Abstract
Thromboembolism is a common, complex, and costly complication in patients with cancer. Management has changed significantly in the past decade, but remains firmly dependent on the use of anticoagulants. Low-molecular-weight heparin is the preferred anticoagulant for prevention and treatment, although its limitations open opportunities for newer oral antithrombotic agents to further simplify therapy. Multiple clinical questions remain, and research is focusing on identifying high-risk patients who might benefit from primary thromboprophylaxis, treatment options for those with established or recurrent thrombosis, and the potential antineoplastic effects of anticoagulants. Risk-assessment models, targeted prophylaxis, anticoagulant dose escalation for treatment, and ongoing research studying the interaction of coagulation activation in malignancy may offer improved outcomes for oncology patients.
Introduction
Management of thrombosis in patients with cancer has changed significantly in the past decade. This is partly due to a better understanding of the pathophysiology, the natural history, and the therapeutic response of this disease to anticoagulation. There is also a greater awareness of the negative impact of this common complication on the quality of life and life expectancy of these patients. However, multiple questions remain concerning the optimal approaches for preventing and treating thrombosis in oncology patients. Most recently, research is focusing on identifying high-risk patients who might benefit from primary thromboprophylaxis, treatment options for those with established or recurrent thrombosis, and the potential antineoplastic effects of anticoagulants. This review summarizes recent evidence on the development and validation of risk-assessment models for predicting the risk of symptomatic venous thromboembolism (VTE), the efficacy and safety of primary prophylaxis in patients with selected tumor types, treatment options in patients with recurrent thrombosis despite anticoagulation, and the survival benefits of anticoagulants in oncology patients.
Risk of VTE and Risk-Assessment Models
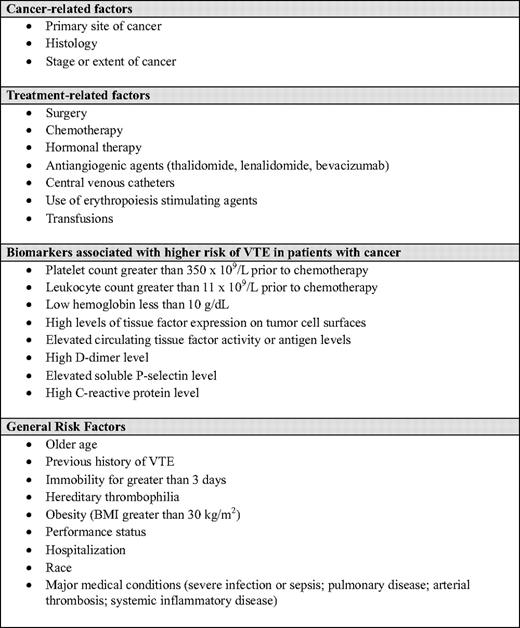
The risk of VTE in patients with cancer varies markedly between patients and even within an individual patient over time. Estimates ranging from 1% to 30% have been reported. This is largely a reflection of the large number of factors that interact and influence the risk of VTE in a heterogeneous population. Such factors have been identified using data from population-based databases, registries, hospital records, retrospective cohorts, prospective observational studies, and clinical trials (Table 1).1 However, the differences in these sources—including patient selection; duration of follow-up; and methods of screening for, diagnosing, and reporting VTE—limit our ability to estimate the true incidence of VTE and compare reported rates even in well-defined groups of oncology patients.
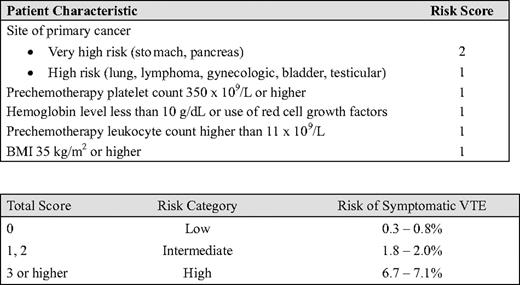
Although knowing the population incidence of VTE is useful, accurately estimating an individual patient's risk for VTE is clinically more relevant because it allows physicians to target thromboprophylaxis in those who are most at risk for VTE. To address this clinical need, a VTE risk-assessment model has been proposed that can be applied to an individual patient who is receiving chemotherapy in an outpatient setting.1,2 This model was developed using prospectively collected data from a multicenter registry, the Awareness of Neutropenia in Chemotherapy Study Group Registry, that was designed to evaluate febrile neutropenia and other chemotherapy-related complications in patients with cancer starting a new chemotherapy regimen. Five independent risk factors were identified that predicted for symptomatic VTE during the first four cycles of chemotherapy: site of cancer, pre-chemotherapy platelet count, hemoglobin level or the use of red cell growth factors, pre-chemotherapy leukocyte count, and body mass index (BMI). Using the regression coefficients from the multivariate model, a risk score model containing these five clinical and laboratory items was developed (Table 2). Patients are classified into three categories based on their total risk score: low-risk (score 0; VTE risk 0.3%–0.8%), intermediate-risk (score 1 or 2; VTE risk 1.8%–2.0%), or high-risk (score 3 or higher; VTE risk 6.7%–7.1%). The major advantage of this model is the easy availability of these common clinical markers, while the major limitation is the generalizability of the results. Because the registry contained only a small number of patients with some tumor types (e.g., brain or renal cancers) and the performance status of the patients was excellent, the model may not be predictive of VTE development in some tumor types or in those who have advanced disease and a poor performance status. Because of the age and static nature of the registry data (collected from 2002 to 2005), the model is also unable to integrate additional risk factors that are subsequently identified, such as new therapies (e.g., bevacizumab or thalidomide). It is also uncertain why some well-established risk factors for VTE, such as metastatic disease or older age, were not associated with VTE in the analysis. Incorporating additional variables such as biomarkers associated with VTE (e.g., D-dimer, tissue factor, soluble P-selectin) has been proposed, and may improve the model,3–6 but it is important to recognize that assays of these markers are not uniformly standardized and adding more score items may complicate the model without improving its accuracy or utility. Recently, the five-item Khorana model was validated and proven to be robust using data from the Vienna Cancer and Thrombosis Study in a much broader range of patients.3 This model is also being tested in an ongoing study funded by the National Heart, Lung and Blood Institute (www.clinicaltrials.gov, trial #NCT00876915). Further research and exploration of risk-assessment models will help to tailor thromboprophylaxis to reduce the burden of VTE and improve the risk-benefit ratio of anticoagulant thromboprophylaxis in individual patients.
Prevention of VTE
Primary anticoagulant prophylaxis is recommended in all oncology patients admitted to the hospital for surgical or medical reasons.7 Although there are data for unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), fondaparinux, and warfarin for primary prophylaxis, contemporary studies have largely studied LMWH.8 To date, one small phase II study has evaluated a new oral anticoagulant, apixaban, in outpatients receiving chemotherapy.9 The initial results are promising, but larger studies are needed to show efficacy and safety in the broad range of oncology patients.
Surgical Patients
It is well established that anticoagulant prophylaxis in the surgical setting reduces clinically important thrombosis.8 In the cancer population, the evidence is consistent but relatively weak because of the paucity of trials focusing on this group of patients. Nonetheless, studies have shown that prophylaxis with either UFH or LMWH will reduce the risk of deep vein thrombosis to approximately 15% after major abdominal or pelvic surgery for cancer.10,11 Also, patients with cancer may tolerate higher doses of LMWH without experiencing increased bleeding compared with patients without cancer.12 Fondaparinux has also been shown to be effective for prophylaxis in the surgical setting. In the subgroup of 1408 patients with cancer enrolled in the PEGASUS trial, fondaparinux was associated with a significant reduction in VTE compared with dalteparin (4.7% vs. 7.7%; p = 0.02), leading to a relative risk reduction of 38.6% (95% confidence interval [CI] 6.7–59.7).13
Other studies have also shown that patients with cancer benefit from a longer duration of prophylaxis for up to 1 month after surgery.14,15 In the ENOXACAN II trial, patients undergoing abdominal or pelvic surgery who received enoxaparin for 30 d after surgery had a 60% risk reduction in VTE compared with those who received the standard duration of 6 to 10 d (4.8% vs 12.0%; p = 0.02).15 The need for an extended duration of prophylaxis is also supported by prospective observational studies reporting the incidence of symptomatic VTE after cancer surgery. In the @RISTOS study that followed 2373 patients who underwent surgery for cancer, 40% of symptomatic VTE events occurred more than 3 weeks after surgery, and 46% of the deaths were due to fatal pulmonary embolism.16 The risk factors that were significantly associated with VTE were: previous history of VTE (odds ratio [OR] 6.0; 95% CI 2.1–16.8); anesthesia lasting 2 h or longer (OR 4.5; 95% CI 1.1–19.0); bed rest for 4 d or longer (OR 4.4; 95% CI 2.5–7.8); advanced tumor (OR 2.7; 95% CI 1.4–5.2); and age 60 years or older (OR 2.6; 95% CI 1.2–5.7). Similarly, the incidence of symptomatic VTE was found to peak at 3 weeks after cancer surgery in the Million Women Study.17 According to this population-based study using the United Kingdom National Health Service hospital admission database, the risk of VTE within the first 7 weeks after surgery for cancer was 92-fold higher than in women who did not have surgery, and the risk remained elevated at 53-fold higher up to 12 weeks after surgery. Overall, it was observed that 1 in 85 women having surgery for cancer developed symptomatic VTE despite standard thromboprophylaxis. Although it remains unproven that extended thromboprophylaxis will improve survival or is cost-effective after cancer surgery, reducing the burden of VTE is an important outcome. Extending prophylaxis up to 4 weeks after cancer surgery is recommended by consensus guidelines, particularly in patients with several risk factors for VTE.7,8 However, the optimal duration of prophylaxis is not known. Although the Million Women Study results suggest that prophylaxis beyond 4 weeks may be indicated, further studies are needed to evaluate the impact of extended thromboprophylaxis on important clinical outcomes.
Medical Inpatients
The risk-benefit of thromboprophylaxis in oncology inpatients has not been formally studied. One randomized controlled trial evaluating LMWH in medical inpatients reported no difference between LMWH thromboprophylaxis and placebo in the small subgroup of patients with cancer.18 Although this was a post-hoc subgroup analysis that was underpowered, it is reasonable to question whether patients with cancer require higher doses of anticoagulants because of their prothrombotic state, and whether they also have a higher risk of bleeding because many have thrombocytopenia and are usually admitted for serious medical conditions. Regardless, consensus recommendation is supportive of thromboprophylaxis in patients with cancer when they are admitted to the hospital.7,8 However, physician compliance with these recommendations is poor, perhaps reflecting the lack of evidence and the concern for serious bleeding.19
Ambulatory Patients
Oncology patients receiving chemotherapy in the outpatient setting are also at risk for VTE. The most recent trials conducted in patients with advanced pancreatic cancer receiving systemic chemotherapy have shown positive results with LMWH prophylaxis. The CONKO-004 trial found a 87% risk reduction of VTE using enoxaparin at 1 mg/kg once daily for 3 months compared with no prophylaxis (9.9% vs 1.3%; p < 0.01),20 while the FRAGEM study reported a 62% risk reduction in VTE using the CLOT therapeutic regimen of dalteparin (31% vs 12%; p = 0.02).21 These results are in contrast to negative findings reported in earlier trials evaluating LMWH given at prophylaxis doses in ambulatory patients with advanced breast cancer, non-small-cell lung cancer, or high-grade malignant gliomas. This conflicting evidence would seem to suggest that standard prophylaxis doses of LMWH may be insufficient to prevent thrombosis in patients with cancer. However, another possible reason is that prophylaxis is beneficial in only certain tumor types. In the PROTECHT study, in which 1166 patients with advanced lung, breast, gastrointestinal, ovary, or head and neck cancers were randomized to receive nadroparin or placebo while receiving outpatient chemotherapy, the prophylaxis dose of nadroparin reduced the risk of venous or arterial thrombosis by 46% from 3.9% to 2.1%,22 but this result was primarily driven by thrombotic events in patients with lung or gastrointestinal cancer. Overall, there is good evidence that LMWH is effective in reducing clinically important VTE in selected outpatients receiving chemotherapy,23 but the optimal dose, duration, and specific patient populations have to be further defined.
The first and only study evaluating an oral, direct inhibitor of activated factor X in oncology patients has been reported. In this phase II randomized trial, patients with metastatic disease receiving first- or second-line chemotherapy received one of three doses of apixaban or placebo.9 Among the 125 patients included, apixaban appeared to be well tolerated, with very few thrombotic and bleeding events observed during the 12-week drug exposure period. Further research exploring the role of this and other new oral anticoagulants is eagerly awaited.
Treatment of Cancer-Associated Thrombosis
The recommended treatment for cancer-associated thrombosis is LMWH. For the initial phase of treatment, post-hoc data from randomized trials suggest comparable efficacy between UFH and LMWH, as well as a 3-month survival advantage in favor of LMWH.24 For long-term treatment, LMWH is more efficacious than warfarin therapy and reduces the risk of symptomatic recurrent VTE by 52%.25 To date, only dalteparin has regulatory approval for the extended treatment of VTE in patients with cancer, but the other LMWHs are also being used in clinical practice. Given its convenience over UFH, the short-term survival benefit, and its superiority over warfarin, LMWH is the preferred anticoagulant for both initial and long-term treatment of cancer-associated thrombosis in major consensus guidelines.7,26 Treatment data for the newer oral anticoagulants, including dabigatran and rivaroxaban, show they are comparable to warfarin in efficacy and safety, but few patients with cancer were enrolled in these studies.27,28 These new agents are attractive alternatives because they do not require laboratory monitoring and have minimal drug interactions, but whether they provide similar efficacy and safety as LMWH or warfarin in oncology patients need to be properly studied.
Treatment of Recurrent VTE
Up to 9% of patients with cancer treated with LMWH and 20% of those treated with warfarin can develop recurrent VTE. Studies have suggested that the presence of metastasis, younger age, or a short interval between VTE and cancer diagnosis (<3 months) are predictors of recurrent thrombosis despite anticoagulation.29,30 Whether the risk factors that increased the risk of a first episode of thrombosis also contribute to a higher risk of recurrent thrombosis is unknown.
Although randomized controlled trial data are lacking to guide optimal management in oncology patients with recurrent thrombosis, observational data and increasing clinical experience support the use of LMWH in this setting. In patients who develop a recurrence while on warfarin therapy, the recommended practice is to switch these patients to LMWH because it is more efficacious than warfarin. Raising the intensity of warfarin therapy is not recommended because of a potential for increasing bleeding without a benefit in reducing recurrent VTE. Patients with cancer have a high risk of bleeding and a high risk of recurrent thrombosis despite achieving therapeutic and even higher international normalized ratios (INRs).
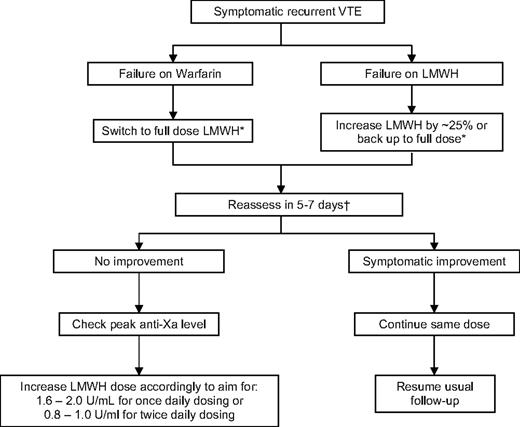
Dose escalation appears to be effective in the majority of patients who develop a recurrence while on LMWH. In a small cohort study of oncology patients with recurrent thrombosis while on LMWH or warfarin, escalating the dose of LMWH by 20% to 25% or switching to LMWH, respectively, was effective in preventing further thrombotic episodes.31 During 3 months of follow-up, 6 of 70 (8.6%) of patients developing another recurrence, while one patient had a major bleeding event and two had minor bleeding. The success of escalated doses of LMWH suggests that the standard weight-adjusted dose regimens are insufficient in some patients with cancer. This is not surprising given the heightened prothrombotic state of these patients. A suggested algorithm for managing oncology patients with recurrent thrombosis is outlined in Figure 1.
Inferior Vena Cava Filter
Insertion of a vena cava filter has been recommended for oncology patients with recurrent VTE despite adequate long-term LMWH therapy. However, adequate studies have not been done to evaluate or document the outcomes. Retrospective series have reported that up to 32% of oncology patients with filters inserted for thrombosis develop recurrent VTE.32 This high recurrence rate is not surprising because filters do not treat the underlying hypercoagulable state in patients with cancer. The single randomized trial studying the efficacy of filters in patients who were also treated with anticoagulation showed a reduction in symptomatic pulmonary embolism but higher rates of recurrent deep vein thrombosis in the filter group.33 Overall, the total VTE rates were the same and a difference in overall survival was not observed. Considering the cost and invasiveness of filters and the lack of proven efficacy, they should be used only in situations in which anticoagulant therapy is contraindicated because of serious, active bleeding, and should be avoided for the treatment of thrombosis. Research is urgently needed to study the use of filters in oncology patients.
Anticoagulants and Cancer Survival
The role of anticoagulants as anticancer agents remains uncertain. Experimental studies have provided compelling evidence, but the pathophysiological mechanisms are not yet understood. Confounded by the heterogeneity of tumor biology and treatments, as well as outcomes of different cancers, clinical studies have not yielded convincing data that anticoagulants have direct or indirect effects on malignancy growth, differentiation, and metastatic potential.
The anticancer potential of anticoagulants was first observed with UFH in animal models over 80 years ago. Only one randomized controlled trial with activated partial thromboplastin time-adjusted UFH has been conducted, and a survival benefit was observed with UFH in patients with small-cell lung cancer who were receiving chemoradiation. Similarly, warfarin was found to improve survival in patients with small-cell lung cancer. However, systematic reviews of clinical trials have failed to show an anticancer effect for UFH or warfarin.34 There are no clinical data available on the effect of fondaparinux on mortality in patients with cancer.
To date, LMWHs have the most consistent yet inconclusive evidence for an anticancer effect. The initial observation that LMWHs may have an antineoplastic effect was reported in meta-analyses of clinical trials that compared LMWH with UFH for the initial treatment of acute VTE. However, none of these trials was designed with survival as the primary outcome, and potential biases or imbalances in prognostic factors in the patients with cancer could not be ruled out. Also, it remains difficult to explain how a 1-week course of an anticoagulant could exert such a dramatic effect on the natural history of malignancy.
To specifically determine whether LMWHs can improve survival of patients with cancer, a number of randomized trials have now been completed and several are ongoing. Published studies have been summarized in a number of meta-analyses.34,35 Overall, the data show that LMWH is associated with a reduction in 1-year overall mortality in patients with cancer, with a relative risk of 0.88 (95% CI 0.78–0.98) and an absolute risk difference of 8%.34 Available evidence also suggests that patients with early-stage disease are more likely to benefit than those with advanced disease. It is important to note, however, that these analyses combined results from studies that used different preparations of LMWH given at different doses, for different durations, and in different patient populations. The clinical heterogeneity among these studies has raised concerns about the appropriateness of combining the results and overinterpretation of the data. Furthermore, recent trials studying primary prophylaxis have failed to demonstrate a difference between the LMWH-treated group and the control group during the first few months of follow-up.20–22 Whether the negative findings are reflective of the advanced-disease state of the patients in these studies is uncertain.
The question of whether LMWHs can improve survival of patients with cancer is also unanswered from a mechanistic standpoint. It is possible that the mechanism may be secondary to nonspecific suppression of thrombin generation or activity, rather than to antitumor effects that are specific and unique to LMWH. This hypothesis is supported by animal-model studies testing other anticoagulants that target specific steps in the coagulation cascade, including hirudin and recombinant nematode anticoagulant peptide c2.36,37 Inhibition of metastatic tumor growth when animals are pretreated with these anticoagulants is impressive. However, such pretreatment data from animal studies may not be extrapolated to clinical medicine, where anticoagulants are only administered after a tumor is well established. In addition, there are also data to suggest that certain anticancer mechanisms, such as interference with metastatic spread through selectin-binding inhibition, are not dependent on antithrombotic activity.38 This line of evidence would indicate that non-anticoagulant functions of large, charged molecules such as heparin may be important for the beneficial effects on survival observed in experimental animals. Other studies also show that intracellular pathways that control cell growth, apoptosis, or angiogenesis are also affected by clot-independent activities.39
Summary
Cancer-associated thrombosis is a challenging clinical problem. Anticoagulants are effective and relatively safe for the prevention and treatment of VTE in this setting. Improving patient outcomes will depend on identifying high-risk patients who would benefit most from primary prophylaxis, discovering more efficacious and safer therapies that are simple to administer, and perhaps finding the elusive common target that triggers both the hypercoagulability and malignant progression in these patients.
Disclosures
Conflict-of-interest disclosure: The author has received honoraria from Bayer, Pfizer, Sanofi Aventis, and Boehringer Ingelheim. She has also been a consultant for Bayer, Pfizer, Sanofi Aventis, Boehringer Ingelheim, and Leo Pharma. The author has also received research funding from Eisai and is associated with the Beohringer Ingelheim speakers' bureau. Off-label drug use: Rivaroxaban for the treatment of cancer-associated thrombosis; dabigatran etexilate for the treatment of cancer-associated thrombosis.
Correspondence
Dr. Agnes Y.Y. Lee, Diamond Health Care Centre, 2775 Laurel Street, 10th floor, Vancouver, BC, Canada V5Z 1M9; Phone: (604) 875-4592; Fax: (604) 875-4696; e-mail: alee14@bccancer.bc.ca