Abstract
Many mechanisms contribute to the complex pathophysiology of sickle cell disease (SCD), with dysfunction of the vascular endothelium as a unifying theme. Specifically, hemolysis-associated low arginine and nitric oxide (NO) bioavailability, amplified by NO synthase uncoupling, elevated arginase activity, superoxide production, oxidative stress, accumulation of arginine analogs such as asymmetric dimethylarginine, ischemia-reperfusion injury, inflammation, apolipoprotein A-1 depletion, and a hypercoagulable state are significant mechanisms contributing to endothelial dysfunction. Genetic polymorphisms also influence disease severity. Clearly the variable spectrum of disease is the consequence of multiple events and genetic susceptibility that go beyond the occurrence of a single amino acid substitution in the beta globin chain of hemoglobin. Recent studies begin to demonstrate overlap among these seemingly unrelated processes. Impaired NO bioavailability represents the central feature of endothelial dysfunction, and is a common denominator in the pathogenesis of vasculopathy in SCD. The consequences of decreased NO bioavailability include endothelial cell activation, upregulation of the potent vasoconstrictor endothelin-1, vasoconstriction, platelet activation, increased tissue factor, and activation of coagulation, all of which ultimately translate into the clinical manifestations of SCD. Evidence supporting vasculopathy subphenotypes in SCD, including pulmonary hypertension, priapism, cutaneous leg ulceration, and stroke, will be reviewed and relevance to other hemolytic disorders including the thalassemia syndromes will be considered.
Introduction
Sickle cell disease (SCD) is as much a disease of endothelial dysfunction1 as it is a hemoglobinopathy that triggers erythrocyte polymerization. Increased expression of adhesion molecules on erythrocytes and endothelial cells, interactions with leukocytes, increased levels of circulating inflammatory cytokines, enhanced microvascular thrombosis, and endothelial damage are all thought to contribute to obstruction of the arterioles by sickled erythrocytes. Nitric oxide (NO) is a free radical and a potent vasodilator2 that regulates vascular homeostasis. Interestingly, NO has properties that can impact every aspect of SCD, from decreasing platelet activation and adhesion receptor expression on the vascular endothelium, to decreasing vascular smooth muscle proliferation, limiting ischemia-reperfusion injury, modulating endothelial proliferation, and regulating inflammation. Given the crucial role of NO depletion in endothelial dysfunction,3 it is not surprising that NO dysregulation is a common denominator among varied mechanisms of sickle vasculopathy.4 NO is produced in the endothelium from its obligate substrate L-arginine, which is converted to citrulline by a family of enzymes, the NO synthases (NOS). Although NOS expression and activity is increased, SCD is characterized by a state of NO resistance, NO inactivation, and impaired NO bioavailability.4–6 Under conditions of increased hemolysis, inflammation and/or oxidative stress, the compensatory upregulation of NO likely becomes overwhelmed and ineffective (Figure 1; see Color Figures, page 498). Vascular dysfunction is the end result, due to complex and multifactorial interactions that ultimately manifest as the clinical phenotypes of SCD (Figure 2; see Color Figures, page 498). Although alternative mechanisms of vasculopathy unique to thalassemia are likely compared with SCD, it is currently not a well-investigated topic in non-sickle cell hemoglobinopathies. This review will focus on the common features of intravascular hemolysis, chronic anemia and endothelial dysfunction in both SCD and thalassemia that lead to vasomotor instability and ultimately produce a proliferative vasculopathy.
Hemolysis: Global Disruption of the Arginine–Nitric Oxide Pathway
A new disease paradigm involving hemolysis-associated endothelial dysfunction7,8 has implication for SCD,7,9 thalassemia,10 and all hemolytic conditions.8 The process of hemolysis initiates a global attack on the arginine-NO pathway.8,11 Under normal conditions, hemoglobin is safely packaged within the erythrocyte plasma membrane; however, during hemolysis it is decompartmentalized and released into plasma, where it rapidly reacts with and destroys NO.5 This results in abnormally high NO consumption and the formation of reactive oxygen species, ultimately inhibiting vasodilation. NO destruction by hemoglobin can also cause further impairment in vascular endothelial function via transcriptional activation of adhesion molecules, including VCAM-1 and E-selectin, and potent vasoconstrictors such as endothelin-1.8 The simultaneous release of erythrocyte arginase during hemolysis12 will limit the availability of arginine to NOS, contributing to a deficiency of NO.
Arginase also redirects the metabolism of L-arginine to L-ornithine and the formation of polyamines and L-proline, which are essential for smooth muscle cell growth and collagen synthesis. Therefore, the induction of arginase may also promote aberrant vessel wall remodelling and neointima formation.13
Proline is an amino acid that is also involved in lung fibrosis, airway remodeling, and asthma in addition to vascular smooth muscle proliferation,12 common features of pulmonary dysfunction in SCD14,15 and thalassemia.16 In SCD, pulmonary complications compromise oxygenation and contribute to a vicious cycle of erythrocyte sickling. By creating a shift towards ornithine metabolism, arginase triggers a process that contributes to the proliferative vasculopathy commonly found in hemolytic disorders.
Mechanisms of Arginine Dysregulation
Normal arginine metabolism is impaired in SCD12 and thalassemia10 for a variety of reasons that contribute to endothelial dysfunction and pulmonary hypertension (PH), (Figure 3; see Color Figures, page 499). Adults with SCD are arginine deficient at steady-state,17,18 while children have plasma levels that are similar to normal controls. An arginine deficiency develops with age and is influenced by acute events. Plasma arginine concentration decreases significantly in both adults and children during vaso-occlusive crisis (VOC) and acute chest syndrome (ACS), and is associated with low NO metabolite levels.18 Ultimately, low arginine bioavailability is associated with early mortality in adults with SCD.12
Increased arginase activity
Arginase is an essential enzyme in the urea cycle, responsible for the conversion of arginine to ornithine and urea. The NOS and arginase enzymes can be expressed simultaneously under a wide variety of inflammatory conditions, resulting in competition for their common substrate.19 Two forms of arginase have been identified: type 1, a cytosolic enzyme highly expressed in the liver, and type 2, a mitochondrial enzyme found predominantly in the kidney, prostate, testis, and small intestine.19 Arginase-1 is also present in human red blood cells. Plasma arginase activity is elevated in SCD as a consequence of inflammation, liver dysfunction and, most significantly, by the release of erythrocyte arginase during intravascular hemolysis, which has been demonstrated by the strong correlation between plasma arginase levels and cell-free hemoglobin levels and other markers of increased hemolytic rate.12 In addition, arginase activity is higher in the erythrocytes of patients with thalassemia and SCD compared to normal controls, and strongly correlates to plasma arginase activity.12 Upregulated expression of arginase-1 also results in increased proliferation rates of vascular smooth muscle and endothelial cells19 and in this capacity, may further contribute to vasculopathy in addition to its unique role during hemolysis. When arginine is catalyzed to NO, NOS produces the intermediate product N-hydroxy-L arginine (NOHA).20 NOHA is a potent arginase inhibitor, reflecting complicated feedback mechanisms in place to maintain homeostasis, with both NOS and arginase playing a regulatory role in NO production.19 As there is only limited arginase-1 found in the murine erythrocyte compared to human red blood cells, the major sources of increased arginase activity in the sickle cell mouse11 originate from cells other than the erythrocyte. It is unfortunately not feasible to extrapolate the contribution of erythrocyte arginase release to complications of hemolysis in SCD from the sickle cell mouse model.
Intracellular arginine transport
Arginase concentration present in the plasma is not reflective of whole-body arginase activity, since the arginases are intracellular enzymes that appear in the circulation after cell damage or cell death. The arginine-to-ornithine ratio, which significantly correlates to plasma arginase activity,12 may represent a superior marker of relative arginine bioavailability than arginine concentration alone. Arginine, ornithine and lysine compete for the same arginine transporter system (cationic amino acid transporter, CAT); therefore, an arginase-triggered rise in ornithine will further impact arginine transport and bioavailability. Plasma arginine concentration in SCD is approximately 40 to 50 mM at baseline,18 about 50% lower than normal values18 and well below the Km for CAT (100–150 mM). Even modest fluctuations in extracellular arginine concentration may significantly impact cellular arginine uptake and bioavailability. A low arginine:ornithine ratio is associated with increased mortality in SCD12 and severity of PH of various etiologies.21
Renal dysfunction
Global arginine bioavailability is diminished further in patients with renal dysfunction through the loss of de novo arginine synthesis from citrulline which occurs primarily in the kidney. Renal dysfunction, a common occurrence in SCD,7,22 will impair the major route for endogenous arginine biosynthesis. Rising creatinine levels correlate strongly to rising citrulline levels, suggesting renal insufficiency.12 Including the impact of renal dysfunction on arginine bioavailability through analysis of the arginine/(ornithine + citrulline) ratio revealed an increased risk of death in patients with low amino acid ratios. These findings suggest that adequate arginine bioavailability is critical for survival and provide clinicians with an objective index of disease severity.12
Endogenous NOS inhibitors
Low arginine bioavailability may be exacerbated further by the presence of elevated asymmetric dimethylarginine (ADMA), which is a competitive inhibitor of arginine transport and all NOS isozymes.23 High levels of ADMA can also contribute to NOS uncoupling.24 Circulating ADMA levels are elevated in several conditions of endothelial dysfunction, including SCD,25 and have been implicated in the pathophysiology of systemic and PH and risk of early mortality. The most elevated ADMA level occurred in SCD patients with the highest hemolytic rate and was associated with PH and mortality (Kato et al, personal communication).
ADMA normally produced in the body is hydrolyzed by dimethylarginine dimethylaminohydrolase (DDAH). Homocysteine inhibits DDAH activity. This may represent a mechanism whereby hyperhomocysteinemia, a known risk factor for vascular disease and thrombosis, leads to elevated plasma ADMA levels and decreased NO production in the cardiovascular system through competitive arginine inhibition. Landburg and colleagues recently demonstrated that elevated ADMA levels in patients with SCD did not increase over baseline during VOC.26 Although they conclude that there is no primary role for ADMA during crisis,26 given that arginine bioavailability decreases significantly during VOC and ACS,18 a rise in the ratio of ADMA-to-arginine may have some impact on global arginine bioavailability and endothelial dysfunction that should be explored further.
Uncoupled Nitric Oxide Synthase
Hemolysis will drive arginine consumption, which will ultimately exacerbate NO sequestration and decreased NO synthesis. Under conditions of hypoxia, high ADMA, low arginine, or low availability of essential NOS cofactors (NADPH and/or tetrahydrobiopterin),27 NOS will be uncoupled, producing reactive oxygen species in lieu of NO, further reducing NO bioavailability and adding the milieu of oxidative stress. An imbalance between endothelial NO (eNO) synthase-derived NO and superoxide generation has been established in the hemizygote sickle cell mouse model by Wood et al.28 These authors were also the first to suggest that abnormal tetrahydrobiopterin function or availability may be yet another mechanism contributing to dysregulation of the arginine-NO pathway in SCD. This is a mechanism now well described in systemic hypertension that has only recently been addressed in PH. Upregulation of NOS would therefore enhance oxidative stress when the local milieu favors NOS uncoupling. Indeed, studies in transgenic sickle cell mice demonstrate that NOS activity is paradoxically increased and uncoupled while NO bioavailability is low.11
Oxidative Stress
Oxidative stress is another prominent mechanism of vasculopathy. In hemolytic disorders, the erythrocyte may be a major determinant of the global redox environment. The sickle and thalassemia erythrocytes have increased concentrations of reactive oxygen species (ROS) compared with normal red blood cells.6,29,30
Overproduction of ROS, such as superoxide, by both enzymatic (xanthine oxidase, NADPH oxidase, uncoupled eNOS) and nonenzymatic pathways (Fenton chemistry), promotes intravascular oxidant stress that can likewise disrupt NO homeostasis and produce the highly oxidative peroxynitrite.4 Increased xanthine oxidase expression in the lung of the sickle cell mouse has also been reported to scavenge NO in this vascular system.31
Altered cell membrane lipids in SCD and abnormal erythrocyte phosphatidylserine (PS) exposure triggered in part by oxidative stress may also contribute to the early demise of the red blood cells in circulation, making them more vulnerable to the enzymatic breakdown by secretory phospholipase A2, an important lipid mediator in inflammation. PS exposure also induces binding of red cells to endothelial cells, leading to sequestration of PS-exposing cells in peripheral blood vessels. This process can contribute to vascular dysfunction, hemolysis, and a pro-thrombotic state.32
Finally, alterations in the glutathione buffering system common to these hemoglobinopathies30,33 may render erythrocytes incapable of handling the increased oxidant burden, thereby predisposing them to hemolysis. Recently we discovered that a depletion of erythrocyte glutamine concentration and aberrations in erythrocyte glutathione metabolism is linked to severity of PH in SCD and bio-markers of hemolytic rate.34 Glutamine, an essential precursor in NADPH biosynthesis, is metabolized to the glutathione substrate glutamate in the process of NADPH production. Glutamine thus plays an antioxidant role through preservation of intracellular NADPH, making it an important amino acid for glutathione homeostasis. Glutamine also serves as a precursor for the de novo production of arginine through the citrulline-arginine pathway. Orally ingested glutamine is metabolized to citrulline in the enterocytes, and is subsequently used by the kidneys to synthesize arginine. Glutamine therapy has already demonstrated promise in SCD.35 Interestingly, oral arginine increased erythrocyte total glutathione levels in both humans36 and sickle cell transgenic mice,37 which may improve the redox state of the sickle erythrocyte. Arginine, demonstrating initial promise for treatment of PH in SCD,38 has recently been shown by Kaul and colleagues to improve vascular function in the sickle cell mouse model by ameliorating hemolysis, oxidant stress and the NO resistance state.39 These findings highlight the complex interactions of hemolysis and oxidative stress, and the close relationship of these two mechanisms in SCD, fueling the debate of which came first.40 It is possible that these two critical and co-dependent mechanisms of vasculopathy may alternate as the primary mechanism of action based on unique individual stresses at a particular point in time. Teasing out these answers may help direct novel therapy that will effectively disrupt the cycle of hemolysis and oxidant injury. Long-term benefits of arginine in hemolytic disorders may, however, be limited by the actions of elevated plasma arginase activity, if the dose is not sufficient to overcome the effects of arginase consumption.
Coagulopathy
Coagulation abnormalities are frequently reported in hemolytic anemias. Although multifactorial, activation of coagulation may be another downstream effect of low NO bioavailability. Red blood cell PS-exposure in thalassemia and SCD patients appears to play a significant role in the etiology of the observed hypercoagulable state.41 A recent study by Setty and colleagues demonstrated that PS-exposing red blood cells induced a twofold increase in tissue factor expression in vivo, an effect they suggest is not due to the direct physical interaction of the red blood cells with the endothelium in patients with SCD, but rather is the result of increased circulating hemoglobin released during hemolysis, bridging the mechanisms of hemolysis and coagulation.42
Splenectomy is an additional risk factor for the development of PH and a hypercoagulable state in hemolytic disorders. Singer and colleagues recently reported an association of PH with splenectomy, increased platelet activation, hypercoagulability and evidence of chronic hemolysis in patients with both beta-thalassemia major and intermedia.43 Intravascular hemolysis has the potential to drive a pro-coagulant state, as NO has properties that inhibit platelet activation, tissue factor expression and thrombin generation.8 Altered arginase activity within the platelets of patients with SCD likely contributes to pathological platelet activation and the hypercoagulopathy and vasculopathy in this disorder.44 High thrombin generation is known to occur in both SCD and thalassemia syndromes. Thrombin itself increases arginase activity in human endothelial cells,45 and will stimulate vascular smooth muscle cell polyamine synthesis by increasing CAT and ornithine decarboxylase gene expression, thus playing a role in endothelial dysfunction. Additionally, Villagra and colleagues have recently demonstrated that increased platelet activation in SCD correlates strongly with both PH severity and biomarkers of hemolysis. The NO scavenging capacity of cell-free hemoglobin will contribute to platelet activation, while sildenafil therapy was shown to inhibit platelet activation.46 Ataga and colleagues found that patients with SCD had higher levels of markers of coagulation (thrombin-antithrombin complex, prothrombin fragment F1+2, D-dimer) and endothelial activation (soluble vascular endothelial cell adhesion molecule, sVCAM) compared to normal volunteers, while measures of hemolytic rate correlated with indices of hypercoagulability.47 A mechanistic model is emerging that links coagulation abnormalities to dysregulation of the arginine-NO pathway in PH that has important implications for hemolytic disorders.
Apolipoprotein Dysregulation in SCD
Serum apolipoprotein levels have long been known to be lower in patients with SCD than in the general population.48 The most abundantly significant of these is apolipo-protein A-I (apoA-I), found primarily on high-density lipo-protein cholesterol (HDL-C) particles, the so-called good cholesterol. In the general population, low apoA-I and HDL-C levels are a risk factor for the development of atherosclerosis, a proliferative vasculopathy primarily affecting the coronary and cerebral vasculature, promoting the risk of myocardial infarction and stroke. A recent study identified a trend that SCD patients with the lowest apoA-I levels have a particularly high prevalence of PH, up to 71% in patients with plasma apoA-I levels in the bottom quartile (< 87 mg/dL), compared to 36% in SCD patients in the top apoA-I quartile (> 111 mg/dL; odds ratio (OR) 2.0, P = .06, log rank test for trend) (Yuditskaya et al, personal communication). Apolipoprotein B (apoB), associated with low-density lipoprotein cholesterol, appears to trend in the opposite direction, as it does in atherosclerosis. The PH prevalence in the upper quartile of apoB levels (> 73 mg/dL) was 69%, compared to 31% in the lowest quartile (< 49 mg/ dL)(OR 2.3, P = .05). The top quartile of the ratio of apoB/ apoA-I (> 0.72) was even more strongly associated with PH prevalence compared to the lowest quartile (< 0.51)(77% vs. 23%, OR 3.3, P = .006).
Consistent with a role in endothelial function, SCD patients with lower-than-median apoA-I levels (< 89 mg/ dL) demonstrate deficient vasodilatory responses to brachial artery infusions of the endothelial agonist acetylcholine compared to SCD patients with higher-than-median apoA-I (percentage increase in blood flow, P < .0001, ANOVA) (Yuditskaya et al, personal communication). This constitutes evidence that SCD patients with low apoA-I have endothelial dysfunction, a parallel finding to the general population. This likely is mediated through apoA-I binding to the scavenger receptor class B type I (SRB-I) receptor on endothelial cells, which activates a poorly characterized signal transduction pathway that ultimately stimulates eNOS to release NO.49 The findings in humans are supported by experiments in the sickle cell mice with peptide mimetics of apoA-I.50 Deficient vasodilatory response to acetylcholine in the sickle cell mouse is restored nearly to normal following treatment with the apoA-I mimetic L4F. This defect in vascular function appears to be distinct from and additive to the NO resistance due to NO scavenging.
Circulating Endothelial Progenitor Cells in SCD
Endothelial progenitor cells (EPCs) are bone marrow–derived cell populations that contribute to re-endothelialization after endothelial injury. In the general population, they are found in low numbers in peripheral blood, even lower in subjects with known risk factors for coronary atherosclerosis.51 One study conducted in 24 patients with SCD with high plasma sVCAM-1 levels showed low numbers of circulating EPCs compared to 10 African-American control subjects, using a colony-forming assay (8.9 ± 12.5 vs. 24.7 ± 31.1 colonies/well, mean ± SEM, P < .05) (Bereal et al, personal communication)). The SCD patients with low EPC had endothelial dysfunction as indicated by blunted vasodilatory effect of 3 doses of acetylcholine (7.5, 15 and 30 mg/min) in forearm blood flow assays compared to high EPC patients (84 ± 21 vs 194 ± 44%; 145 ± 34 vs 305 ± 86%; 255 ± 66 vs 407 ± 113%; P < .05, two-way ANOVA). Finally, the low EPC patients have higher pulmonary pressures as indicated by higher TRV (2.6 ± 0.2 vs 2.4 ± 0.2 m/ sec, P < .05). In summary, circulating EPC counts appear to be lower in SCD patients with vasculopathy than the general population, and the low counts may correlate with endothelial dysfunction and PH.
Vascular Phenotypes of Sickle Cell Disease
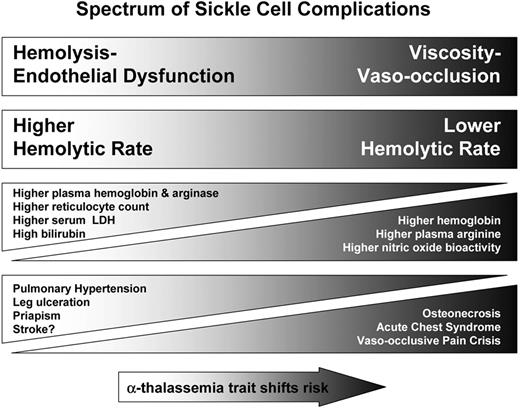
Hemolytic rate is associated with a growing list of clinical complications of SCD that fall into two partially overlapping subphenotypes: a viscosity–vaso-occlusion phenotype versus one of hemolysis-endothelial dysfunction (Figure 4 ).52 The first subphenotype includes the more classic manifestations of SCD, including VOC and ACS. These complications are epidemiologically associated with a high steady-state white blood cell count, high baseline hemoglobin levels and low fetal hemoglobin levels, with protection provided by increasing fetal hemoglobin concentration. The complications are mainly the consequence of microvascular obstruction by sickle erythrocytes and the pathogenesis characterized by ischemia-reperfusion injury, adhesion, infarction and inflammation. The second subpheno-type is one that shares some features with other hemolytic anemias and includes PH, systolic hypertension, priapism, cutaneous leg ulceration, sudden death and possibly stroke and asthma. Consistent with this formulation, co-inheritance of α-thalassemia trait, which reduces hemolytic rate in SCD,53 reduces the risk of leg ulceration, priapism and stroke, and increases the risk of VOC, ACS, and osteonecrosis.52
Serum lactate dehydrogenase (LDH) is released from the erythrocyte along with free hemoglobin and arginase. As such, LDH represents a convenient biomarker of intra-vascular hemolysis and NO bioavailability associated with mortality that Kato and colleagues found helpful in identifying the clinical subphenotypes of hemolysis-associated vasculopathy (Figure 5 ).54
PH is the best characterized clinical complication of acute and chronic hemolysis55 and occurs in about one third of adults7,22,56 and children57–61 with SCD. A Doppler echocardiogram measured tricuspid regurgitant jet velocity (TRV) of 2.5 m/sec or greater, suggesting PH is currently the strongest predictor for early death in SCD, with approximately 10-fold increased risk of early mortality7,62,63 and a 40% mortality risk within 3 years of diagnosis.7 A TRV ≥ 2.5 m/sec is 2 standard deviations greater than normal. Ten percent of adult patients with SCD have a TRV ≥ 3 m/sec and of these most have mean pulmonary artery pressures greater than 25 mm Hg on right heart catheterization.64
A PH prevalence of 60% to 75% has been reported in both thalassemia intermediate and major,10 and occurs in nearly every form of hereditary or acquired hemolytic anemia.8,55 PH has been definitively linked to hemolytic rate and low NO bioavailability in both human and sickle cell transgenic mouse studies.8 Hsu and colleagues have demonstrated that sickle cell transgenic mice develop spontaneous PH associated with a global impairment in NO production as a result of uncoupled NOS, and from NO inactivation by plasma hemoglobin and superoxide.11 An association with low NO and arginase-limited arginine bioavailability via non-hemolytic pathways has been reported in both primary and secondary PH,65 aligning an impaired arginine-NO pathway with PH pathogenesis regardless of the inciting trigger.
Genetic susceptibility likely influences the severity of the clinical phenotype. Ashley-Koch and colleagues recently published evidence that genetic polymorphisms identified in the TGβ superfamily are associated with PH risk in SCD, including activin A receptor, type II-like 1 (ACVRL1) and bone morphogenic protein receptor 2 (BMPR2), single nucleotide polymorphisms found to also be associated with primary PH.66
Priapism and cutaneous leg ulcers have been reported in patients with thalassemia intermedia and other hemolytic anemias in addition to SCD.52 In a recurring theme, this complication was found to be most prevalent in patients with SCD with the highest rates of hemolysis,7,67 and reported by Nolan and colleagues to be associated with a genetic polymorphism in Klotho, a gene that regulates NO bioavailability.68 Patients with conditions associated with lower hemolytic rate, such as hemoglobin SC and S-beta-thalassemia, or high fetal hemoglobin expression were at lower risk for priapism.
While less clearly linked to hemolytic rate, both stroke and asthma share overlapping mechanisms involved in sickle cell vasculopathy. Ischemic stroke and PH have several common risk factors, including a history of prior stroke, systolic hypertension, low transcutaneous oxygen saturation, and severe anemia. Both have histophathological evidence of large vessel arterial disease, featuring smooth muscle hyperplasia with overlying endothelial damage, fibrosis and thrombosis in situ. In addition, in the STOP cohort, chronic transfusion has been shown to reduce plasma free hemoglobin levels and stroke incidence in children with SCD at risk for stroke.69
Asthma exacerbates disease severity in SCD, and has recently has been linked to PH in children58 and mortality in adults.70 Intravascular hemolysis triggers a shift in arginine metabolism away from NO, towards ornithine-dependent pathways12,71 that contributes to structural remodeling of the lungs (Figure 5 ). Asthma is associated with an inflammatory-mediated elevation in arginase activity and an acute arginine72 and NO deficiency. Onyekwere and colleagues found a correlation of FEV1 with TRV on Doppler echocardiography.57 This relationship should be evaluated further, since patients with SCD may potentially be at risk for an asthma-like condition triggered or worsened by hemolysis-driven release of erythrocyte arginase, in addition to classic familial asthma.73 Regardless of the mechanism, asthma in SCD should be aggressively managed based on published NIH guidelines.
Conclusions
The variable clinical spectrum of SCD is the consequence of multiple events and genetic susceptibility that goes beyond the occurrence of a single amino acid substitution in the beta globin chain of hemoglobin. Any attempt to identify the primary mechanism will certainly generate debate; however, it is clear that there are complex interrelationships among the many mechanisms discussed that make it difficult to argue that any single event occurs in isolation. Impaired NO bioavailability represents the central feature of endothelial dysfunction, and pervasively contributes to the overlapping mechanisms of vasculopathy in SCD. The biological consequences of hemolysis on NO bio-availability ultimately translate into the clinical manifestation of PH, priapism, leg ulcers and stroke, now recognized as the hemolytic subphenotype of SCD.52 Insight into the pathogenesis of sickle vasculopathy many guide future therapies and has broad applications for other hemolytic anemias. Some interventions that might improve vasculopathy in hemoglobinopathies are listed in Table 1 , while novel therapeutic strategies are discussed in depth in an accompanying article by Dr. Gregory Kato,88 beginning on page 186. Combination therapies that target hemolysis and oxidative stress should be considered in future studies.
Therapeutic interventions that might improve vasculopathy.
| Intervention . | Mechanism . | Evidence . |
|---|---|---|
| Sildenafil | ↑ NO generation ↓ Platelet activation | Human clinical trials46,74 Case reports75,76 |
| Transfusion | ↓ Hemolysis ↓ Inflammation | Human clinical trials69,77,78 |
| Arginine | ↑ NO generation ↓ Hemolysis ↓ Platelet activation ↓ Oxidative stress | Human clinical trials36,38,79–81 Sickle cell mouse studies37,39,82 |
| Glutamine | ↓ Hemolysis ↓ Oxidative stress | Human clinical trials83 Endothelial culture experiments35 |
| Statins | ↓ Oxidative stress ↑ NO generation | Sickle cell mouse studies84 |
| Niacin | ↑ ApoA-1 ↑ HDL | Human clinical trials85 |
| ApoA-1 mimetic (L-4F) | ↓ Inflammation | Sickle cell mouse studies50 |
| Hydroxyurea | ↑ NO generation ↓ Hemolysis 2° ↑ Fetal Hb | Human clinical trials86 Endothelial culture experiments87 |
| Intervention . | Mechanism . | Evidence . |
|---|---|---|
| Sildenafil | ↑ NO generation ↓ Platelet activation | Human clinical trials46,74 Case reports75,76 |
| Transfusion | ↓ Hemolysis ↓ Inflammation | Human clinical trials69,77,78 |
| Arginine | ↑ NO generation ↓ Hemolysis ↓ Platelet activation ↓ Oxidative stress | Human clinical trials36,38,79–81 Sickle cell mouse studies37,39,82 |
| Glutamine | ↓ Hemolysis ↓ Oxidative stress | Human clinical trials83 Endothelial culture experiments35 |
| Statins | ↓ Oxidative stress ↑ NO generation | Sickle cell mouse studies84 |
| Niacin | ↑ ApoA-1 ↑ HDL | Human clinical trials85 |
| ApoA-1 mimetic (L-4F) | ↓ Inflammation | Sickle cell mouse studies50 |
| Hydroxyurea | ↑ NO generation ↓ Hemolysis 2° ↑ Fetal Hb | Human clinical trials86 Endothelial culture experiments87 |
Spectrum of sickle cell subphenotypes affected by hemolytic rate. The viscosity–vaso-occlusion subphenotype is associated with a lower hemolytic rate, marked by a higher hemoglobin level, and low plasma hemoglobin, lactate dehydrogenase (LDH), bilirubin and arginase levels. Patients with these features have a higher incidence of vaso-occlusive pain crises, acute chest syndrome, and osteonecrosis. In contrast, patients with the hemolysis-endothelial dysfunction subphenotype exhibit markers of high hemolytic rate, including low hemoglobin level, high plasma hemoglobin, LDH, bilirubin, and arginase, culminating in low nitric oxide bioavailability and high prevalence of pulmonary hypertension, leg ulceration, priapism, and stroke. Co-inheritance of α-thalassemia trait with sickle cell disease reduces the hemolytic rate, minimizes the risk of hemolysis-associated complications and increasing the risk of viscosity-related complications. Adapted with permission from Kato GJ, Gladwin MT, and Steinberg MH. 52
Spectrum of sickle cell subphenotypes affected by hemolytic rate. The viscosity–vaso-occlusion subphenotype is associated with a lower hemolytic rate, marked by a higher hemoglobin level, and low plasma hemoglobin, lactate dehydrogenase (LDH), bilirubin and arginase levels. Patients with these features have a higher incidence of vaso-occlusive pain crises, acute chest syndrome, and osteonecrosis. In contrast, patients with the hemolysis-endothelial dysfunction subphenotype exhibit markers of high hemolytic rate, including low hemoglobin level, high plasma hemoglobin, LDH, bilirubin, and arginase, culminating in low nitric oxide bioavailability and high prevalence of pulmonary hypertension, leg ulceration, priapism, and stroke. Co-inheritance of α-thalassemia trait with sickle cell disease reduces the hemolytic rate, minimizes the risk of hemolysis-associated complications and increasing the risk of viscosity-related complications. Adapted with permission from Kato GJ, Gladwin MT, and Steinberg MH. 52
Relationship of serum lactate dehydrogenase (LDH) levels and history of vasculopathic complications. (A) Kato and colleagues reported LDH levels in 213 patients with sickle cell disease. The frequency distribution by LDH level in hundreds is indicated by the vertical bars. For comparison of the prevalence of selected sickle cell complications, data from 213 patients are divided into three groups according to serum LDH levels. The low LDH is defined by LDH levels lower than one standard deviation below the overall mean (range 121–189 IU/L), medium LDH by overall mean LDH level plus or minus one standard deviation (range 190–511 IU/L) and high LDH higher than one standard deviation above the overall mean (range 512–1171 IU/L). The prevalence of pulmonary hypertension (B), leg ulcers (C), and, in males, priapism (D) are also related to LDH group. All statistics are significant by chi square test for trend. The number of patients in each group (n) is indicated above each bar. Adapted with permission from Blood.54
Relationship of serum lactate dehydrogenase (LDH) levels and history of vasculopathic complications. (A) Kato and colleagues reported LDH levels in 213 patients with sickle cell disease. The frequency distribution by LDH level in hundreds is indicated by the vertical bars. For comparison of the prevalence of selected sickle cell complications, data from 213 patients are divided into three groups according to serum LDH levels. The low LDH is defined by LDH levels lower than one standard deviation below the overall mean (range 121–189 IU/L), medium LDH by overall mean LDH level plus or minus one standard deviation (range 190–511 IU/L) and high LDH higher than one standard deviation above the overall mean (range 512–1171 IU/L). The prevalence of pulmonary hypertension (B), leg ulcers (C), and, in males, priapism (D) are also related to LDH group. All statistics are significant by chi square test for trend. The number of patients in each group (n) is indicated above each bar. Adapted with permission from Blood.54
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Department of Emergency Medicine, Children’s Hospital & Research Center Oakland, Oakland, CA