Abstract
Navigation of transplanted stem cells to their target organs is essential for clinical bone marrow reconstitution. Recent studies have established that hematopoietic stem cells (HSCs) dynamically change their features and location, shifting from quiescent and stationary cells anchored in the bone marrow to cycling and motile cells entering the circulation. These changes are driven by stress signals. Bidirectional migrations to and from the bone marrow are active processes that form the basis for HSC transplantation protocols. However, how and why HSCs enter and exit the bone marrow as part of host defense and repair is not fully understood. The development of functional, preclinical, immune-deficient NOD/SCID (non-obese diabetic-severe combined immunodeficiency) mice transplantation models has enabled the characterization of normal and leukemic human HSCs and investigation of their biology. Intensive research has revealed multiple tasks for the chemokine SDF-1 (stromal cell-derived factor-1, also known as CXCL12) in HSC interactions with the microenvironment, as well as the existence of overlapping mechanisms controlling stress-induced mobilization and enhanced HSC homing, sequential events of major physiological relevance. These processes entail dynamically interacting, multi-system aspects that link the bone marrow vasculature and stromal cells with the nervous and immune systems. Neural cues act as an external pacemaker to synchronize HSC migration and development to balance bone remodeling via circadian rhythms in order to address blood and immune cell production for the physiological needs of the body. Stress situations and clinical HSC mobilization accelerate leukocyte proliferation and bone turnover. This review presents the concept that HSC regulation by the brain-bone-blood triad via stress signals controls the bone marrow reservoir of immature and maturing leukocytes.
Introduction
“It is not the strongest of the species that survives, nor the most intelligent that survives. It is the one that is the most adaptable to change” (L.C. Megginson, 1963, commenting on the studies of Charles Darwin). “The light is what guides you home, the warmth is what keeps you there”
Ellie Rodriguez
The potential of hematopoietic stem cells (HSCs) to repopulate ablated bone marrow is the basis for their definition as true stem cells. They are dynamic and versatile cells with changing phenotypes. These primitive progenitor cells can modulate their motility, location, and cell cycle status. They respond to signals from their microenvironment as well as from remote organs. Such modulations include intensive changes in the repertoire of cytokines and their cell surface receptors, adhesion molecules, proteolytic enzyme activation, and cytoskeletal rearrangement. The changes are aimed at facilitating hematopoietic stem and progenitor cell (HSPC) migration and development, which enables their transformation from quiescent, tissue-anchored cells to motile, proliferating cells. These processes are also associated with the cell differentiation needed for the replenishment of the blood on demand with new cells with a finite life span, which is required for host defense and repair mechanisms.
The terms “homing” and “mobilization” are used to describe the in vivo migration of circulating HSPCs into their physiologic site of hematopoiesis, the bone marrow, and their enhanced exit from this organ back to the blood, respectively. HSPCs egress from the bone marrow during steady-state homeostasis is at a very low rate. However, stress conditions such as bleeding, inflammation, and injury greatly amplify and intensify this process. These processes are mimicked by clinical mobilization, in which HSPCs are recruited from the bone marrow to the circulation by means of chemotherapy induction and repeated cytokine stimulation to expand and harvest HSPCs for clinical transplantations.
HSPC mobilization and enhanced homing share various regulators and overlapping mechanisms showing that these two processes are sequential events with physiological relevance. Both directional migration processes to and from the bone marrow involve adhesion to the vascular wall and crossing over the endothelial blood-bone marrow barrier. Recently, multiple studies have established that murine HSPCs are preserved in their primitive phenotype within the bone marrow. These cells are in close contact with various stromal cells, including endosteal bone-lining osteoblasts, endothelial and peri-arterial reticular cells, as well as nestin-positive cells, all robustly expressing the chemokine SDF-1, which is essential for maintaining murine HSC quiescence.1 These nursing microenvironments, defined as “stem cell niches,” protect HSCs and provide them with signals that maintain their quiescent, slow-dividing and stationary state.1–4 Detachment of HSCs from these niches is believed to be associated with their entry into the cell cycle, proliferation, and differentiation, which is also accompanied by increased migration and recruitment to the circulation (as discussed in 5–7 ).
The nervous system, which is the body's master regulator, has been implicated in controlling the immune system (discussed in8 ). Neural and immune cells also regulate HSPC motility and development, both directly via secretion of neurotransmitters and myeloid cytokines and indirectly by way of bone remodeling processes. These include bone formation by osteoblasts, bone resorption by osteoclasts, circadian rhythms, and the dynamic nature of the stem-cell niches. The current understanding of leukocyte production on demand, the regulation of HSPC homing and mobilization, and the central role of the brain-blood-bone triad in these processes will be discussed herein.
Essential Tools for Human HSPC Research: Functional, Preclinical, Immune-Deficient Mouse Models
Understanding the mechanisms that regulate human HSPC homing and mobilization in vivo required the development of animal models for human cell engraftment. The development of functional, preclinical models of transplanted, immune-deficient NOD/SCID (non-obese diabetic-severe combined immunodeficiency) mice promoted significant progress in normal and leukemic human HSC research. Particularly, these models enabled the generation of chimeric mice engrafted with human HSPCs, which provided mechanistic insights into their phenotypic characterization, as well as the identification of molecules participating in the regulation of HSPC homing, retention, proliferation, differentiation, and mobilization. The SCID, NOD/SCID, and the later-derived immune-deficient murine models constitute an experimentally relevant microenvironment for human HSPCs due to their immunodeficiency, which makes them permissive for transplanted human cells. In addition, high homology and partial cross-reactivity exist between human and murine regulating factors, such as cytokines, chemokines, adhesion molecules, and enzymes. This enhances the success of transplanted human cells in these mice. NOD/SCID-repopulating human HSPCs, mostly defined as CD34+/CD38-/low/CXCR4+ cells, can home in the bone marrow of sub-lethally irradiated NOD/SCID hosts and imbue the mouse with a high degree of multi-lineage hematopoiesis. This process can be repeated in secondary transplanted recipient mice.5 However, these models still suffer xenogeneic limitations, because HSPCs in mice do not always produce the same results as in humans. For example, human cord blood CD34+ cells engraft in these mice better than bone marrow or mobilized CD34+ cells. Chimeric, immune-deficient mice have also been used for establishing both homing and mobilization assays that identify and characterize molecular and cellular components participating in human HSPC motility processes. The NOD/SCID mouse model was also established as a functional, preclinical model for leukemic HSPC cells obtained from some acute myeloid leukemia (AML) and precursor B-acute lymphocytic leukemia (ALL) patients. The severity of leukemia in transplanted mice correlates highly with the aggressiveness of the disease from the derived patient, and thus can be used to predict clinical outcomes. Finally, NOD/SCID mice are also used to monitor homing of human stromal progenitors. After certain manipulations, human CD44 cells could navigate to the murine bone marrow via interactions with selectins.9
Non-motile, Tissue-Anchored Quiescent HSPCs: Bone Marrow Residents
HSPCs reside primarily in the bone marrow in contact with supportive stromal cells, which maintain their undifferentiated phenotype and developmental potential. Proliferation, differentiation, and release to the circulation during the steady state occur at low levels, providing the body with short-lived hematopoietic cells as well as immature and maturing leukocytes as part of host immunity. Stress signals accelerate and intensify these processes dramatically, producing leukocytes on demand as part of host defense and repair10 and to address the body's immediate needs during emergency situations.10
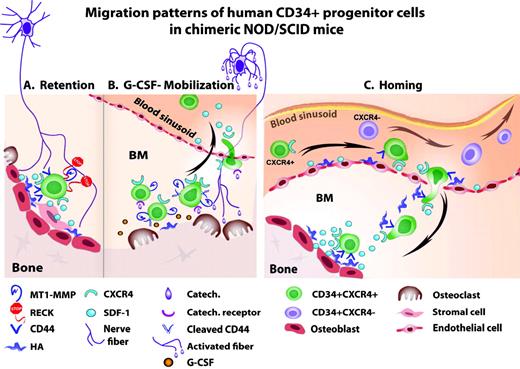
Various molecular anchors keep HSPCs in contact with stromal cells in the bone marrow.4 The chemokine SDF-1 plays a central role in HSPC regulation, with a dose, tissue, and context dependency. SDF-1 is expressed by murine bone marrow osteoblasts, endothelial, reticular, nestin-positive, and other stromal cell types. At the homeostatic basal expression level, SDF-1 acts via its major receptor, CXCR4, as a survival factor for bone marrow HSPCs, inducing their quiescence and retention. Mouse embryos that do not express SDF-1 or CXCR4 have multiple lethal defects, including the lack of bone marrow seeding by HSPCs. Human and murine SDF-1 are cross-reactive, which explains why human HSPC can home in and be retained within the bone marrow of transplanted NOD/SCID mice (as discussed in 1,5 ) HSPCs residing in the bone marrow express a wide array of adhesion molecules for attachment to the stromal supportive network. For example, immature human CD34+ cells express CD44 at high levels while retained in the bone marrow.11,12 This adhesion molecule anchors HSPCs to endothelial cells of human bone marrow blood sinusoids or endosteal osteoblasts via CD44's major ligand, hyaluronan. Proteolytic enzymes, such as the cell-surface MT1-MMP (membrane type 1 matrix metalloproteinase), cleave CD44, thus negating its function. These proteolytic enzymes are also expressed by immature human CD34+ HSPCs and by various myeloid cells.12 During steady state, these interactions are tightly controlled by RECK (reversion-inducing-cysteine-rich protein with Kazal motifs), which inhibits MT1-MMP and MMP-2/9 activity. Human CD34+ progenitors residing in the bone marrow do not secrete MMP-2/9, while circulating progenitors do produce these enzymes (discussed in5 ). These processes are also controlled by RECK, which is also expressed by human CD34+ progenitors.12 Several other molecules, such as β1-integrin and Rac1/Rac2,13 have also been implicated in regulating HSPC retention. Mechanisms mediating the retention of human CD34+ HSPCs in the bone marrow are illustrated in Figure 1A.
(A) Retention of human CD34+ HSPCs via SDF-1/CXCR4 interactions, CD44/HA interactions, and inhibition of MT1-MMP and MMP2/9 by RECK, all leading to tissue-anchored, quiescent CD34+ HSPCs. (B) G-CSF-induced mobilization of human CD34+ HSPCs via activation of osteoclasts, proteloytic enzymes (including up-regulation of surface MT1-MMP), cleavage of CD44 and SDF-1, and CXCR4 up-regulation, leading to CD34+ HSPC proliferation, differentiation, and increased recruitment to the circulation. (C) Homing of human CD34+ HSPCs in transplanted, immune-deficient NOD/SCID mice preconditioned with total body irradiation. Increased SDF-1 levels in the murine bone marrow endothelium and endosteum region attract human CD34+/CXCR4+ HSPC, while CXCR4-/low HSPCs are mostly trapped in the circulation
(A) Retention of human CD34+ HSPCs via SDF-1/CXCR4 interactions, CD44/HA interactions, and inhibition of MT1-MMP and MMP2/9 by RECK, all leading to tissue-anchored, quiescent CD34+ HSPCs. (B) G-CSF-induced mobilization of human CD34+ HSPCs via activation of osteoclasts, proteloytic enzymes (including up-regulation of surface MT1-MMP), cleavage of CD44 and SDF-1, and CXCR4 up-regulation, leading to CD34+ HSPC proliferation, differentiation, and increased recruitment to the circulation. (C) Homing of human CD34+ HSPCs in transplanted, immune-deficient NOD/SCID mice preconditioned with total body irradiation. Increased SDF-1 levels in the murine bone marrow endothelium and endosteum region attract human CD34+/CXCR4+ HSPC, while CXCR4-/low HSPCs are mostly trapped in the circulation
Stress Signals: The Impetus for HSPC Migration and Development
Stress conditions due to bleeding, inflammation, or DNA damage introduce dramatic systemic and local changes, which have a large impact on HSPC regulation. These changes include detachment of the progenitors from their bone marrow docking sites, cell cycle entry, increased motility, and recruitment. These cells follow the stress signals via the circulation toward the injured tissues. Interestingly, long-term repopulating HSCs extensively proliferate in response to in vivo bacterial infection. Both infection and interferon (IFN)-γ administration also facilitate HSPC mobilization, demonstrating an important role of IFN-γ in HSPC quiescence and mobilization.14
Inflammation is also associated with increased production of granulocyte colony-stimulating factor (G-CSF), which triggers HSPC mobilization. This physiologic effect is clinically utilized to expand and mobilize HSPCs from the bone marrow to the circulating blood in order to harvest progenitors for stem-cell transplantations. Experiments in parabiotic mice with a shared circulation demonstrate that HSC mobilization and homing are sequential events that are both triggered and enhanced by G-CSF, since HSPC mobilization by repeated G-CSF stimulation also leads to increased homing and engraftment of the partner mouse's bone marrow.5–7
The bone marrow is a highly vascularized organ. Sinusoidal vessels are believed to be the site of HSPC exchange between the bone marrow and the circulating blood. Thus, endothelium integrity and the regulation of its permeability are pivotal in the endothelium's role as the “gatekeeper” of the blood-bone marrow barrier. Bone marrow endothelium permeability is increased by total body irradiation, chemotherapy, and inflammation (as discussed in 3,5 ) all of which enhance HSPC bidirectional transmigration.
HSPC Mobilization: Extensive Recruitment
Mobilization by repeated G-CSF stimulation requires awakening the quiescent HSPCs, generating guiding signals, locally repressing the inhibitory attachment machinery, and gaining motility. Stress signals induce the detachment of HSCs from their docking sites and inhibit bone marrow retention-inducing factors. These signaling molecules can be delivered via the circulating blood from a remote site in an endocrine-regulated fashion. However, stress signals can also be transmitted in a paracrine-regulated fashion to HSPC via stromal cells within the neighboring bone marrow vicinity. The chemokine SDF-1 also provides a pivotal guiding signal. The basal expression of SDF-1, which retains HSPC quiescence during the steady state, is reduced during mobilization. Studies by our group and others have shown that G-CSF-induced mobilization is associated with a transient increase in SDF-1 levels, which is then followed by reduced production and secretion and cleavage of this chemokine in the bone marrow by a host of proteolytic enzymes.6,11,15,16 In a complex series of events, bone marrow HSPCs increase their CXCR4 expression and motility. Inflammatory signals also expand and activate mature bone-resorbing osteoclasts. In addition to their traditional role in bone turnover, these cells are also implicated in HSPC release and mobilization. Particularly, cathepsin K, the enzymatic hallmark of bone degradation by osteoclasts, can also degrade SDF-1, osteopontin, and the membrane-bound kit ligand, all of which are molecules that regulate HSPCs.17
Thrombolytic agents are also involved in G-CSF mobilization.18 Interestingly, G-CSF stimulation leads to a reduction in SDF-1 production in the bone marrow due to morphological and functional changes of the endosteal osteoblasts, major producers of this chemokine that are also involved in signaling from the nervous system.16,19 These events are followed by the proteolytic deactivation of SDF-1 and, concomitantly, HSPC proliferation and differentiation. While SDF-1 is reduced in the bone marrow and CXCR4 is increased, the level of CXCR4 in the circulation does not change significantly. Bone marrow HSPCs become more motile, gain CXCR4 expression, and the proteolytic machinery is activated (as discussed in 11 ). In parallel, human CD34+ cell adhesion via CD44 is now down-regulated due to CD44's cleavage by MT1-MMP. This activation of human MT1-MMP in chimeric NOD/SCID mice is made possible by the down-regulation of its inhibitor, RECK.12 All of these adaptations act in synergy to facilitate HSPC egress from the bone marrow. The levels of MT1-MMP expression by myeloid cells in the patient's blood circulation are correlated with clinical mobilization, and mobilized human CD34+ cells have reduced CD44 expression.12 In recent studies, the CXCR4 antagonist AMD3100 (plerixafor) was shown to rapidly mobilize human CD34+ HSPC. AMD3100 synergizes with G-CSF to increase HSPC motility.20,21 This process also involves innate immunity components such as the complement cascade.22 It is interesting that the mobilization of HSPCs and endothelial progenitors share common mechanisms.23,24
One of the pathways that G-CSF activates in HSPC is the reactive oxygen species (ROS) signaling pathway. By activating the c-Met/HGF (hepatocyte growth factor) axis, G-CSF increases production of ROS, which induces HSPC mobilization. Accordingly, ROS inhibition reduces both G-CSF-induced mobilization and the enhanced motility of HSPC,25 demonstrating the importance of ROS as a regulator of enhanced HSPC migration, proliferation, and differentiation, leading to reduced long-term stem cell repopulation. However, this transient increase in ROS is reversible, and the long-term repopulation potential can be restored by in vitro ROS inhibition (as discussed in 25 ).
Guided by stress-induced signals, HSPCs are recruited to injured sites and tissues. We have shown that local damage induced in the liver of NOD/SCID chimeric mice increased the expression of SDF-1 in the injured liver. This increase caused the recruitment of human CD34+ progenitors from the engrafted murine bone marrow. The human cells were observed localizing in close proximity to SDF-1-expressing bile duct epithelial cells. Interestingly, SDF-1 expression in the human liver is dramatically up-regulated by hepatitis C virus infection and in the murine liver by irradiation, which suggests that orchestrated changes induced by stress signals can potentially participate in host defense and repair by as-yet-unknown mechanisms (as discussed in 26 ).
The bone marrow microstructure in general, and the trabecular endosteum region in particular, are critical regulators of HSPC fate and retention. Upon osteoclast malfunction, both steady-state release and G-CSF-induced progenitor mobilization are preferentially reduced.17 Osteoclast perturbations can be genetically introduced in young PTPε KO (protein tyrosine phosphatase-epsilon knockout) females or can be induced by anti-osteoclast drug therapy, which imposes changes in bone turnover.17
G-CSF administration activates osteoclasts, which were recently shown to be involved in mediating the increased proliferation of bone marrow stromal progenitors.27 These observations suggest that osteoclasts participate not only in bone resorption and HSPC mobilization, but also in the regulation of the bone marrow-supporting niches. Similarly, mice lacking CD45 have a distorted morphology and a reduction in osteoclast activity, resulting in mild osteopetrosis. These mice display extramedullar hematopoiesis and abnormal bone microstructure, mainly reflected in modified distribution and density of the bone trabecules, implicating reduced levels of murine HSPCs in the bone marrow. CD45 knockout mice poorly mobilize HSPCs in response to G-CSF, RANKL (receptor activator for nuclear factor κB ligand), and AMD3100 stimulation.28 Mechanisms of G-CSF-induced mobilization of human CD34+ HSPCs in NOD/SCID mice are illustrated in Figure 1B. Taken together, these studies demonstrate the essential function of balanced osteoblast/osteoclast regulation and the bone marrow microenvironment to support and allow the mobilization process. These results suggest that via their progeny, osteoclasts, neutrophils, and other myeloid cells, HSCs indirectly participate in regulating the dynamic bone marrow microenvironment, the immature stromal and hematopoietic pool size, and the mobilization process.
Several other mobilizing agents, such as antibodies for VLA-4 (very-late antigen-4) and IL-8 (interleukin-8), have been identified; however, in this review, we chose to focus on those acting via the SDF-1/CXCR4 axis, which is a pivotal regulator of HSPC function.
Homing: The Active Crossing of the Blood-Bone Marrow Barrier and Retainment in the Bone Marrow Microenvironment
Clinical protocols of bone marrow transplantations are dependent on the homing skills of the transplanted cells. Intravenously infused into the peripheral blood, HSPCs find their way to the bone marrow and lodge there to initiate hematopoiesis and bone marrow reconstitution, which is a multi-step, coordinated process.
The homing of human CD34+ HSPCs to immune-deficient murine bone marrow requires preconditioning of the host mice with total body irradiation or other means of ablation, such as chemotherapeutic-induced DNA damage. This induces a dramatic elevation of SDF-1 production by bone marrow stromal cells within 1 to 2 d leading to activation of proteolytic enzyme machinery. Navigating human HSPCs can successfully migrate to the murine bone marrow if CXCR4 is functionally expressed on the CD34+ cell surface (as discussed in 5 ). Expression of this receptor is dynamic and can be up-regulated by short term, 1- to 2-d pre-stimulation of CD34+ cells ex vivo with SCF (Stem Cell Factor), IL-6, HGF, and other cytokines. Homing of human CD34+ HSPCs in transplanted NOD/SCID mice is regulated by the availability of both SDF-1 as the guiding signal and of CXCR4 as the detector of this signal. Importantly, the migration potential of a patient's CD34+-enriched cells toward a gradient of SDF-1 in vitro is also correlated with their repopulation potential in clinical autologous transplantations. These results suggest that the repopulation potential of human CD34+ cells in patients and in chimeric NOD/SCID mice is dependent on CXCR4 signaling (as discussed in 5 ). CXCR4 is also functionally expressed by many malignant human cells and is a poor prognostic factor for AML patients.
Bone marrow endothelial cells produce and present SDF-1, as well as its major receptor, CXCR4. Endothelial CXCR4 binds this chemokine and functionally transfers it from the circulation via the vessel lumen into the bone marrow. Bone marrow endothelial CXCR4 transfer of SDF-1can also facilitate the homing of transplanted human CD34+ HSPCs to the murine bone marrow.29 Interestingly, preliminary results reveal that SDF-1 injected directly into the bone was transferred by endothelial cells and released into the circulation in a CXCR4-dependent manner, facilitating rapid murine HSPC mobilization.11 This feature of the endothelial blood-bone marrow barrier is believed to constitute a major vectorial regulator directing HSPCs in or out of the bone marrow. This vectorial regulation follows SDF-1's dynamic fluctuations due to stress-induced enhanced production in the bone marrow or in other organs. In agreement with these observations, the inhibition of CD26, a peptidase with SDF-1 cleavage activity, improves homing and engraftment of human CD34+ progenitors in transplanted, immune-deficient mice.30,31
Similarly to HSPC mobilization, bone marrow homing also requires an active crossing of the blood-bone marrow barrier, namely the vascular wall. Many molecules expressed by endothelial cells take an active part in this process. HSC attachment to the vascular lumen requires binding of selectins and integrins to their receptors, which facilitates cell adhesion to the vessel wall under blood flow shear stress prior to their crossing. Presentation of SDF-1 by endothelial cells mediates increased expression and activation of the HSPC adhesion machinery, which involves cytoskeleton rearrangement to enable cell spreading (as discussed in 5 ). Anchors required for docking in the bone marrow include CD44, which is also expressed by human CD34+ HSPCs. CD44 is required for cell spreading and adhesion to hyaluronan and osteopontin, both of which are expressed by blood vessel walls and along the endosteum, and are implicated together with SDF-1 and membrane-bound SCF in murine HSC homing and repopulation.32–34 Indeed, blocking CD44 function prevents homing of immature human CD34+ cell to the murine bone marrow and spleen (as discussed in 5 ). Mechanisms regulating the homing of human CD34+ HSPCs to the NOD/SCID mouse bone marrow are illustrated in Figure 1C.
Activation of the adhesion machinery is dynamic and requires a tight regulation to allow HSPC attachment and detachment, both of which are essential for cell migration. For example, genetic modulation of CD45 leads to a constant and highly adhesive HSPC phenotype due to Src hyperactivation, which prevents their normal homing to the bone marrow of wild-type hosts.28 Lodging of homing HSPCs to the bone marrow endosteum also requires sensing of the calcium levels,35 which links bone turnover with the regulation of murine stem cell homing. Intracellular rho-GTPases are also important regulators of HSC motility and adhesion.13
The Brain-Blood-Bone Triad: A Circular Hierarchy Regulator via Dynamic Interactions
The nervous system via its various pathways has been implicated as a major regulator of the immune system. However, activated leukocytes, including lymphocytes and lipopolysaccharide-stimulated macrophages, also secrete neurotransmitters that exert bifunctional effects on the immune cells and provide feedback to the nervous system. The bone marrow is highly innervated, with nerve fibers running along the brachiated blood vessels and into the bone marrow parenchyma. Thus, catecholamines produced by the sympathetic system can be delivered to the bone marrow by the blood circulation, but some are also secreted from nerve endings directly in the bone marrow to act on bone marrow resident cells in a paracrine fashion.8,36 Accumulated data depict a major role for signals coming from the sympathetic system in the regulation of HSPC retention, homing, and mobilization, which include direct and indirect effects exerted via the bone-remodeling processes. Human CD34+ HSPCs dynamically express catecholaminergic receptors, with higher expression observed by the more primitive CD34+CD38-/low subset. Repeated stimulation with the mobilizing myeloid cytokines G-CSF and GM-CSF (granulocyte-macrophage colony stimulating factor) also increases this expression. Therefore, mobilized human CD34+ cells isolated from the peripheral blood have higher catecholaminergic receptor expression compared with tissue-anchored bone marrow CD34+ progenitor cells. These receptors play a functional role in the homing process, because stimulation with neurotransmitters triggers in vitro CD34+ cell proliferation and increased motility and engraftment capabilities, as measured in chimeric NOD/SCID mice. Moreover, catecholaminergic stimuli also enhance the expression of the membrane-bound enzyme MT1-MMP and SDF-1-directed migration.36,37
The Wnt canonical signaling pathway and beta-catenin stabilization are also activated by this catecholaminergic stimulation to take part in the homing process. Interestingly, we found that these neural signals can also induce murine HSPC mobilization.37 Indeed, antagonizing the Wnt signaling pathway with Dickkopf-1 during steady-state homeostasis mobilized both hematopoietic and endothelial murine progenitor cells.23,38 In contrast, during alarm situations and repeated G-CSF-induced mobilization, inhibition of Wnt signaling inhibits mobilization.37 Driving forces of HSPC mobilization also include the suppressive effects that G-CSF stimulation exerts on bone-lining osteoblasts and their capacity to produce SDF-1. This requires peripheral beta-adrenergic signals.19
Sympathetic signals also control bone-remodeling processes, which constitute part of the HSPC paracrine regulatory milieu, including bone formation by osteoblasts and degradation by osteoclasts. Nerve endings with norepinephrine secretion capacity are observed in close proximity to osteoblasts and osteoclasts near the bone-growing plates, at regions enriched with HSPCs. Bone mass and remodeling processes aimed at maintaining bone integrity are known to be regulated by signals from the nervous system. Particularly, beta-adrenergic signals that are involved in HSPC migration and mobilization tilt the remodeling balance toward osteoclast activation and osteoblast suppression. For example, parathyroid hormone regulates bone turnover and in vivo HSPC expansion and mobilization (as discussed in 8 ).
The emerging relationship among the nervous, immune, and bone-maintaining systems may allow us to conceive a well-orchestrated hierarchal network by which the body functions in the context of HSPC regulation. The evidence that exogenous signals transmitted by light/dark cycles are detected by the nervous system, which then translates these circadian cues to neurotransmitter and hormone secretion, both major regulators of HSPCs and their microenvironment, places the nervous system at the top of this regulatory hierarchy. SDF-1, the major HSPC chemokine, is produced and secreted in the bone marrow in association with circadian rhythms in a mechanism that includes beta3-adrenergic (AdR) activity, which is not yet fully understood.8,39 HSPC release from the bone marrow to the circulation in steady-state conditions is also synchronized with and follows bone marrow SDF-1 production, with anti-phase fluctuations.39 Accordingly, expression of CXCR4 by human bone marrow CD34+ HSPC cells also demonstrates a circadian oscillation pattern.40 The effects of immune cells and bone disorders on the nervous system demonstrate the circular, bidirectional manner by which this network exerts its regulation.
Concluding Remarks and Future Directions
The nervous system is the fastest detection and message-delivery framework, which makes it capable of immediate response to environmental changes throughout the body. This regulatory network demonstrates multidirectional streaming. Thus, exogenous stimuli and other stimuli originating from the immune system, bone, bone marrow, and the stromal microenvironment all regulate HSPCs. However, the nervous system dominates the brain-bone-blood triad.
Prospectively, the major question is “what does the body gain by responding to circadian signals in controlling bone turnover and HSPC trafficking?” This question may be answered by seeing these “body defense tools” as constantly working at low gear to maintain homeostatic balance. Bone maintenance requires continuous formation and degradation. HSC regulation requires their quiescence and retention in the bone marrow, but also their migration, self-renewal, differentiation, and recruitment to the circulation. These opposing processes may require an on/off switch that reliably inverts the regulatory machinery in opposite directions. To continuously keep this network active, there could be a need for repetitive, low rates of mild, stress-like signals that are generated in constant intervals. These intervals are provided by circadian peaks, serving as an external pacemaker to synchronize blood cell production, bone turnover, and immunity. These circadian rhythms also keep the body ready and well trained for dynamic and acute changes, including intensified stem-cell migration and development, which are triggered by stress signals during acute situations. This concept is relevant to clinical mobilization and homing during stem-cell transplantation. Future protocols may harness these insights to increase the success of HSPC transplantation by taking into consideration normal circadian rhythms, the timing of G-CSF and AMD3100 treatments, and unique features of the patient's bone, immune, and neural status.
Acknowledgments
We regret that due to the citation limit of 40 references published during the last 5 years, we could not cite and discuss many important studies on stem-cell homing and mobilization.
Disclosure
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Off-label drug use: None disclosed.