Abstract
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) differs in histological and clinical presentation from classical Hodgkin lymphoma (cHL). The typical morphologic signs of NLPHL are atypical “lymphocytic and histiocytic” (L&H) cells, which are surrounded by a non-neoplastic nodular background of small lymphocytes of B-cell origin. The NLPHL cells are positive for CD45, CD19, CD20, CD22 and CD79a, but lack expression of CD15 and CD30, the typical markers for cHL. NLPHL patients are predominantly of male gender with a median age of 37 years. Patients often present in early stages (63%) and rarely have B-symptoms (9%). Treatment of NLPHL patients using standard Hodgkin lymphoma (HL) protocols leads to complete remission (CR) in more than 95% of patients. Survival and freedom from treatment failure (FFTF) are worse in advanced-stage patients than in early-stage patients. Thus, patients in advanced and in early stages with unfavorable risk factors are treated similarly to cHL patients. In contrast, patients with early-stage NLPHL without risk factors can be sufficiently treated with reduced intensity programs having less severe adverse effects. As a result, treatment of early NLPHL is less clearly defined, including radiotherapy in extended field (EF) or involved field (IF) technique, combined modality treatment, and, more recently, monoclonal antibody rituximab. Watch and wait strategy plays an important role in pediatric oncology to avoid adverse effects associated with therapy.
The incidence of Hodgkin lymphoma (HL) accounts for approximately 2.2/100,000 in the European Union.1 HL includes two different disease entities: the rare nodular lymphocyte-predominant HL (NLPHL) accounting for approximately 5% of cases and cHL making up to 95% of all cases. NLPHL differs from cHL in its histopathological and clinical characteristics. Patients with NLPHL usually present with early clinical stage, cervical or inguinal involvement, and few if any adverse prognostic factors. They are predominantly male and most frequently in the 25- to 45-year group. The disease progresses slowly, with more frequent relapses, which are rarely fatal.2,3
This chapter describes histopathological and clinical characteristics, prognosis, and management of NLPHL.
Morphology
NLPHL is characterized by atypical “lymphocytic and histiocytic”(L&H), or “popcorn” cells, which are embedded in background of progressively transformed follicles. These contain follicular dendritic cell networks, follicular CD57+ T cells and plenty small lymphocytes of B-cell origin. Furthermore, clusters of epithelioid histiocytes may be observed at the rim of the tumor nodules. Plasma cells, eosinophils and neutrophils are rarely seen.4 According to the current WHO definition, at least a partial nodular pattern is required for the diagnosis of NLPHL.5 Diffuse areas are present in minority of cases, and it remains controversial whether purely diffuse cases really exist.6 The histopathological pattern of NLPHL differs from that of cHL, which is characterized by a small number of morphologically abnormal mononucleated and multinucleated giant cells (Hodgkin and Reed-Sternberg [RS] cells) surrounded by a reactive background composed of T cells, histiocytes, eosinophils and plasma cells7 (Table 1 ). If the lymph node architecture is partially effaced, the tumor cells are situated in the marginal zone around follicles.
It has been shown that both NLPHL and cHL are malignant B-cell lymphomas of germinal center origin.8 More recent data demonstrate that a number of B-cell signal transducing molecules are absent or reduced in Reed-Sternberg cells, whereas they are largely preserved in L&H cells and in non-Hodgkin B-cell lymphomas (NHL).9 This may support the idea of distinct oncogenic mechanisms underlying the two forms of HL, i.e., cHL and NLPHL.
Immunophenotype
The NLPHL cells are CD45+, express the B-cell associated antigens CD19, CD20, CD22, CD79a and epithelial membrane antigen (EMA), but lack expression of CD15 and CD30, the characteristic markers for cHL.10 J-chain has also been demonstrated in many cases.11 In contrast to typical NHL, L&H cells are usually Ig-negative by routine techniques. Popcorn cells also express the nuclear protein encoded by the bcl-6 gene, which is required for normal germinal center B-cell development.12 The nodules of NLPHL represent progressively transformed germinal centers. The small lymphocytes in these nodules are a mixture of polyclonal B cells with a mantle zone phenotype (IgM and IgD+), and numerous T cells, many of which are CD57+, that surround the tumor cells13 (Table 2 ). Although several reports suggest that the T cells surrounding popcorn cells are mostly CD57+,6,14,15 this can be difficult to demonstrate in many cases and absence of CD57 in the rosettes does not argue against diagnosis.
NLPHL and large B-cell lymphoma
A slightly increased incidence of NHL in patients with NLPHL has been demonstrated in several studies. The British National Lymphoma Investigation (BNLI) Group reported on 22 of 3033 HL patients randomized into the BNLI trials who developed NHL after 16 years of follow-up (incidence 0.7%). The frequency of NHL varied from 3.8% in NLPHL to 0.3% in nodular sclerosing HL.16 Orlandi et al found 5 NHL in the group of 68 NLPHL patients. The cumulative risk of NHL was 9% at 10 years.17 Furthermore, the large B-cell lymphomas that arise in NLPHL patients do not always show the typical L&H cells and usually resemble other diffuse, large B-cell lymphomas.18 Most patients with secondary NHL have a B-cell immunopheno-type, with B-lineage antigen expression in the majority and monotypic Ig expression in approximately 30% to 50% of cases. A clonal relationship between NLPHL and diffuse large B-cell lymphoma was observed by molecular genetic analysis in some cases.19
NLPHL and T-cell/histiocyte rich B-cell lymphoma
T-cell/histiocyte-rich B-cell lymphoma (T/HRBCL) represents a variant of diffuse large B-cell lymphoma in which neoplastic CD20+ B cells are scattered among the majority of nonneoplastic T cells with or without histiocytes.5 There are morphological and probably biological similarities between NLPHL and T/HRBCL, although they are distinct tumors and are treated differently. The morphologic and biologic similarities may lead to difficulties in differential diagnosis. In NLPHL, most tumor cells have an L&H appearance; RS cells are rarely identified. In T/HRBCL, the neoplastic cells mostly resemble centroblasts, L&H cells, or immunoblasts, RS cells are scarce. There are no strikingly predominating tumor cell variants to subcategorize T/HRBCL according to their morphology.6 Regarding the immunophenotype, both CD79a and bcl-2 are more frequently expressed in T/HRBCL than in NLPHL.20,21 In contrast, PU.1, a transcription factor necessary in early B-cell differentiation, is expressed in NLPHL but reduced in, or absent from, T/HRBCL.22,23 Interestingly, the subtle disparity between the phenotypes of tumor cells seems to reflect their relationship to follicular dendritic cells (FDC) meshworks. In NLPHL, neoplastic cells inside FDC networks showed a higher expression of J chain and PU.1 than cells in the same tumors that grew diffusely outside; the latter more frequently expressed CD79a and bcl-2. However, immunophenotypic differences in the tumor cells cannot currently be used for diagnostic purposes.6
On the other hand, the reactive background greatly helps the diagnosis. A follicular environment is retained in NLPHL, documented by the presence of meshworks of FDCs, but is absent from T/HRBCL.24 By definition, small B cells are abundant in NLPHL, but rare in T/HRBCL. The nature of the T-cell background is diverse24; in NLPHL, T cells are mainly CD4+CD57+ follicular T cells that commonly formed the rosettes,14 while in T/HRBCL, CD8+ cytotoxic T cells and histiocytes predominate25 and T-cell rosettes are rarely seen.
Clinical Presentation of NLPHL
Age and gender
Many aspects of this rare disease were described in an international intergroup analysis conducted by the European Task Force on Lymphoma (ETFL). In their data set, NLPHL had a similar age distribution (median 35 years) to that for cHL. Approximately 70% of NLPHL patients are male,3 a finding that is similar to the gender distribution of mixed cellularity cHL, but different from that for nodular sclerosis cHL, which has nearly equal gender balance. A more recent analysis performed by the German Hodgkin Study Group (GHSG) comparing 394 patients with NLPHL and 7904 cHL patients treated within the clinical trials between 1988 and 2002 showed similar age and gender distribution with a median age of 37 in the NLPHL group and 33 years in the cHL group. NLPHL patients were again predominantly of male gender (75%)26 (Table 3 ).
Stage and systemic symptoms
The ETFL project showed that NLPHL more often presented in early stages: 53% of NLPHL patients were in stage I and only 6% in stage IV. B symptoms were observed in 10% of NLPHL. This contrasts with cHL. The recent GHSG analysis found even higher rates of early stage NLPHL patients: of 394 NLPHL patients, 63% were in early stage, 16% in intermediate and 21% in advanced stage of disease. This contrasts with the 7904 cHL patients analyzed, of whom 22% were in early, 39% in intermediate and 39% in advanced stages, respectively. About 9% of NLPHL patients had B symptoms at presentation compared to 40% cHL patients26 (Table 3 ). In other published studies stage I NLPHL accounted for 34% to 59% and stage IV for 1% to 12% of cases. Thus, the proportion of early-stage disease is consistently higher when compared with cHL, and stage IV is much rarer. B symptoms were present in 6% to 15% of patients. The average distribution of stages was as follows: stage I, 49%; stage II, 24%; stage III, 20%; stage IV, 7%; B symptoms, 11%.27–30
Organ involvement and other negative prognostic factors
The ETFL analysis showed that site-specific organ involvement occurred with very low frequency in NLPHL patients: 8% of patients had spleen involvement, 1% bone marrow, 3% liver, 1% lung, 1% skeletal and 2% of patients showed other organ involvement. Stage IV disease is more prevalent among patients with cHL, reflecting predominantly lung involvement in nodular sclerosis cHL and bone marrow involvement in mixed cellularity cHL. The prognostically significant factors of bulky disease and mediastinal involvement are rare in NLPHL (13% and 7%). NLPHL seems to be confined more often to peripheral sites, such as upper neck, epitrochlear, and inguinal nodes.3
Clinical Course and Treatment Outcome
LPHL in early favorable stages
Clinical course and treatment outcome differ substantially between different NLPHL risk groups.3,26 The ETFL project found differences in clinical outcome between early favorable, early unfavorable (early stages with risk factors) and advanced stage groups.3 According to this analysis, NLPHL early favorable stage patients can be sufficiently treated with reduced intensity programs having less severe side effects. Analyses performed by the European Organization for Research and Treatment of Cancer (EORTC) suggested that the disease related death rate in HL patients decreases during the years after treatment. The overall mortality is higher compared to the general population, largely due to cardiac failures and secondary cancers.31 Consequently, several new treatment approaches aimed at reducing toxicity were evaluated for early favorable stage NLPHL patients to avoid side effects of standard treatment. Establishing a standard treatment for NLPHL in early favorable stages is difficult due to the low incidence of NLPHL and the very few events observed in this entity. As a result, treatment of early favorable NLPHL stages is heterogeneous including extended and involved-field radiation, combined modality treatment and more recently monoclonal antibodies.32–36
Pediatric study groups reported nonrandomized case studies of NLPHL with small numbers of patients,37,38 suggesting that a watch-and-wait strategy after initial lymph node surgery may be an appropriate treatment. Pellegrino et al retrospectively analyzed 27 children (median age 10 years), most of whom had localized NLPHL who received either standard treatment or were not treated beyond initial lymphadenectomy. With a median follow-up of 70 months, overall survival (OS) was 100% and event-free survival (EFS) was 69% (42% ± 16% for patients after surgical adnectomy and 90% ± 8.6% in combined treatment group). EFS was not significantly different between the two groups. Patients with a residual mass after initial surgery had worse EFS if they did not receive additional treatment. Thus, treatment in these cases reduces the number of relapses but has no impact on overall survival.37
An American group of pediatric oncologists treated 15 children and adolescents (median age 11 years) with localized NLPHL. Patients received selected therapy: those with stage I diseases who were disease-free after excision of the involved lymph node were carefully followed without further treatment. Patients with incomplete resection in stages I or II were treated with a brief chemotherapy consisting of vincristine, doxorubicin, cyclophosphamide and prednisone. All treated patients in this nonrandomized trial reached CR; 1 patient in stage II relapsed 6 years after the initial diagnosis.38
These retrospective studies on small number of pediatric patients indicate promising results for early favorable NLPHL. Treatment of this rather benign malignancy in pediatric patients strongly aims at avoiding adverse events such as growth retardation, infertility, hypothyroidism, cardiopulmonary complications and second malignancies. However, these small nonrandomized sample sizes precluded meaningful comparisons. The watch-and-wait strategy remains an experimental approach and currently is not appropriate in standard praxis. The patients in early favorable stage should be treated in clinical trials with proven inclusion criteria to avoid leaving this potentially curable neoplasm untreated.
For adult early favorable NLPHL patients, Schlembach et al conducted a retrospective analysis on 36 cases with non-bulky IA, supradiaphragmatic IIA or subdiaphragmatic disease and suggested involved-field or regional radiotherapy alone as an adequate treatment for stage IA patients. The 5-year relapse-free and overall survival rates for the 20 patients with stage IA NLPHL after involved field or regional radiotherapy were 95% and 100%, respectively.36 An Australian group reported on 202 NLPHL patients in stage I and II who were treated with radiotherapy alone. The treatment included mainly mantle and inverted-Y field techniques. The OS at 15 years was 83%, and freedom from progression was observed in 82% of the patients. Causes of death at 15 years were NLPHL in 3% of patients, NHL in 2%, in-field malignancy in 2%, in-field cardiac/respiratory disease in 4% and other in 6% of patients. The authors suggested that radiotherapy may be curative for patients with stage I/II NLPHL and suggested that limited-field radiotherapy may be used without loss of treatment efficacy in this patient group.35
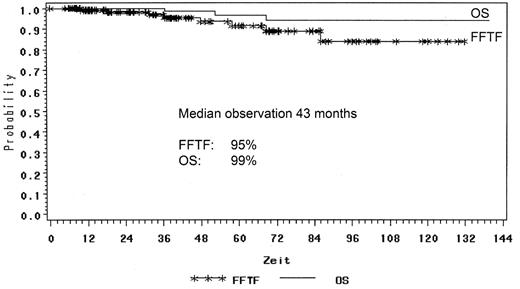
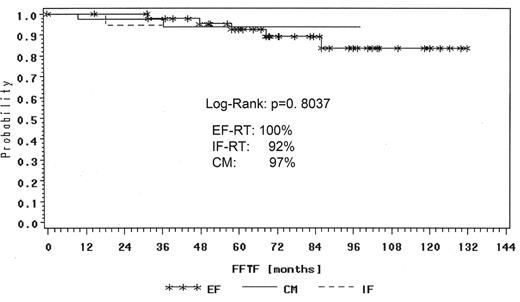
The GHSG retrospectively reviewed all NLPHL cases registered and compared the different treatment approaches such as extended and involved-field radiation as well as combined modality treatment from chemotherapy and radiation for NLPHL stage IA patients. One hundred thirty-one patients with NLPHL in clinical stage IA without risk factors were analyzed. Forty-five patients were treated with EF radiotherapy, 45 patients had IF radiation and 41 patients received combined modality treatment. The median follow-up was 78 months in the EF group, 40 months after combined modality and 17 months after IF, respectively. A total of 129 patients achieved complete remission (CR and CRu): 98% after EF radiotherapy, 100% after IF radiation and 95% after combined modality. Toxicity of treatment was generally mild with most events observed after combined modality. With a median follow-up of 43 months there were 5% relapses and only 3 patient deaths. The freedom from treatment failure (FFTF) rate was 95% and OS rate 99% for all patients (Figure 1 ). FFTF at 24 months was 100% for EF, 92% for IF and 97% for combined modality therapy (Figure 2 ). In terms of remission induction, IF radiotherapy for stage IA NLPHL is as effective as extended field or combined modality treatment. However, longer follow-up is needed before final conclusions can be drawn regarding the optimal therapy in this series.34 After their H7 trial (1998–93), the EORTC has also adopted IF radiotherapy as standard treatment for stage IA NLPHL.39
LPHL in early unfavorable and advanced stages
Due to the excellent treatment results in early favorable NLPHL stages, several more recent analyses have focused on outcome differences between NLPHL and cHL to define the best current treatment for advanced NLPHL patients.36,40,41
The ETFL project showed 96% complete remission for NLPHL, somewhat higher than for the cHL cases (nodular sclerosis, 89%; mixed cellularity, 93%). The HL specific survival and failure-free survival of NLPHL cases at 8 years were 95% and 74%, respectively, with an OS of 89%. An indolent course with frequent recurrences has long been regarded typical of NLPHL. The ETFL showed a tendency, albeit not statistically significant, to more frequent late relapses and better long-term survival in NLPHL compared with cHL.
A French group analysis of 500 HL patients including 42 NLPHL, 144 cHL without mediastinal involvement (MI) and 314 cHL patients with MI showed identical clinical characteristics for NLPHL patients and for cHL patients without MI. This pattern was significantly different from that of cHL patients with MI. Fifteen-year HL mortality rates were similarly low in NLPHL and cHL patients without MI. The study suggests that NLPHL and cHL patients without MI have a similar prognosis after a brief anthracycline-based chemotherapy regimen followed by extended field radiation.41
The more recent comprehensive GHSG analysis evaluated clinical trail results of 8298 HL patients, of whom 394 were NLPHL and 7904 cHL. CR/CRu was reached in 91% of NLPHL patients vs. 86% cHL patients in early stages, 86% vs 83% in early unfavorable, and 79% vs 75% in advanced stages. Of NLPHL patients, 0.3% developed progressive disease (PD) compared to 3.7% cHL patients. The relapse rate of NLPHL patients was very similar to cHL patients (8.1% vs 7.9%), but there was significant difference in terms of early relapses (0.76% vs 3.2%, P = 0.02) in favor of NLPHL. There were 2.5% secondary malignancies in NLPHL and 3.7% in cHL; 4.3% NLPHL and 8.8% cHL patients died. The FFTF rates for NLPHL and cHL patients at a median observation of 41 or 48 months were 88% and 82%, respectively (P = 0.0093). The OS for NLPHL and cHL patients was 96% and 92%, respectively (P = 0.016)26(Table 4).
In view of its low incidence and few associated secondary events, it is very difficult to conduct randomized trials for NLPHL patients to compare stage-adapted treatment schedules in NLPHL. The published data show that the treatment outcome of NLPHL in early unfavorable and advanced stages is not substantially different from cHL. The current treatment recommendation is to treat early unfavorable and advanced stages of NLPHL according to the treatment protocols for cHL.
Role of Monoclonal Antibodies in NLPHL
Therapy with monoclonal antibodies would be an option possibly associated with lower treatment-related toxicity and little if any late adverse effects. Since L&H cells stain strongly for CD20, clinical trials using the chimeric anti-CD20 antibody rituximab have been conducted. The GHSG evaluated rituximab in a phase II trial of relapsed or refractory NLPHL patients.32 Fourteen adults with CD20+ HL at a median of 9 years after initial diagnosis received rituximab at standard doses once weekly for 4 weeks. The overall response in 14 assessable patients was 86%, with 8 complete remissions and 4 partial remissions, and 2 patients with progressive disease. At a median follow-up of 12 months, 9 of 12 responders were in remission.
The Stanford group also reported their results in a study including 22 adult patients with either untreated or previously treated NLPHL who also received rituximab at standard doses.33 The overall response rate in this study was 100%. However, with a short median follow-up of 13 months, 9 patients had relapsed. Rituximab appeared to be less effective in patients with larger lymph nodes, stage III or IV disease and more than 2 involved nodal regions. Both the GHSG study and the Stanford trial showed little toxicity and good feasibility and suggest that rituximab might become a new treatment option for CD20+ HL patients either alone or in combination with cytotoxic drugs or radiotherapy.
Prognosis after Relapse
In the ETFL study, NLPHL patients showed a tendency to more favorable survival after relapse compared with cHL patients (P = 0.05). This, however, should be interpreted with caution because the NLPHL patients more often have early stage at first diagnosis and therefore on average received less intensive first-line therapy. Multiple relapses were observed in 12 of 45 relapsing patients (27%) in the NLPHL group. Information on the sequence of relapse diagnoses was incomplete but suggested that transformation to cHL is rare.3 The recent GHSG analysis showed differences in terms of early relapses between NLPHL and cHL patients treated in three GHSG study generations (0.76 vs 3.2%, P = 0.02), but the overall relapse rate of NLPHL was very similar to cHL patients (8.1% vs 7.9%).26 Furthermore, the FFTF rates and OS rates were significantly better for NLPHL than for cHL patients (88% vs 82%, P = 0.0093; 96% vs 91%, P = 0.0166).
Secondary Non-Hodgkin Lymphoma After NLPHL
The enhanced number of secondary NHL after NLPHL might influence the follow-up after primary treatment and, more importantly, the choice of primary treatment. In the ETFL project, complete data on occurrence of second malignancies after NLPHL were not collected, but all deaths from second malignancies were recorded. There were two fatal NHL following NLPHL (n = 219). Median follow-up was 6.8 years for all NLPHL patients. Four additional non-fatal secondary NHL were documented, 2 directly following primary treatment and 2 after relapse, giving a total of at least 6 NHL after 219 cases of primary NLPHL (2.9%).
These figure has been compared with cHL from the International Database (IDHD).42 Of 12,411 HL patients, 106 had documented secondary NHL (0.9%), and the cumulative incidence rate for NHL was 1.0% after 10 years. On the basis of this evidence, a two- to threefold higher rate of secondary NHL following NLPHL compared with cHL is observed.
Retrospective evaluation of data from patients with NLPHL who developed NHL indicates an aggressive clinical course with poor survival. Huang et al retrospectively analyzed 21 patients with diffuse large B-cell lymphoma arising either concurrently or subsequently to a diagnosis of NLPHL. The median OS and failure-free survival for the entire group was only 35 and 11 months, respectively, with 5-year OS and failure-free survival of 31 and 18%. Although the median survival was poor for the whole group, patients who achieved a CR with aggressive combination chemotherapy had a more favorable outcome regardless of risk factors. Regimens for cHL appeared to be ineffective.43
With regard to other secondary malignancies, the recent comprehensive GHSG analysis showed no differences in terms of overall incidence of secondary malignancies between NLPHL and cHL in early favorable (2.8% vs 3.3%), early unfavorable (1.6% vs 4%) and advanced stages (2.4% vs 3.7%).
In summary, there is evidence that NHL are seen more frequently following NLPHL than other cHL subtypes.
Conclusions
The resemblance of NLPHL to nonmalignant disorders (favorable clinical presentation, and good survival rates even after relapse) suggests that the optimal initial treatment strategy particularly for early-stage patients should be less intensive than that for cHL. Late toxicities, which contribute considerably to the overall mortality, could thus be reduced. The long survival of early favorable stage NLPHL patients without any treatment beyond lymph node excision could favor a watch-and-wait strategy in this very selected group of patients, although only after rigorous staging. This still unproved approach should be reserved for clinical trials. New experimental therapies such as monoclonal antibody rituximab immunotherapy might also become an alternative therapeutic option, either alone or in combination with chemo- or radiotherapy.
The GHSG and EORTC recommend IF-radiotherapy for NLPHL in early favorable stages. However, one should note that there are still no randomized trials to confirm this treatment approach. For early unfavorable stages and advanced stages, no differences in treatment outcome were found between NLPHL and cHL. These stages should be treated according to standard HL therapy.
Morphologic characteristics of NLPHL and cHL.
| . | NLPHL . | cHL . |
|---|---|---|
| Modifed from DeVita et al. Cancer. Principles & Practice of Oncology. Lippincott Williams & Wilkins; 2000. | ||
| Abbreviations: NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; L&H, lymphocytes and histiocytes; cHL, classical Hodgkin lymphoma; RS, Reed-Sternberg | ||
| Pattern | Nodular (at least in part) | Diffuse, interfollicular nodular |
| Tumor cells | L&H, or “popcorn cells” | RS cells, mononuclear or lacunar cells |
| Background | Lymphocytes, histiocytes | Lymphocytes, histiocytes, eosinophils, plasma cells |
| Background lymphocytes | B cells > T cells | T cells > B cells |
| . | NLPHL . | cHL . |
|---|---|---|
| Modifed from DeVita et al. Cancer. Principles & Practice of Oncology. Lippincott Williams & Wilkins; 2000. | ||
| Abbreviations: NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; L&H, lymphocytes and histiocytes; cHL, classical Hodgkin lymphoma; RS, Reed-Sternberg | ||
| Pattern | Nodular (at least in part) | Diffuse, interfollicular nodular |
| Tumor cells | L&H, or “popcorn cells” | RS cells, mononuclear or lacunar cells |
| Background | Lymphocytes, histiocytes | Lymphocytes, histiocytes, eosinophils, plasma cells |
| Background lymphocytes | B cells > T cells | T cells > B cells |
Immunophenotypic characteristics of NLPHL and cHL.
| . | NLPHL . | cHL . |
|---|---|---|
| Modifed from DeVita et al. Cancer. Principles & Practice of Oncology. Lippincott Williams & Wilkins; 2000. | ||
| Abbreviations: NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; cHL, classical Hodgkin lymphoma | ||
| CD15 | − | + |
| CD30 | − | + |
| CD20 | + | +/− |
| CD45 | + | − |
| EMA | + | − |
| CD57+ T cells | + | − |
| . | NLPHL . | cHL . |
|---|---|---|
| Modifed from DeVita et al. Cancer. Principles & Practice of Oncology. Lippincott Williams & Wilkins; 2000. | ||
| Abbreviations: NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; cHL, classical Hodgkin lymphoma | ||
| CD15 | − | + |
| CD30 | − | + |
| CD20 | + | +/− |
| CD45 | + | − |
| EMA | + | − |
| CD57+ T cells | + | − |
Patient characteristics of NLPHL and cHL.
| Patients . | NLPHL (n= 394) . | cHL (n= 7904) . |
|---|---|---|
| Data from the German Hodgkin Study Group. | ||
| Abbreviations: NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; cHL, classical Hodgkin lymphoma | ||
| Age, median (years) | 37 | 33 |
| Female (%) | 25 | 44 |
| Male (%) | 75 | 56 |
| B symptoms (%) | 9 | 40 |
| Early stage (%) | 63 | 22 |
| Intermediate stage (%) | 16 | 39 |
| Advanced stage (%) | 21 | 39 |
| Patients . | NLPHL (n= 394) . | cHL (n= 7904) . |
|---|---|---|
| Data from the German Hodgkin Study Group. | ||
| Abbreviations: NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; cHL, classical Hodgkin lymphoma | ||
| Age, median (years) | 37 | 33 |
| Female (%) | 25 | 44 |
| Male (%) | 75 | 56 |
| B symptoms (%) | 9 | 40 |
| Early stage (%) | 63 | 22 |
| Intermediate stage (%) | 16 | 39 |
| Advanced stage (%) | 21 | 39 |
Treatment outcome of NLPHL and cHL patients in the German Hodgkin Study Group.
| . | NLPHL (n= 394) . | cHL (n= 7904) . |
|---|---|---|
| Abbreviations: NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; cHL, classical Hodgkin lymphoma; FFTF, freedom from treatment failure; OS, overall survival | ||
| Progress (%) | .03 | 3.7 |
| Relapse (%) | 8.1 | 7.9 |
| Secondary Malignancies (%) | 2.5 | 3.7 |
| Death (%) | 4.3 | 8.8 |
| FFTF (%) | 88 | 82 |
| OS (%) | 96 | 92 |
| Median follow-up (months) | 41 | 48 |
| . | NLPHL (n= 394) . | cHL (n= 7904) . |
|---|---|---|
| Abbreviations: NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; cHL, classical Hodgkin lymphoma; FFTF, freedom from treatment failure; OS, overall survival | ||
| Progress (%) | .03 | 3.7 |
| Relapse (%) | 8.1 | 7.9 |
| Secondary Malignancies (%) | 2.5 | 3.7 |
| Death (%) | 4.3 | 8.8 |
| FFTF (%) | 88 | 82 |
| OS (%) | 96 | 92 |
| Median follow-up (months) | 41 | 48 |
FFTF and OS for stage IA NLPHL patients in the German Hodgkin Study Group trials.
Abbreviations: FFTF, freedom from treatment failure; OS, overall survival; NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma
FFTF and OS for stage IA NLPHL patients in the German Hodgkin Study Group trials.
Abbreviations: FFTF, freedom from treatment failure; OS, overall survival; NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma
FFTF according to different treatment of IA NLPHL patients in the German Hodgkin Study Group trials at 24 months.
Abbreviations: FFTF, freedom from treatment failure; NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; EF-RT, extended field radiotherapy; IF-RT, involved field radiotherapy; CM, combined modality treatment.
FFTF according to different treatment of IA NLPHL patients in the German Hodgkin Study Group trials at 24 months.
Abbreviations: FFTF, freedom from treatment failure; NLPHL, nodular lymphocyte-predominant Hodgkin lymphoma; EF-RT, extended field radiotherapy; IF-RT, involved field radiotherapy; CM, combined modality treatment.