Abstract
Recombinant human factor VIIa (rFVIIa) is approved by the US Food and Drug Administration for use in the setting of hemorrhage associated with factor VIII or factor IX inhibitors in patients with congenital or acquired hemophilia. This indication represents only a small number of bleeding conditions. Since it became available, rFVIIa has been increasingly used in the management of off-label indications, ranging from emergent hemostasis in traumatic hemorrhage to prophylactic hemostasis in patients undergoing major surgery. Prominent off-label indications include the management of patients with coagulopathies, such as occurs in trauma patients experiencing massive and uncontrolled hemorrhage, and in patients undergoing cardiovascular surgery with cardiopulmonary bypass. Other occasions for use occur in patients with intact coagulation systems, with nontraumatic intracranial hemorrhage being the most common in this group. Uncertainties regarding the efficacy and safety associated with use of rFVIIa in these off-label scenarios have led to evidence-based assessments of patient outcomes, including mortality, the rate of thromboembolic adverse events, and posttreatment functional status. We review the evidence regarding the efficacy and safety of this important, but controversial, hemostatic agent in the off-label setting.
Physicians have long had access to pharmacologic agents to manipulate the coagulation system. Heparin, named for its original isolation from liver in 1918, was introduced into clinical use in 1937, and has since become a mainstay of therapy for a wide range of medical and surgical conditions.1 The original formulation of this pharmaceutical, unfractionated heparin, has now been supplanted in some areas of clinical use by the low molecular weight heparins, introduced in the 1990s. The oral vitamin K antagonist coumarin (later warfarin), originally derived from naturally occurring dicoumarol in sweet clover, was first developed as a rodenticide, but was later applied to clinical use in the 1950s.1 Heparin and coumarin were first tested in a randomized clinical trial in 1960.2 Today, the management of heparin or warfarin anticoagulation is considered no more specialized than the selection of antibiotics to treat community-acquired pneumonia. On the other hand, procoagulant therapies—including desmopressin acetate, tranexamic acid, aminocaproic acid, aprotinin (withdrawn from the market in 2007), activated prothrombin complex concentrates, and recombinant human factor VIIa (rFVIIa)—have, by and large, not been so widely adopted into common practice patterns in general internal medicine and perioperative surgical management. rFVIIa is emerging as an exception, however, with ever-increasing use by a wide range of physicians and surgeons.
Native factor VIIa isolated from human plasma was first shown to be effective for halting bleeding in patients with hemophilia with inhibitors to factor VIII (antihemophilic factor) in 1983,3 and later became available as a recombinant product in 1988.4 The currently marketed product, rFVIIa (Novo Nordisk A/S, Bagsvaerd, Denmark) was approved in the United States by the Food and Drug Administration (FDA) in 1999 for use in treating bleeding episodes in patients with congenital hemophilia A (deficiency of factor VIII) or B (deficiency of factor IX) with inhibitors. In 2005, the label was expanded to include use in patients who were congenitally deficient for factor VII and also for prophylaxis of surgical bleeding in patients with hemophilia A or B. The most recent modification to the FDA label in 2006 added approval for use in patients who have acquired hemophilia with inhibitors to factor VIII or IX. The agent is also approved in the European Union and Japan for control of hemorrhage in patients with Glanzmann's thrombasthenia. In spite of these very proscribed indications for use of rFVIIa, physicians have demonstrated great interest in using rFVIIa as a “universal” prothrombotic agent. Consequently, rFVIIa is now used for a wide range of off-label indications involving uncontrolled hemorrhage and various surgical scenarios, including prophylaxis against bleeding and treatment response to excessive intraoperative and postoperative bleeding.
Mechanism of Action
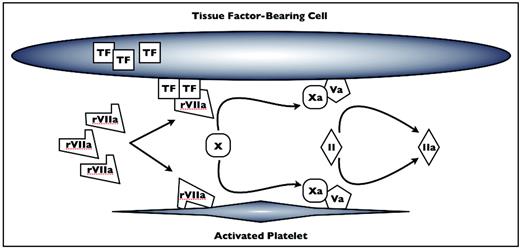
rFVIIa is thought to act via two mechanisms, both of which serve to restrict coagulation activation to the site of tissue damage (Figure 1).5,6 First, rFVIIa complexes directly with tissue factor (TF) released from the subendothelium at sites of vascular disruption. The TF–rFVIIa complex then activates the remainder of the common coagulation cascade via activated factor X. Alternatively, rFVIIa may bind to activated platelets, which also concentrates factor X activation to sites of tissue injury.7 The factor Xa generated by these two mechanisms ultimately drives the thrombin burst, which cleaves fibrinogen to fibrin, thus initiating the formation of the fibrin meshwork critical to secondary coagulation and clot stabilization. The potential role for rFVIIa in tissue factor-independent clotting has raised concern for its site specificity and the risk for off-target thrombosis. Accordingly, the FDA required the addition of a black box warning to the package insert in 2005 warning physicians of the risk of thromboembolic complications when the agent is used. In view of these safety concerns for thromboembolic adverse events (TAEs), along with continued uncertainty regarding its level of efficacy, we will review the evidence regarding rFVIIa efficacy and risks in off-label settings.
rFVIIa mechanisms of action. At pharmacologic levels, rFVIIa complexes with tissue factor (TF) from the subendothelium and on the surface of cells at the site of tissue damage. rFVIIa also binds to the surface of activated platelets. Factor X (FX) is activated by rFVIIa-TF and rFVIIa on the surface of platelets to FXa, which complexes with FVa to catalyze the conversion of prothrombin (FII) to thrombin (FIIa). Xa indicates FXa; Va, Fva; II, FII; Iia, FIIa. Adapted from Poon.6
rFVIIa mechanisms of action. At pharmacologic levels, rFVIIa complexes with tissue factor (TF) from the subendothelium and on the surface of cells at the site of tissue damage. rFVIIa also binds to the surface of activated platelets. Factor X (FX) is activated by rFVIIa-TF and rFVIIa on the surface of platelets to FXa, which complexes with FVa to catalyze the conversion of prothrombin (FII) to thrombin (FIIa). Xa indicates FXa; Va, Fva; II, FII; Iia, FIIa. Adapted from Poon.6
Randomized Clinical Trials of rFVIIa in Off-Label Settings
For most of the indications thus far studied in randomized clinical trials (RCTs), the outcomes have been negative for a clinically meaningful effect from use of rFVIIa, or the quality of data has been inadequate to guide use of rFVIIa in off-label settings due to inconclusive findings. Many studies have been underpowered or have assessed questionably relevant endpoints, while failing to address hard endpoints such as mortality, adverse events, or functional outcomes. We thus will review relevant RCTs, with emphasis on the endpoints they evaluated and their pertinent weaknesses.
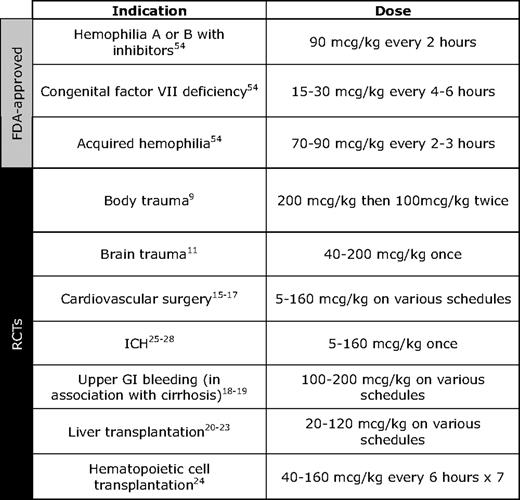
Another reason the data have been difficult to interpret, even when multiple studies have addressed a specific indication, is the heterogeneity in the dosing and schedules of rFVIIa that have been used. The approved dose for use in hemophilia with inhibitors is 90 μg/kg repeated every 2 hours as needed, whereas the recommended replacement dose for those with congenitally deficient FVII is 15 to 30 μg/kg every 4 to 6 hours until hemostasis is achieved. Clinical trials of rFVIIa for prophylaxis of surgical bleeding in settings such as liver transplantation and cardiac surgery with use of cardiopulmonary bypass have ranged 5 to 160 μg/kg, whereas trials using rFVIIa for treatment of active bleeding in trauma, spontaneous intracranial hemorrhage (ICH), and liver transplantation have ranged 5 to 200 μg/kg (Table 1).
rFVIIa Use in Trauma
In the setting of trauma, excessive hemorrhage is accompanied by a coagulopathy due to hemodilution, consumption of coagulation factors, and fibrinolysis, particularly when resuscitative transfusions do not include sufficient blood component therapy.8 Additional contributing causes of impaired clotting factor function in this context include acidemia and hypothermia. Although rFVIIa is considered to have great promise as an emergency hemostatic agent in this setting, there have been surprisingly few controlled trials to assess safety and efficacy.
Boffard and colleagues9 simultaneously reported two randomized, double-blind, placebo-controlled trials (one with patients suffering blunt trauma and one with patients suffering penetrating trauma) to assess the safety and efficacy of rFVIIa for controlling traumatic bleeding. Patients in both trials were randomized to receive rFVIIa 200 μg/kg after transfusion of their eighth unit of red blood cells (RBCs), followed by two doses of 100 μg/kg given at 1 and 3 hours following the initial dose. In these 32 center tandem trials, 69 patients with blunt trauma and 70 patients with penetrating trauma received rFVIIa. Although there was a significant decrease in the number of RBC transfusions per rFVIIa-treated patient within 48 hours of presentation in those with blunt trauma in comparison to patients receiving placebo treatment (reduction of 2.6 RBC units, P = .02), there was no significant difference for patients with penetrating trauma. Both groups experienced a roughly 50% relative risk reduction in the proportion of patients requiring massive transfusion (defined as more than 20 RBC units). Treatment with rFVIIa did not affect the number of platelet, plasma, or cryoprecipitate transfusions in either group. In the blunt trauma group, the incidence of acute respiratory distress syndrome (ARDS) was decreased significantly (3% with rFVIIa vs 16% with placebo; P = .03); however, there were no significant differences in 48-hour and 30-day mortality, rates of multiorgan system failure, or duration of intensive care unit stay.
Unfortunately, no further RCTs have been published that address whether clinical outcomes more significant than those addressed in the Boffard study are altered by treatment with rFVIIa in the setting of blunt or penetrating trauma. A large manufacturer-sponsored trial involving 150 international sites that was designed to assess the potential for mortality and morbidity benefits associated with use of rFVIIa in blunt and penetrating trauma was recently terminated when a preplanned interim data analysis predicted a low likelihood of observing a significant difference in the primary mortality endpoint.10 Dosing in this trial was identical to that in the Boffard trial (200 μg once, followed by 100 μg at 1 and 3 hours following the initial dose). Prior to termination of the trial, 218 patients were treated with rFVIIa, and 242 patients received placebo for blunt trauma; the mortality rate was 11% in both groups (P = .934). Too few patients with penetrating trauma were enrolled to permit statistical analyses.
Narayan and colleagues11 recently published the only RCT addressing the efficacy of rFVIIa in the management of traumatic brain injuries. Five dosages of rFVIIa (40, 80, 120, 160, and 200 μg/kg) were compared with placebo at 38 centers in patients with traumatic brain contusions and ICHs larger than 2 mL in volume. Thirty-six patients received placebo, and 11 to 14 patients were treated with rFVIIa at each dose level for a total of 61 patients receiving rFVIIa. Although there was a trend toward decreased hematoma volume in patients receiving more than 80 μg/kg of rFVIIa, there were no significant differences in Glasgow Coma Scores or 15-day mortality rates. A possible increase in the rate of deep vein thrombosis in the rFVIIa treatment groups was noted.
rFVIIa Use in Cardiovascular Surgery
Like patients with traumatic injuries, those undergoing cardiovascular surgical procedures facilitated by cardiopulmonary bypass (CPB) may be coagulopathic; however, in this case, the coagulopathy is iatrogenic due to the use of heparin and to platelet consumption and dysfunction that occurs during CPB.12,13 Significant crystalloid administration during surgery also contributes to dilutional coagulopathy.14 Due to these factors, individuals undergoing cardiovascular surgery may experience significant bleeding and roughly 80% require allogeneic RBC transfusions.15
Diprose and colleagues15 reported a small RCT in which 20 patients were randomized to placebo or treatment with a single dose of rFVIIa (90 μg/kg) after the reversal of CPB heparinization with protamine. Although the original power calculations for their planned trial required a minimum of 32 patients, due to inadequate funding for the trial agent, they opted to proceed with an underpowered pilot study with fewer patients. The number of patients requiring RBC transfusion was significantly lower in the rFVIIa group than in the placebo group; but, given the small sample size, there were no other statistically significant differences between the groups. Despite an 80% decrease in the cost of transfusion-related services for the rFVIIa group, there were no net savings, due to the high cost of rFVIIa treatment.
Gill and colleagues16 recently published the only other RCT in adult patients undergoing cardiovascular surgery with CPB. In this 30 center trial, patients with ongoing bleeding following reversal of heparinization after CPB were randomized to receive placebo (n = 68) or rFVIIa at doses of 40 μg/kg (n = 35) or 80 μg/kg (n = 69). Treatment with the higher rFVIIa dose significantly decreased the need for reoperation (P = .04), and patients in both groups received significantly fewer allogeneic RBC transfusions (P = .01). Mortality was not significantly different between groups, but there was a trend toward a higher rate of serious adverse events, particularly stroke, in the patients receiving rFVIIa. The authors concluded that rFVIIa should not be used for cardiovascular surgery until larger controlled clinical trials have been performed.
Addressing the experience in pediatric patients, a single randomized trial was conducted to assess the benefit of prophylactic rFVIIa during and after surgical correction of congenital heart defects while on CBP.17 Seventy-six infants younger than age 1 were randomized between 40 μg/kg of rFVIIa versus placebo. This age group was selected based on their susceptibility to dilutional coagulopathy and belief that rFVIIa therapy would avoid the need for treatment with other coagulation factors. Patients randomized to receive the agent were given a dose immediately upon reversal of heparinization with protamine and were eligible for a second dose if significant bleeding was still present after 20 minutes. Surprisingly, rFVIIa administration prolonged the time from heparin reversal to chest closure (the primary endpoint), and there was no difference in the secondary endpoints of RBCs, platelet, or fresh frozen plasma transfusions.
rFVIIa Use in Liver Disease
Patients with end-stage liver disease represent another group with coagulopathy in whom rFVIIa has been studied in various settings. Two trials by Bosch and colleagues18,19 administered rFVIIa to patients with Child-Pugh class B or C liver cirrhosis experiencing bleeding from the upper gastrointestinal tract. In both trials, rFVIIa administered after endoscopic intervention for variceal bleeding did not have a significant impact on need for RBC transfusions, emergent reprocedures, 5-day mortality, or rates of TAEs. Patients with end-stage liver disease were also enrolled in four RCTs evaluating prophylactic administration of rFVIIa during orthotopic liver transplantation.20–23 These studies are heterogeneous with respect to study design, primary outcomes, and doses of rFVIIa used, but none demonstrated a difference in mortality or TAEs. Only one study demonstrated a benefit of rFVIIa on RBC transfusion requirements (300 mL ± 133 in the rFVIIa group vs 570 mL ± 111 in the control group; P < .017).22
rFVIIa Use in Thrombocytopenia
No randomized trials have been performed in patients with bleeding related to immune-mediated or malignancy-related thrombocytopenias. Pihusch and colleagues24 conducted a randomized trial in patients experiencing bleeding between 2 and 180 days following hematopoietic cell transplantation (HCT). Treatment groups were placebo (n = 23) versus rFVIIa at 40 (n = 20), 80 (n = 26), or 160 (n = 31) μg/kg every 6 hours for seven doses. Patients underwent transplantation for a range of hematolymphoid malignancies, including acute leukemias, chronic myeloid leukemia, non-Hodgkin lymphomas, multiple myeloma, and myelodysplastic syndrome, and were closely split between full intensity (56%) and reduced-intensity (43%) conditioning regimens. The most common site of bleeding was the lower gastrointestinal tract, with other common sites being the bladder (chemotherapy-induced hemorrhagic cystitis), upper gastrointestinal tract, and the lungs (diffuse alveolar hemorrhage). The primary endpoint was bleeding score at 38 hours, and no differences were observed between the placebo group and the three rFVIIa groups combined. A post-hoc analysis demonstrated a significant improvement in bleeding score in the 80 μg/kg group when compared with those who received placebo (P = 0.02), but this effect was not seen with the 160 μg/kg group. There was no difference in transfusion requirements between any of the groups.
rFVIIa Use in Spontaneous ICH
In contrast to trauma, end-stage liver disease, cardiovascular surgery using CPB, and the post-HCT period, spontaneous ICH (in the absence of anticoagulant therapy) represents a scenario in which the coagulation system is generally intact. These bleeding episodes occur, instead, due to complications of poorly controlled hypertension, or due to anatomic lesions (eg, aneurysms or arteriovenous malformations). Four RCTs using rFVIIa in the management of spontaneous ICH have been published, all of which were reported by Mayer and colleagues.25–28 These trials excluded patients receiving anticoagulation for any reason. The first of two large, multicenter phase II dose-seeking trials was published in 2005 and involved 399 patients randomized between placebo or rFVIIa at 40, 80, or 160 μg/kg within 4 hours of stroke onset.25 Hematoma volume expansion 24 hours after presentation was significantly decreased in the patients receiving rFVIIa, compared with placebo (P = .01 for placebo vs the three rFVIIa groups combined). Mortality at 90 days was improved from 29% in the placebo group, compared with 18% in the combined rFVIIa groups (P = .02). Functional outcomes were also improved.
The favorable outcomes in this trial contrast with findings of a subsequent phase III trial performed by the same investigators in which 841 patients were equally distributed between placebo (n = 268) versus 20 (n = 276) or 80 (n = 297) μg/kg of rFVIIa within 4 hours of stroke onset.28 Despite significant reduction in expansion of hematoma size 24 hours after treatment with 80 μg/kg of rFVIIa (P < .001), there were no differences in survival or the frequency of poor functional outcome between the placebo and rFVIIa groups at 90 days measured by the modified Rankin scale. Based on the results of the earlier trial,25 these results were contrary to what was expected. Although the overall frequency of venous thromboembolic events was similar between groups, there was a significant increase in arterial TAEs in the group treated with 80 μg/kg of rFVIIa (9% vs 4% with placebo; P = .04).
In a minority of cases of ICH, patients may be coagulopathic due to, for instance, anticoagulation with warfarin for other medical conditions, such as atrial fibrillation or venous thromboembolism. This patient population was excluded from all four studies conducted by Mayer and colleagues, and a RCT of rFVIIa use in this setting has yet to be reported. The only available data for this population comes from individual case reports and uncontrolled case series. One report describes the use of rFVIIa in seven adult patients with prolonged international normalized ratio (or INR), three of whom required surgery. The doses administered ranged from 20 to 90 μg/kg, and all patients were reported to have a positive outcome.29 Two published reports of 15 total patients treated with rFVIIa for reversal of excessive anticoagulation with Coumadin support a dosage of 20 μg/kg, or 1.2 mg for an adult patient.30–32 A recent review of 12 patients with acute warfarin-associated ICH over this same time period at one institution, all of whom received rFVIIa (30–135 μg/kg) as well as vitamin K (10 mg/day for 3 days) and fresh-frozen plasma (1307 ± 652 mL) for treatment, found that treatment was associated with rapid correction of INR, and that single doses appeared safe in this high-risk population.32 These observations indicate that rFVIIa may be used to reverse the effect of warfarin or other vitamin K-antagonist therapy in cases in which the administration of vitamin K alone has been found to be insufficient. Nevertheless, available data are limited and a 2008 American Society of Hematology Evidence-Based Review recommended against use of rFVIIa for acute warfarin reversal.33
TAEs with Off-Label rFVIIa
When used for the approved indications, rFVIIa is rarely associated with TAEs. Abshire and Kenet34 reviewed the incidence of TAEs in patients with congenital and acquired hemophilia. TAEs in hemophilia patients reported to NovoNordisk, the manufacturer of rFVIIa, following the licensure of rFVIIa in 1999 until 2003, numbered only 25 of an estimated 700,000 doses administered. Additional data from clinical trials in patients with inhibitors that were conducted between 2003 and 2006, as well as additional reports (voluntary and solicited by the manufacturer) during postmarketing surveillance brought the prevalence of TAE in hemophilia patients to 4 of 100,000.35
In contrast, the risk of TAEs observed in clinical trials evaluating off-label uses of rFVIIa has raised concerns. Many off-label uses of rFVIIa are associated with derangements of coagulation status that include disseminated intravascular coagulation or severe functional impairment with immobility, both of which are independent factors contributing to risk of thromboembolic complications. Patients included in RCTs are generally highly selected and thus may not represent the general population with respect to risk for thromboembolic complications from rFVIIa therapy (eg, individuals with a history of venous or arterial thromboembolism are most commonly excluded from rFVIIa trial enrollment).36 In several of the previously mentioned trials, the occurrence of TAEs was noted, although given the small size of most trials, these events were generally not prevalent in high enough numbers to reach statistical significance.
Despite these caveats, the risk of patient harm is a primary concern with unregulated use of rFVIIa in off-label settings. In practice, many such TAEs may go unreported.37 TAEs associated with use of rFVIIa reported to the FDA Adverse Event Reporting System during the first 5 years the drug was available (1999–2004) were reviewed and found to be associated predominantly with off-label usage (151 of 168 reports).38 Arterial events were most common (54% of total), including thromboembolic stroke (21%), myocardial infarction (19%), and other arterial thromboses (14%). The remaining TAEs consisted of pulmonary emboli (18%), deep vein thrombosis and other venous thromboses (23%), and device thromboses (5%). Seventy-two percent of fatal events were attributed to the thromboembolic complication.
Thomas and colleagues39 evaluated TAE rates in patients receiving rFVIIa for control of traumatic bleeding and found that 9.4% experienced TAEs. Ten of 14 deaths at this center were thought to be due, at least in part, to the thromboembolic complication. Data from three of the four RCTs of rFVIIa for treatment of spontaneous ICH were pooled and analyzed by Diringer and colleagues.40 Although there was no increase in overall TAEs, in comparison with the placebo groups, there was a trend toward increased arterial thromboses. A systematic review of RCTs, comparative and noncomparative cohort studies, retrospective cases series, and individual case reports of rFVIIa use in the setting of cardiovascular surgery also identified stroke to be the most common TAE with use of rFVIIa (2.6% of patients receiving rFVIIa experienced stroke, whereas 1.5% developed myocardial infarction, 0.8% had deep vein thrombosis, and 0.4% had intracardiac thrombosis).41 Meta-analysis by Hsia and colleagues42 of TAE rates across 22 clinical trials for various indications revealed a statistically significant increase in arterial thromboembolism (4.5% with rFVIIa vs 2.0% with placebo; P < .01).
Current Approaches to Guidelines for Use of rFVIIa
Goodnough and colleagues43 were the first to report usage patterns and develop guidelines for use of rFVIIa at a single institution. They found that 93.8% of rFVIIa use at their academic hospital was off-label. Maclaren and colleagues44 then reported a multicenter review of rFVIIa use, including both academic and nonacademic hospitals, and found that 92% of use was for off-label indications. Several other studies have reported off-label use to similar degrees.37,38,45–48 We and others at our own institution recently completed a retrospective study of off-label rFVIIa usage patterns throughout the United States as part of a comparative effectiveness review for the Agency for Healthcare Research and Quality.49 We found that, in 2008 (the most recent year of our analysis), 97% of inpatient uses of rFVIIa were off-label.
This rate of off-label use and the high cost associated with rFVIIa have led several institutions to develop their own guidelines for off-label rFVIIa use to minimize nonreimbursable medication expenditures. At the national level, several professional organizations have also developed evidence-based guidelines for use of rFVIIa in off-label settings. Guidelines of this nature frequently direct physicians to follow an algorithm for blood component replacement prior to the use of rFVIIa. Antifibrinolytic agents may also be recommended prior to the use of rFVIIa. In some cases, consultation with a hematologist, transfusion medicine specialist, or a pharmaceutical oversight committee is required, because it has been demonstrated that involvement of a transfusion medicine43 or hematology50 consultation service leads to fewer doses of rFVIIa being administered. This may be due to consultations resulting in use of alternative blood component replacement therapy or other procoagulant therapies with which practitioners in other fields have less experience.
An example of consensus recommendations for the off-label use of rFVIIa was published by a multidisciplinary panel convened jointly by the Society for the Advancement of Blood Management and the University HealthSystem Consortium.8 In patients not taking anticoagulation therapy, the panel deemed most uses to be inappropriate or of uncertain benefit. Rescue therapy was deemed appropriate for patients with uncontrolled bleeding in the setting of cardiac, aortic, hepatic, or orthopedic surgery, if they failed to achieve hemostasis with significant clotting factor replacement (20 mL/kg or 6 units of fresh frozen plasma, 6 units of platelets twice for platelet counts less than 50,000, and/or 10 bags of cryoprecipitate twice when fibrinogen is low).8 Frontline use was deemed appropriate in blunt trauma, as supported by the finding of decreased transfusion requirements and ARDS in this population, although there was no demonstrated mortality benefit.9 Finally, frontline use was advised for nontraumatic ICH based on the positive results of a multicenter trial of rVIIa in patients with spontaneous ICH25 ; however, it is extremely important to note that these guidelines antedated the follow-up trial of rFVIIa therapy in ICH, which demonstrated no survival or functional outcome benefit.28
Given the difficulty of rapidly reversing anticoagulation with warfarin or low molecular weight heparins, the consensus recommendations allowed use if severe active bleeding is present in this patient population. The American College of Chest Physicians has also endorsed use of rFVIIa in the reversal of therapeutic anticoagulation with severe bleeding,51 and similar guidelines for use of rVIIa as an adjunct treatment for massive bleeding were recently assembled by a multinational group of physicians representing the European Society of Hematology, the European Society of Intensive Care Medicine, the European Society for Emergency Medicine, the European Resuscitation Council, the European Haematology Association, and the European Association of Trauma and Emergency Surgery.52 Unfortunately, efficacy and clinical outcomes in this setting have not been well-studied.
Alternatives to such guidelines, or perhaps a complement to them, are patient registries. Physicians are presently required to enter patients in pharmaceutical company registries, when prescribing medications such as thalidomide, lenalidomide, the thrombopoietin agonists, romiplostim, eltrombopag, and most recently erythropoietic-stimulating agents. The difficulty that critical reviewers of the literature continue to have enumerating the risk of TAEs with rFVIIa use in off-label settings demonstrate why such registries may be necessary. Although such systems bestow significant administrative burdens on treating physicians, they may ultimately be a valuable system for identifying rare, but significant, adverse events.
Conclusions
rFVIIa received orphan drug status during early application for approval from the FDA, because it was applied initially to the management of a rare disease: hemophilia with inhibitors. The drug now is used for a diverse range of off-label indications, with gross sales in the United State of nearly $140 million per year from in-hospital use.53 The clinical benefit of rFVIIa in most conditions for which it is used in off-label settings remains uncertain. As we have reviewed herein, most of the clinical trials evaluating rFVIIa in the off-label setting have not demonstrated a mortality benefit with use of this agent. Indeed, most trials have evaluated indirect outcomes, such as blood product utilization as their primary endpoints, which is only one of many important factors treating physicians must consider when deciding whether to use (or recommend use of) rFVIIa in off-label clinical scenarios. Additionally, the trial experience with rFVIIa used in the setting of ICH is instructive. Initial phase I/II data suggested a benefit, but phase III data from a large, appropriately powered study demonstrated no significant benefit from the use of rFVIIa.
Along with this uncertainty regarding efficacy, available data suggest that the risk of TAEs is increased when rFVIIa is used in off-label settings, with particular concerns being raised about arterial thromboses.55 Unfortunately, the majority of trials have thus far been underpowered to clarify the true risks. Furthermore, the underlying causes of TAEs when rFVIIa has been used off-label remain unclear. It may be due to an increased risk of thromboembolic events in these patient populations in spite of trial eligibility criteria generally excluding those at highest risk, or it may be related to the generally higher doses of rFVIIa used in off-label trials than even those recommended by the manufacturer for individuals with the severe coagulapathy associated with hemophilia with inhibitors.
In the absence of clearly supportive data, and with much data suggesting an increased risk of TAEs, we recommend that practicing hematologists exercise restraint in the use of rFVIIa in off-label settings. Until better quality and more statistically significant data regarding the use of rFVIIa in different off-label scenarios become available, we believe it is useful for professional organizations to develop conservative evidence-based guidelines for use of rFVIIa in off-label settings that should be updated frequently to reflect findings from new trials. We hope that patient safety will be appropriately guarded by this mechanism, while encouraging physicians to adapt their clinical practice to fresh evidence.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Off-label drug use: rFVIIa, efficacy/safety considerations outside of approved indications.
Correspondence
Lawrence T. Goodnough, MD, Department of Pathology, Stanford University School of Medicine, 300 Pasteur Dr., H1402, MC 5626, Stanford, CA 94305; Phone: (650) 723-5848; Fax: (650) 723-9178; e-mail: LGoodnough@stanfordmed.org