Abstract
The prevention of venous thromboembolism (VTE) in patients recovering from major trauma, spinal cord injury (SCI), or other critical illness is often challenging. These patient groups share a high risk for VTE, they often have at least a temporary high bleeding risk, and there are relatively few thromboprophylaxis trials specific to these populations. A systematic literature review has been conducted to summarize the risks and prevention of VTE in these three groups. It is concluded that routine thromboprophylaxis should be provided to major trauma, SCI and critical care patients based on an individual assessment of their thrombosis and bleeding risks. For patients at high risk for VTE, including those recovering from major trauma and SCI, prophylaxis with a low molecular weight heparin (LMWH) should commence as soon as hemostasis has been demonstrated. For critical care patients at lower thrombosis risk, either LMWH or low-dose heparin is recommended. For those with a very high risk of bleeding, mechanical prophylaxis should be instituted as early as possible and continued until pharmacologic prophylaxis can be initiated. The use of prophylactic inferior vena caval filters is strongly discouraged because their potential benefit has not been shown to outweigh the risks or substantial costs. Implementation of thromboprophylaxis in these patients requires a local commitment to this important patient safety priority as well as a highly functional delivery system, based on the use of pre-printed orders, computer prompts, regular audit and feedback, and ongoing quality improvement efforts.
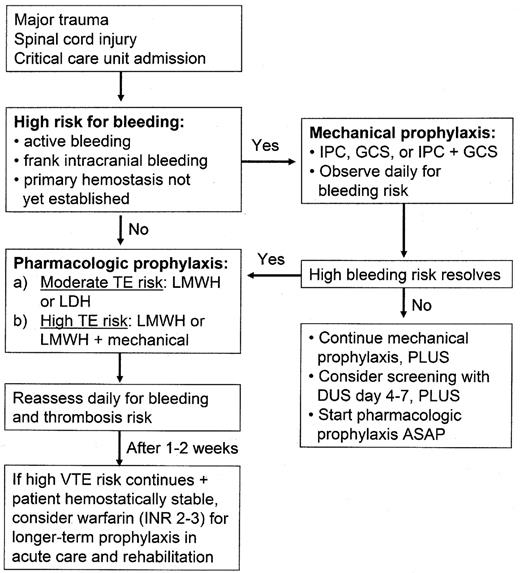
Across the spectrum of hospitalized patients, the rates of venous thromboembolism (VTE) vary substantially.1 Although a large number of patient-specific thrombosis risk factors have been shown to contribute to this variability, the principal factor that determines VTE risk is the patient’s primary reason for hospitalization, whether this is a surgical procedure or an acute medical illness. This paper will discuss the risks of VTE in three populations: major trauma, spinal cord injury (SCI) and critical care patients. The reasons for selecting these patient groups are as follows: each is associated with a high risk of VTE; there is often also a temporary high risk of bleeding; and there have been relatively few studies of prophylaxis in each of these groups. Despite the challenges involved in preventing VTE in these patients, recent evidence allows clinicians to provide effective and safe prophylaxis (see Figure 1 ).1
MajorTrauma
Among hospitalized patients, those recovering from major trauma have the highest risk of VTE.1–3 Without prophylaxis, patients with major trauma have a risk of deep vein thrombosis (DVT) that exceeds 50% and pulmonary embolism (PE) is the third most common cause of death in trauma patients who survive beyond the first day.1,2 In a prospective study of 443 major trauma patients who did not receive any thromboprophylaxis, the prevalence of DVT, using routine contrast venography, was 58%; 18% of patients had proximal DVT.2 Despite the routine use of thromboprophylaxis, another study reported the prevalence of DVT and proximal DVT to be 27% and 7%, respectively, in trauma patients who underwent weekly Doppler ultrasound (DUS).4
The specific risk factors that are independently associated with an increased risk of VTE in trauma include the following: SCI, lower extremity or pelvic fracture, the need for a surgical procedure, femoral venous line or major venous repair, increasing age, and prolonged immobility.1,2,4,5 Limited data suggest that patients with penetrating injuries have a lower risk of thrombosis than those who sustain blunt trauma.
Although the routine use of thromboprophylaxis in trauma was first recommended more than 60 years ago, there are few randomized trials of prophylaxis in this patient group. Mechanical prophylaxis methods are widely used in trauma because they do not increase the risk of bleeding. Limited evidence suggests that intermittent pneumatic compression (IPC) devices are probably effective in trauma patients without lower extremity injuries, especially those with head injuries.1 A recent randomized trial in patients with acute intracerebral hemorrhage found that the combination of IPC and graduated compression stockings (GCS) was associated with a 70% reduction in the rate of asymptomatic DVT compared with GCS alone.6 However, a number of studies have reported no protection from IPC compared with no prophylaxis,1 and a meta-analysis also concluded that IPC did not reduce the rate of DVT compared with no prophylaxis.7 Other important limitations of IPC include its inability to be used in approximately one third of trauma patients (due to lower extremity injuries), and consistent evidence of poor compliance with proper use of the devices by patients and nursing staff. In trauma, the use of mechanical prophylaxis alone cannot be recommended except in patients with active bleeding or in those at high risk for bleeding (until anticoagulants can be given later when the bleeding risk decreases). Mechanical prophylaxis may also be considered in combination with anticoagulant prophylaxis to try to further reduce the high thromboembolic risk (although this has not been proven in such patients). Neither graduated compression stockings (GCS) nor venous foot pumps have been shown to provide protection in trauma patients when used alone. However, a recent randomized trial demonstrated that the use of a foot pump early after trauma with delayed initiation of low molecular weight heparin (LMWH) was as effective as early commencement of LMWH alone.8 A device that flexes and extends the ankle joint every 2 seconds also appears to improve the efficacy of low-dose heparin (LDH) based on one study.9
Low-dose heparin is not particularly effective in major trauma as evidenced by a large randomized trial that showed LMWH provided greater protection than LDH10 and by a meta-analysis that demonstrated that LDH was no better than no prophylaxis.7 The superiority of LMWH over LDH as thromboprophylaxis in trauma is also consistent with observations in other high-risk patient groups.1 LMWH was also shown to be three times more effective than IPC in preventing VTE in trauma patients, with no difference in major bleeding.11
Routine screening of high-risk trauma patients for asymptomatic DVT using DUS is neither feasible nor is it effective as a strategy to prevent clinically important VTE.12 Furthermore, the false-positive rate of screening DUS in asymptomatic patients is high13 and routine screening is very costly. With appropriate use of thromboprophylaxis, there appears to be no incremental value of DUS screening.12,14,15 However, selective screening might be beneficial in a limited proportion of high-risk patients in whom early prophylaxis has not been possible.1,14
Prophylactic inferior vena caval filter (IVCF) insertion has never been shown to be protective in trauma patients and there is no evidence that use of an IVCF is of any benefit when added to the most effective thromboprophylaxis that is appropriate for the patient’s clinical status.1,16 A meta-analysis of prospective studies found no difference in the rates of PE in patients with and without prophylactic IVCFs.17 Furthermore, IVCF use is associated with short- and long-term complications, PE still occurs in patients with filters, there may be a tendency to inappropriately delay effective prophylaxis if an IVCF is inserted, and there is an increased incidence of thrombosis at the vascular access site and at the filter itself. The use of retrievable filters and bedside insertion techniques for filter placement increases the temptation to use these devices with greater frequency. However, the most important concerns about IVCF use continue to be the absence of any direct evidence of benefit, the inability to predict which trauma patients might be protected by filter insertion, particularly as more effective prophylaxis strategies are being utilized, and the substantial costs involved. Until these issues are resolved, I (and others) do not recommend the use of IVCFs as prophylaxis, even in patients at high risk for VTE.1,16,18 IVCF insertion is indicated for patients with proven proximal DVT who either have absolute contraindications to full anticoagulation or who require major surgery in the near future. In these situations, even with an IVCF, therapeutic anticoagulation should be commenced as soon as the contraindication resolves.
The routine use of thromboprophylaxis in trauma patients is now standard of care.1,3 As such, every trauma unit should develop a management guideline for the prevention of thromboembolism, and compliance with this local guideline should be assessed periodically as a quality of care measure. Every trauma patient should be assessed for his or her thromboembolic risk and most should receive prophylaxis. It is important to start as soon as possible, since symptomatic VTE and fatal PE occur when suboptimal or delayed prophylaxis are used.1,12
The use of LMWH, started when primary hemostasis has occurred, is the most efficacious and simplest prophylaxis option for the majority of trauma patients.1,3,10 Current contraindications to the early initiation of LMWH prophylaxis include the following: (1) intracranial bleeding, (2) ongoing, uncontrolled bleeding, and (3) incomplete SCI associated with spinal hematoma. The presence of head injury without frank hemorrhage, lacerations or contusions of internal organs (such as the lungs, liver, spleen, or kidneys), the presence of a retroperitoneal hematoma associated with pelvic fracture, or complete SCIs do not contraindicate the use of LMWH prophylaxis as long as the patient has no evidence of active bleeding. For example, a study of patients with splenic trauma, managed nonoperatively, showed that commencement of prophylactic LMWH within 48 hours of hospital admission was not associated with a greater need for surgical intervention or blood transfusion than LMWH started later.19 As trauma surgeons become more experienced with the use of LMWH, concerns about bleeding appear to be decreasing.
Most trauma patients can be started on LMWH within 36 hours of injury. For patients with contraindications to LMWH prophylaxis, IPC should be considered in spite of its limited protection. If IPC is utilized, it should be started as early as possible after hospital admission, applied to both legs, and used continuously except when the patient is actually walking. After an initial period of mechanical prophylaxis, during which primary hemostasis becomes established, these patients can usually be started on prophylactic LMWH.
Although the optimal duration of thromboprophylaxis is not known for these patients, it should generally continue until discharge from hospital. If the duration of hospital stay (including rehabilitation) continues beyond approximately 2 weeks, and if there is no longer a major risk of bleeding and no further surgical procedures are planned for the near future, continuing inpatient prophylaxis with either LMWH or switching to an oral anticoagulant should be considered.
Acute Spinal Cord Injury
Without prophylaxis, patients with acute SCI have the highest risk of VTE among trauma patients (and therefore also the highest risk among all hospital patient groups).1 DVT occurs in 60% to 100% of SCI patients subjected to routine screening,1,13 and PE remains the third most common cause of death. In addition to the SCI itself, other risk factors for DVT include the following: increasing age, paraplegia vs tetraplegia, complete vs incomplete SCI, concomitant lower extremity fractures, and delayed initiation of thromboprophylaxis. VTE in SCI patients results in considerable long-term disability because these patients have low rates of recanalization of their venous thrombi and they are subject to bleeding complications associated with prolonged anti-coagulation.
A number of small randomized trials suggest that LDH and IPC are not effective methods of prophylaxis when used alone in SCI patients, while LMWH is substantially more efficacious.1 In the largest thromboprophylaxis trial, 476 patients with acute SCI enrolled in 27 centers were randomized to receive either the combination of heparin 5,000 Units q8h plus IPC or enoxaparin 30 mg q12h.13 DVT was demonstrated in 63% of the LDH-plus-IPC group and in 66% of the enoxaparin patients, while the rates of major VTE (proximal DVT plus PE) were 16% and 12%, respectively. No study patient had fatal PE. At least in part, these high DVT rates are related to the delayed initiation of prophylaxis (up to 72 hours after injury). Major bleeding was seen in 5% of LDH-plus-IPC patients and in 3% of those who received enoxaparin.
The insertion of prophylactic IVCFs has been discussed earlier. If suboptimal prophylaxis is used, IVCFs might reduce the occurrence of PE (although this has not been proven). However, these devices are unlikely to be necessary if appropriate prophylaxis is given, filter use is associated with major complications that may be at least as common as massive PE, and they are extremely costly.1,18 It has been estimated that filters would have to be placed in 100 SCI patients receiving prophylaxis to prevent 2 non-fatal PE at a cost of $500,000.18
Although the period of greatest risk for VTE following SCI is the acute-care phase, symptomatic DVT, PE, and fatal PE also occur in the rehabilitation phase.1,20,21 In a recent, nonrandomized study, 119 patients who had a normal DUS 2 weeks after acute SCI, were continued on prophylaxis with either LDH or LMWH for another 6 weeks at which time the DUS was repeated.20 The rates of new VTE were 22% (one fatal PE) and 8% in the LDH and the enoxaparin groups, respectively.
The very high risk of DVT and PE following SCI, combined with the results of currently available prevention studies, support the use of early prophylaxis in all SCI patients.1 LDH and IPC do not provide adequate protection when used alone. LMWH alone or the combination of LMWH (or LDH) and IPC are the recommended early options.1 Before commencing anticoagulant prophylaxis, there should be clinical evidence that primary hemostasis has taken place. If there are concerns about bleeding at the injury site or elsewhere, mechanical prophylaxis with IPC and/or GCS should be initiated as soon as possible after admission with the addition of anticoagulant prophylaxis when the bleeding risk has decreased.
Studies have not addressed the value of routine screening of SCI patients with DUS, although this is a reasonable consideration for patients in whom prophylaxis has been delayed for several days. After the acute injury phase, continuing LMWH or conversion to full-dose warfarin (target INR 2.5, range 2.0–3.0) for the duration of the rehabilitation phase is likely to protect patients from delayed thromboembolic events. It is recommended that DVT prophylaxis be continued for a minimum of 3 months (or until the completion of the in-patient rehabilitation phase).1
Critical Care Patients
The VTE risks in critically ill patients vary considerably, although most intensive care unit (ICU) patients have multiple risk factors for VTE and an overall moderate-to-high risk.22–24 Some of the patient risk factors that predate the ICU admission include recent surgery, trauma, sepsis, malignancy, immobilization, increased age, heart or respiratory failure, and previous VTE. Other thrombotic risk factors that may be acquired during the ICU stay include immobilization, pharmacologic paralysis, central venous lines, surgical procedures, sepsis, mechanical ventilation, vasopressor use, and hemodialysis.22,23 Neither D-dimer levels nor tests of hypercoagulability (activated protein C resistance ratio, Prothrombin 20210A gene mutation, levels of protein C, protein S, or antithrombin, anticardiolipin antibody titer, and lupus anticoagulant) had any predictive value for DVT in critically ill patients.25
There are only two published randomized trials of thromboprophylaxis in critical care patients in which routine screening with an objective diagnostic test was used to detect DVT.26,27 These trials have shown that LDH and LMWH were significantly more effective than no prophylaxis in ICU patients. Unfortunately, these two anticoagulants have never been directly compared in critical care patients. A large, multinational randomized trial is now underway to compare the effectiveness and safety of LDH and LMWH in this setting.28
When LMWH is used as thromboprophylaxis in ICU patients, both generalized patient edema and the use of vasoconstrictor drugs are associated with significantly reduced anti-Xa levels, which might contribute to reduced prophylaxis effectiveness. However, the link between low (or high) anti-Xa levels and either thrombosis or bleeding has never been established in ICU patients (and there is a paucity of such data from other patient groups). Further studies are required to assess the clinical relevance of these observations.
The selection of an appropriate method of thromboprophylaxis should be assessed on admission to the critical care unit.1 This decision involves a consideration of the thromboembolic and bleeding risks, both of which may vary in the same patient, from day to day. For ICU patients at high risk for bleeding, mechanical prophylaxis with IPC and/or GCS is recommended until the bleeding risk decreases, although this has never been studied in a general ICU setting. For ICU patients not at high risk for bleeding with a moderate thrombosis risk (e.g., medically ill or general surgical problems) either LMWH or LDH is recommended. For patients at higher risk (e.g., following major trauma or orthopedic procedures), LMWH provides greater protection than LDH and is recommended. To prevent interruption of thromboprophylaxis, specific prophylaxis recommendations should be included in the patients’ orders when they are transferred from the ICU.
Venous thromboembolism is a common, potentially lethal complication of hospitalization for major trauma, SCI and other critical illnesses. Despite the availability of evidence-based prophylaxis recommendations for these groups,1 the use of this important patient safety intervention is frequently suboptimal. Effective strategies to ensure that high-risk patients receive appropriate thromboprophylaxis include the creation of a local written prophylaxis policy, and the use of preprinted orders or computer decision support systems with mandatory fields addressing prophylaxis.29 Engaging the assistance of pharmacists and nurses to reinforce optimal prophylaxis use on a daily basis will also improve compliance. Only when hospitals incorporate the prevention of thromboembolism into the daily care culture of patients will this important nosocomial complication be controlled.