Abstract
A hemolysis-linked subphenotype of sickle cell disease (SCD), characterized by pulmonary hypertension, stroke, priapism and leg ulcers, is associated with decreased nitric oxide bioavailability and vasculopathy. Vasculopathy appears to have a multifactorial etiology, including mechanisms primarily that involve deficient nitric oxide (NO) signaling, but also involving altered function of NO synthase related to substrate availability and cooperating factors such as apolipoproteins. Improved understanding of the vascular pathophysiology of SCD has led to new vascular targets for translational research in SCD. This growing vascular therapeutics field in SCD is complementary to the ongoing efforts to reduce the morbidity of vaso-occlusive pain crisis. This presentation will review the current biology and translational clinical development of novel small molecules targeting sickle cell vasculopathy. Strategies targeting the hemeoxygenase-carbon monoxide pathway, the arginine-NO synthase-cGMP-phosphodiesterase 5 pathway, the nitrate-nitrite-NO pathway, and the apolipoprotein A-I pathways will be reviewed. In this context, current clinical trials of inhaled NO, CO, nitrite, sildenafil and apoA-I mimetics will be discussed.
Introduction
It is increasingly clear in recent years that a complex pathology affects the vessel wall in sickle cell disease (SCD), an indirect consequence of sickle hemoglobin polymerization in erythrocytes. A chronic vasculopathy in patients with SCD, first proposed by Hebbel and Kaul in 2004,1 characterizes a hemolysis-linked subphenotype of SCD. This subphenotype, comprising pulmonary hypertension (PH), stroke, priapism and leg ulcers, is associated with decreased nitric oxide (NO) bioavailability.2 Sickle vasculopathy appears to have a multifactorial etiology, including mechanisms that primarily involve deficient NO signaling, but also involving other pathways (see accompanying article by Morris, beginning on page 177). Steady-state hemolysis is an invariant feature of SCD, but over a wide spectrum of severity in individual patients. The patients with the most severe chronic hemolysis, characterized by the lowest total red cell hemoglobin levels and highest levels of reticulocytes, serum bilirubin and lactate dehydrogenase (LDH), have the highest prevalence of PH, priapism, leg ulcers and cerebrovascular disease. This is due in large part to decreased NO bioavailability induced by a multi-pronged attack on NO production and turnover.
Intravascular hemolysis results in ectopic localization of red cell contents into plasma, including three of proven significance: hemoglobin, arginase and LDH. Hemoglobin consumes NO in a rapid stoichiometric reaction,3 and arginase depletes the plasma pool of arginine, the substrate for NO synthesis.4 Serum LDH levels serve as a convenient surrogate marker of hemoglobinemia, arginasemia, and decreased NO bioavailability.5 It also has been proposed that sickle erythrocytes have deficient transduction of NO signaling through nitrosation of the beta globin cysteine-93 thiol,6 but mutation of this cysteine does not reduce red cell–mediated hypoxic vasodilation, raising questions about this pathway.7 Improved understanding of the vascular pathophysiology of SCD has led to new vascular targets for translational research in SCD. This growing vascular therapeutics field in SCD is complementary to the ongoing efforts to reduce the morbidity of vaso-occlusive pain crisis. This review covers the current biology and translational clinical development of novel small molecules targeting sickle cell vasculopathy.
Arginine-NO Synthase-cGMP-Phosphodiesterase-5 Pathway
In this pathway, ordinarily, L-arginine is converted by the isoforms of nitric oxide synthase (NOS) to citrulline plus nitric oxide (NO), which then diffuses to the smooth muscle cell, where NO binds to its principal receptor, soluble guanylyl cyclase (sGC), which in turn, converts guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP), inducing vascular smooth muscle relaxation and vasodilation through multiple mechanisms (Figure 1; see Color Figures, page 500). Counter-regulation is provided in pulmonary and penile vascular smooth muscle by phosphodiesterase-5 (PDE5), which hydrolyzes cGMP to 5′ GMP. Attempts are being made to target each of these steps in recent or ongoing clinical trials.
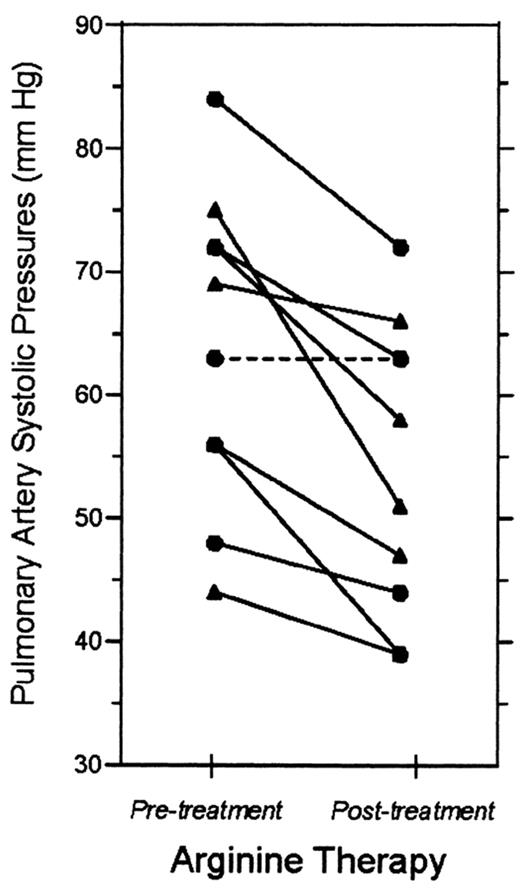
Morris and colleagues have published preliminary evidence supporting oral L-arginine for pulmonary hypertension (PH) in SCD.8 They administered oral L-arginine at 0.1 g/kg 3 times a day to 10 adolescent and adult SCD patients with PH, as indicated by high baseline estimated pulmonary artery systolic pressure (PASP, 63.9 ± 13 mmHg). Plasma arginine levels were low at study entry (50 ± 19 μ M), but tripled after 15 doses of L-arginine (P < .05). After 5 days of treatment, the PASP declined to 54.2 ± 12 mmHg (P = .002) (Figure 2 ).
Kaul and Fabry have also found beneficial effects of arginine supplementation in the sickle cell mouse. They fed various strains of sickle transgenic mice chow containing 5% arginine and made several interesting observations. First, the hemolytic rate was decreased as indicated by a 50% decrease in plasma hemoglobin,9 an apparent consequence of inhibition of the Gardos channel in sickle erythrocytes.10 Second, they found evidence of improvements in NO resistance, likely as a consequence of decreased NO scavenging due to reduced cell-free plasma hemoglobin. After arginine treatment, an NO donor induced a 60% increase in arteriolar diameter, compared to only 15% increase from NO donor exposure before arginine therapy.9 Third, they found that arginine therapy in the mice partially normalized several blood and tissue markers of oxidative stress, including NOx, lipid peroxides, nitrotyrosine and glutathione. Arginine also boosted levels of the catalytic antioxidants superoxide dismutase, catalase and glutathione peroxidase.9,11 The doses of arginine used in these studies is very high, and the high doses might be necessary to obtain the beneficial effects. The positive results of high-dose arginine therapy in the mouse model might be favored by the reportedly lower concentration of arginase in murine red cells. Humans with SCD have variably high red cell and plasma arginase activity that might interfere with consistent attainment of high plasma levels of arginine.
Negative results have also been seen with arginine therapy. In our study of adults already on hydroxyurea, addition of high-dose arginine for 3 months doubled the plasma arginine levels (47 ± 16 to 96 ± 58 μ M, n = 8, P < .005), but did not change the tricuspid regurgitant jet velocity (TRV), a measure of pulmonary pressure (2.57 ± 0.32 to 2.72 ± 0.40 m/sec, n = 9, P = n.s.) (Little et al, manuscript submitted). Arginine supplementation elevated plasma ornithine levels (74±13 to 128±77, n = 8, P = .03), presumably because of high plasma arginase from hemolysis. No improvement was seen in red cell MCHC. Arginine did restore more normal levels of total erythrocyte glutathione after 8 weeks (1222 ± 425 μ M to 1593 ± 406 μ M, n = 8, P = .03), a possible indication of a reduction in oxidant stress, similar to the sickle mice treated with arginine. It is not clear whether in this trial, hydroxyurea use might in some way obscure beneficial effects of arginine supplementation on TRV or hematologic parameters. At the 2008 American Society of Hematology meeting, in the Transfusion Medicine Scientific Subcommittee, Dr. Lori Styles will present additional data from a clinical trial of lower dose, long-term arginine supplementation in adults and children with SCD (ClinicalTrials.gov identifier NCT00513617).
The true efficacy and potential adverse effects of long-term very high dose arginine has not been examined adequately, and although it is available as a dietary supplement without a prescription, providers and patients should be cautious about adopting it until more research is completed. Other amino acid supplements have been tried in SCD, but the data are highly preliminary. Oral citrulline (0.1 mg/kg/day) has been administered to 5 SCD patients, increasing plasma arginine levels by 65%.12 Although no changes were observed in hemoglobin, bilirubin, or reticulocyte count, citrulline supplementation was associated in 3 patients with a 38% decrease in plasma hemoglobin, reminiscent of the changes seen with arginine supplementation in the sickle mouse.13 This strategy relies on efficient renal conversion of citrulline to arginine, which appears to be impaired in many of the adult SCD patients with pulmonary hypertension, one of the target populations for NO pathway therapy (Kato et al, manuscript submitted). In fact, citrulline accumulation is a marker for early mortality in adults with SCD.4 Clearly, there are not adequate published data to understand the efficacy and risks of citrulline supplementation in SCD. Similarly, small pilot clinical trials have been performed with glutamine supplementation in SCD, with a goal of improving oxidant stress in sickle erythrocytes.14,15 Interest in glutamine supplementation has risen with the recent association of low red cell glutamine levels with low red cell glutathione, high plasma hemoglobin and PH in adults with SCD.16
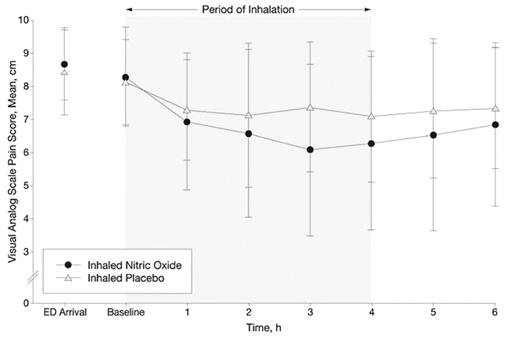
Another option under investigation is restoring more normal levels of NO by direct administration of inhaled NO in patients with SCD. Administration of inhaled NO to patients with SCD has been shown to oxidize plasma heme and ameliorate in vitro NO consumption by plasma from those patients.3 In a double-blind, randomized, placebo-control pilot study in vaso-occlusive pain crisis, Weiner and colleagues have tested the effect of inhaled NO at 80 ppm for 4 hours on 10 children with SCD presenting to the emergency department, compared to 10 treated with placebo.17 Using a 10-cm Visual Analog Pain Scale, they found a 1 cm/hr greater pain reduction in the inhaled NO group compared to those treated with standard therapy plus placebo, which was statistically significant (Figure 3 ). They also found that over the first 6 hours after initiating treatment, cumulative morphine use was less in the treatment group (0.29 vs. 0.44 mg/kg, P = .03) with similar trends at 4 hours (0.26 vs. 0.32 mg/kg, P = .21) and 24 hours (0.63 vs. 0.91 mg/kg, P = .15). They also observed a shorter length of stay in the treatment group, although this was not statistically significant (78 vs. 100 hours, P = .19). No toxicities were observed. A multicenter study of inhaled NO in VOC is currently in progress, a public-private partnership of NHLBI with INO Therapeutics (ClinicalTrials.gov identifier NCT00094887).
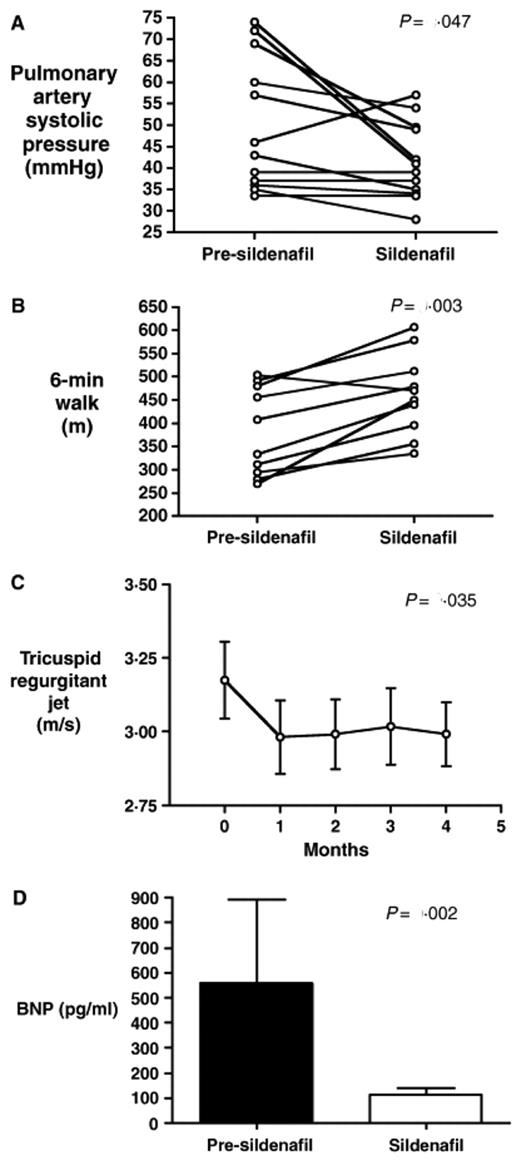
Another strategy that has been tested is to amplify the effect of endogenous NO by inhibiting the breakdown of its downstream signal transduction mediator, cyclic GMP. Sildenafil, a drug marketed primarily for its efficacy in erectile dysfunction, inhibits PDE5, an enzyme in the vascular wall of the penis, lungs, and sinus mucosa that normally limits the duration of cGMP signals. Our group has found preliminary evidence for its efficacy in 12 patients with SCD and PH.18 Sildenafil therapy for a mean duration of 6 ± 1 months decreased the estimated pulmonary artery systolic pressure (50 ± 4 to 41 ± 3 mmHg, difference 9 mmHg, 95% confidence interval (CI): 0.3–17, P = .04)(Figure 4 ). Strikingly, sildenafil use also was associated with a 20% increase in the distance walked in 6 minutes, a functional test of cardiopulmonary reserve that is well accepted in the pulmonary literature (384 ± 30 to 462 ± 28 m; difference 78 m, 95% CI: 40–117, P = .001). Side effects were limited to transient headaches in 2 patients, and transient eye-lid edema in 4 patients. None of the 3 males in the study experienced priapism with sildenafil use, although 2 were at particularly low risk. One of the males was on chronic red cell exchange therapy, and another had pre-existing complete erectile dysfunction, which did not improve on sildenafil. Efficacy of sildenafil in PH has also been seen in another small case series in thalassemia and SCD.19 Now approved by the FDA for treatment of PH in the general population under the trade name Revatio, sildenafil is currently being tested in an NHLBI-sponsored, large scale, multicenter study in patients with SCD and PH (ClinicalTrials.gov identifier NCT00492531).
Although there remain lingering concerns over the theoretical possibility that sildenafil might induce priapism in men with SCD, there are two published case series suggesting its counterintuitive benefit in recurrent priapism.20,21 One of the groups has proposed a rationale for this therapeutic effect based on experiments in mice.22 They reported that priapism occurs not only in the sickle cell mouse, but also in mice deficient in endothelial NOS, or both endothelial and neuronal NOS. Priapism in mice with low NO bio-activity due to deficient NO production in the NOS knockout supports the hypothesis that priapism in SCD is due to the low NO state that arises due to NO scavenging and high plasma arginase. PDE5 mRNA in penile tissue is normally induced by cGMP, to exert negative feedback on cGMP levels. In the NOS-deficient mouse, they find that the penile PDE5 mRNA level is low, apparently due to loss of cGMP stimulation of the PDE5 gene promoter. Consequently, there is not enough cGMP hydrolysis to appropriately terminate significant pulses of NO signaling, resulting in priapism. However, treatment of the mice with sildenafil prolongs the cGMP signals enough to stimulate the PDE5 promoter, restoring sufficient PDE5 enzyme to buffer sudden rises in NO signaling. Sildenafil merits further investigation in priapism in SCD, a frustrating and difficult problem in which existing treatments are largely unrewarding.
Nitrate-Nitrite-NO Pathway
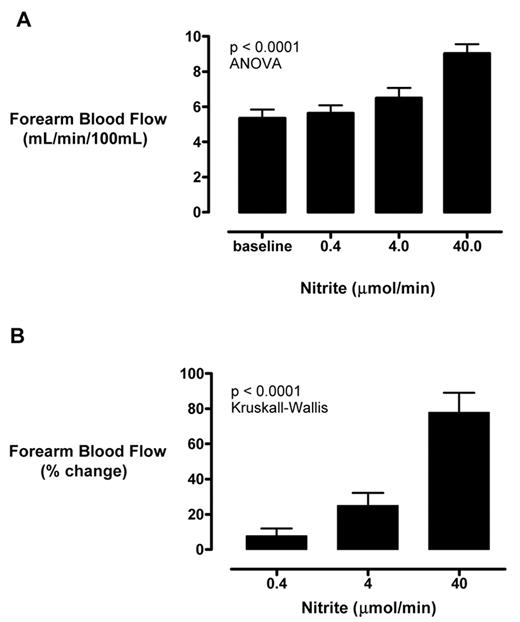
Although microbes demonstrate the ability to interconvert nitrate, nitrite and NO, these pathways were believed to be very limited in humans. It is now clear that under certain conditions in mammals, deoxyhemoglobin, deoxymyoglobin, and xanthine oxidoreductase can convert nitrite into NO.23 Nitrite not only can function as a physiological reservoir for NO production in ischemic tissue, pharmacologic administration can induce vasodilation in healthy volunteers. Our group has recently observed that nitrite infusion in the brachial artery stimulates regional blood flow in 14 patients with SCD.24 In a phase I/II open label study, we found statistically significant, dose-dependent rises in forearm blood flow by venous occlusion strain gauge plethysmography with sodium nitrite infusions of 0.36, 3.6 and 36 μ mol/min, up to 77 ± 11% above baseline (P < .0001) (Figure 5 ). Consistent with a NO donor mechanism, the nitrite response in SCD was blunted significantly compared to healthy volunteers, and correlated to the vasodilatory response to another well-known NO donor, sodium nitroprusside (r = 0.75, P = .002). The systemic plasma nitrite level rose to 5.3 ± 0.9 μ M, a level that appears in animal models to be protective against tissue infarction.25 However, none of the patients developed clinically significant methemoglobinemia, low blood pressure or any significant symptoms. These data are very helpful to plan future clinical trials of sodium nitrite in patients with SCD.
Oral nitrate, abundant in leafy green vegetables, is concentrated in human saliva and converted by oral bacteria to nitrite. In turn, nitrite can be converted by gastric acid into NO, believed to have a beneficial effect to stimulate production of protective gastric mucus and normal gastroesophageal motility.25 The efficacy of dietary or pharmaceutical nitrate on vascular disease in the general population is being assessed, but there has been early speculation that the high nitrate content of the DASH and Mediterranean diets may contribute to their vasculoprotective properties.26 To date, the role of oral nitrate has not been evaluated in SCD vasculopathy.
Apolipoprotein A Pathway
As discussed in the previous presentation, our group has uncovered preliminary evidence for higher prevalence of endothelial dysfunction in SCD patients with apoA-I levels below 99 mg/dL, and in particular, a trend toward higher prevalence of PH, 71% in SCD patients with apoA-I levels below 87 mg/dL (Yuditskaya et al, manuscript submitted). In the general population, endothelial dysfunction and higher risk of atherosclerosis is associated with lower apoA-I and HDL cholesterol (HDL-C) levels, and the FDA has approved use of niacin in this population. In hypercholesterolemic subjects without SCD, niacin increases apoA-I and HDL-C levels, correlated with improvements in blood flow physiology.27 Our group is currently testing the efficacy of niacin to improve agonist-stimulated forearm blood flow in patients with SCD with low apoA-I or HDL-C levels (ClinicalTrials.gov identifier NCT00508989). This trial is a product of a bedside-to-bench investigation, producing a testable bench-to-bedside hypothesis that could result in new treatment.
Consistent with our findings of endothelial dysfunction in SCD patients with low apoA-I levels, Pritchard, Hillery and colleagues have found that a peptide mimetic of apoA-I, L4F, ameliorates endothelial dysfunction in a sickle cell mouse model.28 This is also a promising area for possible clinical investigation in patients.
Endothelin Receptor Blockade
Endothelin-1, a small peptide hormone, is the most potent vasoconstrictor known in the human body. It exerts it effects on the systemic vasculature primarily through the endothelin receptor A (ETA), and primarily in the renal medulla through endothelin receptor B (ETB). Three ET blockers are approved for use in pulmonary hypertension: bosentan, a combined ETA/ETB blocker, and two ETA blockers, ambrisentan and sitaxsentan, the latter approved only in Europe and Australia. A randomized trial of bosentan in sickle cell PH has been conducted, and results are awaited (Clinicaltrials.gov identifiers NCT00310830, NCT00313196, NCT00360087).
Endothelin-1 levels are high at steady state in patients with SCD and rising further during vaso-occlusive crisis.29,30 Endothelin receptor blockade reduces pathologic Gardos channel activation and dense red cell formation.31 These findings have prompted a study recently reported by Sabaa and colleagues from Paris in which they found that in the SAD sickle cell mouse that bosentan decreases the consequences of hypoxia and reoxygenation, particularly on renal blood flow.32 Bosentan prevented renal and pulmonary microvascular congestion, systemic inflammation, formation of dense red cells, and infiltration of activated neutrophils into tissues and subsequent nitrative stress. Bosentan also prevented death of sickle-cell mice exposed to a severe hypoxic challenge. The development of clinical trials of endothelin receptor antagonists in patients with SCD merits serious consideration.
Heme-Oxygenase-Carbon Monoxide Pathway
Hemolysis results in the turnover of hemoglobin, in turn releasing heme, a potentially toxic substance with oxidative and vasculopathic properties. In return, mammals have evolved an intricate and redundant program of heme metabolism that is vasculoprotective. Heme breakdown starts with heme oxygenase-1 (HO-1), which breaks the heme ring, generating carbon monoxide (CO) as a byproduct, the only mammalian chemical reaction that generates CO. HO-1 has anti-inflammatory, anti-proliferative, anti-apoptotic and antioxidant effects on the vasculature, and protects against atherosclerosis in the general population. These effects are mediated through products of its activity, including CO, biliverdin and bilirubin.33 CO also inhibits vaso-constriction, platelet aggregation and vascular smooth muscle proliferation, preventing abnormal vascular remodeling.
In SCD, HO-1 expression was shown by Nath and colleagues to be high in the renal vasculature of a patient and in circulating endothelial cells.34 Our group also has demonstrated a twofold increase in levels of HO-1 mRNA in peripheral blood monocular cells from 27 patients with SCD compared to African-American controls.35 This was accompanied by a 1.5-fold increase in the subunits of biliverdin reductase (BVR), and a 3-fold increase in total bilirubin, the end product of this pathway. HO-1 gene expression correlated significantly with carboxyhemoglobin levels, a marker of carbon monoxide production, (r = 0.51, P = .01) and with total bilirubin levels (r = 0.66; P < .001). HO-1 and BVR gene expression correlated with a marker of hemolysis, lactate dehydrogenase (r = 0.66, P < .0001 and r = 0.58, P < .0001). High level HO-1 expression was also found by Belcher and colleagues in the S+S-Antilles and BERK sickle cell mice.36 These data suggest that HO-1 and BVR expression are induced in response to hemolytic rate, detoxifying the oxidant stress posed by free heme.
Animal data suggest a potential therapeutic application for inhaled CO in SCD. Belcher and colleagues showed that CO administration to the S+S-Antilles sickle cell mouse suppressed vascular stasis (Figure 4A ), activation of NF-κ B (Figure 4B ), and expression of VCAM-1 and ICAM- 1.36 Consistent with these results, CO has been shown by several groups to mediate much of the downstream effects of HO-1 on vasorelaxation, anti-inflammation, anti-proliferation and neurotransmission (Figure 4C ).33 Although inhaled CO is toxic in high concentrations, at low concentrations it appears to have promising activity as a vascular or anti-inflammatory therapeutic. Our group is presently studying its potential utility in reducing acute airway inflammation in the human lung (ClinicalTrials.gov identifier NCT00094406).
Anti-hemolytic Drugs
Hypothetically, drugs that diminish hemolysis in SCD should reduce plasma hemoglobin levels and NO scavenging, thereby improving vasculopathy and PH. Hydroxyurea is well known to increase fetal hemoglobin levels and reduce hemolysis, but in epidemiologic studies by our group, its use was not associated with lower PH prevalence.37 However, in a similar study Ataga and colleagues observed a trend toward possible association of hydroxyurea use to less frequent PH.38 Consistent with this interpretation, we have observed anecdotally in some adult SCD patients with PH that initiation of hydroxyurea therapy has been associated with the rise in fetal hemoglobin levels and concurrent fall in the TRV. A prospective randomized controlled study would be required to definitively resolve this point, but this concept provides an additional rationale for initiating hydroxyurea in patients for whom it is already indicated.
The investigational Gardos channel blocker, senicapoc (ICA-17043), reduces hemolysis in patients with sickle cell anemia.39 According to our model, decreasing hemolysis and increasing hemoglobin and hematocrit might increase whole blood viscosity and the rate of vaso-occlusive phenomena, an effect seen with co-inheritance of SCD with alpha-thalassemia trait.40,41 According to the same model, senicapoc might be predicted to improve sickle vasculopathy and PH, but this remains to be determined.
The effects of hydroxyurea become paradoxical in our model, since it also increases hematocrit and thereby might be predicted to increase vaso-occlusive crisis frequency. However, the well-described effects of fetal hemoglobin to inhibit sickle hemoglobin polymerization obviously play a dominant effect, since hydroxyurea clearly reduces, not increases, the pain crisis rate.42 This implies an additional prediction of the model, that anti-hemolytic drugs will increase pain crisis rate unless sufficient anti-sickling effect is obtained simultaneously by the same drug, or in combination with other drugs. This prediction also remains to be validated. The partial success of hydroxyurea in “curing” SCD and the complete mitigation of SCD seen in patients who fortuitously co-inherit pancellular hereditary persistence of fetal hemoglobin suggest that a successful strategy to induce high-level pancellular fetal hemoglobin could eliminate both vaso-occlusion and hemolysis-associated vasculopathy.
Conclusion
The recent identification of disordered NO signaling in SCD is providing new targets for therapeutic interventions. Clinical investigations at various stages are under way for many agents that have potential efficacy in this pathway. Approaches that restore more normal NO bioavailability or compensate for its deficiency have important therapeutic potential in SCD, but much investigation is still needed at both the bench and the bedside. Strategies to reduce hemolysis are even more appealing to mitigate the hemolysis-associated defect in NO bioavailability, but with caution for the potential to increase viscosity-vasoocclusion phenomena. Clinical trial endpoints should be considered carefully with the understanding that hemolysis–vascular dysfunction outcomes may occur independently of, or even conversely to, vaso-occlusive outcomes. The morbidity and mortality associated with intense hemolysis and NO scavenging in SCD emphasize the importance of translating concepts in sickle vasculopathy to effective drug therapy in patients.
Pilot clinical trial of arginine treatment in sickle cell pulmonary hypertension. Baseline measurements were made of estimated pulmonary artery systolic pressure by Doppler echocardiography in 10 adults with sickle cell disease. After treatment with oral arginine (0.1 mg/kg 3 times a day for 5 days), measurements were repeated and had decreased by a mean of 15% (P = .002).8
Pilot clinical trial of arginine treatment in sickle cell pulmonary hypertension. Baseline measurements were made of estimated pulmonary artery systolic pressure by Doppler echocardiography in 10 adults with sickle cell disease. After treatment with oral arginine (0.1 mg/kg 3 times a day for 5 days), measurements were repeated and had decreased by a mean of 15% (P = .002).8
Reduced pain scores in a pilot clinical trial of inhaled nitric oxide (NO). Visual analog pain scores are shown over the first 6 hours of emergency department treatment of vaso-occlusive pain crisis in 10 children with sickle cell disease treated with inhaled NO 80 ppm for the first 4 hours, compared to 10 treated with placebo gas. The reduction in pain score in the treatment group is significant by repeated measures analysis of variance (P = .02).17
Reduced pain scores in a pilot clinical trial of inhaled nitric oxide (NO). Visual analog pain scores are shown over the first 6 hours of emergency department treatment of vaso-occlusive pain crisis in 10 children with sickle cell disease treated with inhaled NO 80 ppm for the first 4 hours, compared to 10 treated with placebo gas. The reduction in pain score in the treatment group is significant by repeated measures analysis of variance (P = .02).17
Results of a pilot clinical trial of sildenafil for pulmonary hypertension in sickle cell disease (SCD). Baseline measurements were made in 12 patients with SCD on hydroxyurea, and then they were treated with oral sildenafil (25 to 100 mg 3 times daily) for up to 6 months. Sildenafil therapy significantly decreased estimated pulmonary artery systolic pressure (A) and improved 6-min walk distance (B). (C) Time course of the effects of sildenafil on tricuspid regurgitant jet velocity. (D) Decrease in plasma NT-pro-BNP levels with sildenafil therapy.18
Results of a pilot clinical trial of sildenafil for pulmonary hypertension in sickle cell disease (SCD). Baseline measurements were made in 12 patients with SCD on hydroxyurea, and then they were treated with oral sildenafil (25 to 100 mg 3 times daily) for up to 6 months. Sildenafil therapy significantly decreased estimated pulmonary artery systolic pressure (A) and improved 6-min walk distance (B). (C) Time course of the effects of sildenafil on tricuspid regurgitant jet velocity. (D) Decrease in plasma NT-pro-BNP levels with sildenafil therapy.18
Sodium nitrite infusion improves regional blood flow in patients with sickle cell disease. Sodium nitrite was infused into the brachial arteries of 14 patients with SCD sequentially at 0.4, 4, and 40 μ mol/minute. (A) Absolute forearm blood flow increase was detected by venous occlusion strain gauge plethysmography. (B) These changes were even more prominent when calculated as percentage increase over baseline.24
Sodium nitrite infusion improves regional blood flow in patients with sickle cell disease. Sodium nitrite was infused into the brachial arteries of 14 patients with SCD sequentially at 0.4, 4, and 40 μ mol/minute. (A) Absolute forearm blood flow increase was detected by venous occlusion strain gauge plethysmography. (B) These changes were even more prominent when calculated as percentage increase over baseline.24
Disclosures Conflict-of-interest declaration: The author declares a cooperative research and development agreement between the NIH and Ikaria. Off-label drug use: Sodium nitrite to enhance blood flow in sickle cell disease. Atorvastatin to affect blood flow in sickle cell disease. Niacin-ER to increase HDL in sickle cell patients without hypercholesterolemia.
References
Author notes
Critical Care Medicine Department, Clinical Center and the Pulmonary and Vascular Medicine Branch, National Heart, Lung and Blood Institute, National Institute of Health