and prolonged anticoagulant treatment in patients testing positive for thrombophilia, testing for such defects to prolong anticoagulant therapy cannot be justified. Diagnosing APS in women with recurrent miscarriages usually leads to treatment with aspirin and LMWH, although the evidence to support this treatment is also limited.
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Saskia Middeldorp, MD, Professor of Medicine, Department of Vascular Medicine, Academic Medical Center, F4-276, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; Phone: 31-20-5665976; Fax: 31-20-6968833; e-mail: s.middeldorp@amc.uva.nl.
