Abstract
Use of positron emission tomography (PET) or PET/ computed tomography (CT) in Hodgkin lymphoma (HL) continues to expand worldwide. PET is currently widely utilized for response assessment after completion of therapy and, to a lesser extent, for pretreatment staging and assessment of response during therapy (therapy monitoring). In pretreatment staging, PET cannot replace CT or bone marrow biopsy (BMB); however, it can provide complementary information to both CT and BMB, potentially resulting in a modification of disease stage (usually upstaging) in about 15–20% of patients with impact on management in about 5–15%. PET for response assessment at the conclusion of treatment is substantially more accurate than CT because of its ability to distinguish between viable tumor and necrosis or fibrosis in posttherapy residual mass (es) that are present in about two-thirds of patients with HL without any other clinical or biochemical evidence of disease. PET, therefore, provides more accurate response classifications compared with CT-based assessment. The role of PET for therapy monitoring is still evolving but may prove to be the most exciting with potentially high impact on patient management and outcome. PET evaluation during therapy appears to be at least as accurate for predicting patient outcome as evaluation after completion of therapy and its use is clearly justified if the purpose is to provide an early and yet accurate assessment of response with the clear intent of tailoring therapy according to the information provided by the scan. The role of PET scanning for post-therapy surveillance without clinical, biochemical or radiographic evidence of disease remains controversial, primarily because of the potential for a disproportionate fraction of false-positive findings, potentially resulting in increasing cost without proven benefit from earlier PET detection of disease compared to standard surveillance methods. Large prospective studies are therefore needed to determine whether routine surveillance by PET is both cost-effective and whether it results in meaningful changes in patient management and/or outcome.
Use of 18F-fluorodeoxyglucose PET scanning (FDG-PET) in lymphoma has clearly expanded over the last few years. In North America, Europe and Australia, and increasingly also in developing countries, PET is currently the most widely used and accepted functional imaging modality for assessment of patients with Hodgkin (HL) and non-Hodgkin lymphoma (NHL), largely replacing the now “outdated” 67Ga-scintigraphy.1,2 Not only has PET been shown to be superior to 67Ga-scintigraphy in lymphoma staging and restaging, but it is an easier test to perform (requiring only about 2 hours from the time of tracer injection) and is associated with a substantially lower radiation dose to the imaged patient.3 In this short report, I will review the most important applications and potential applications of PET in patients with HL, the strengths and pitfalls of this technique, as well as ongoing efforts to standardize its use, particularly in response assessment.
Applications of PET in HL
Major applications of PET in HL may reasonably be divided into pretreatment staging, restaging, therapy monitoring and posttherapy surveillance and will be reviewed separately below. However, it is useful to outline the difference between the applications of therapy monitoring and restaging, at least based on definitions of the US Center for Medicare and Medicaid Services (CMS), which determines which clinical PET applications are reimbursable for Medicare and Medicaid patients. According to CMS, PET for therapy monitoring is performed during the planned course of therapy, for example, after 2–3 cycles of a 6–8 cycle chemotherapy regimen, to provide an early assessment of response with the goal of tailoring therapy based on the PET results. In contrast, restaging is performed after completion of treatment either for a final response assessment, typically within 1–3 months of completing treatment, or to determine the extent of suspected or known recurrence, usually several months or years thereafter. In lymphoma, restaging PET scans are reimbursed essentially without any restriction whereas PET for therapy monitoring is not yet approved as a standard clinical indication. Nevertheless, it is important to emphasize that CMS will provide coverage for therapy-monitoring PET scans that are performed under the conditions of specifically defined clinical trials, for example, those conducted by National Cancer Institute Cooperative Groups, or a prospective registry, such as the National Oncologic PET Registry (NOPR) administered by the American College of Radiology Investigative Network (ACRIN). The latter provides ample opportunity to perform PET scanning for monitoring in the clinical practice setting whenever the scan results may be used to modify treatment of a HL patient.
PET for Pretreatment Staging
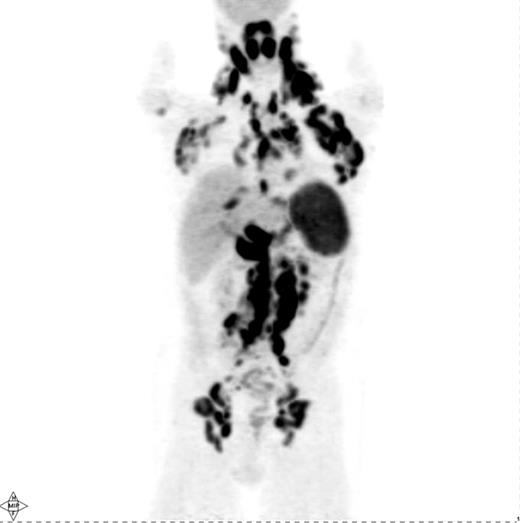
Several investigations have shown that PET is quite sensitive in detecting nodal and extranodal manifestations of HL prior to treatment (Figure 1 ).4–14 Most of these studies have reported on mixtures of HL and NHL patients, although a few have specifically addressed the utility of PET for staging of HL.6–13 In aggregate, these studies demonstrate that PET is able to detect an additional number of presumed HL sites compared with conventional staging methods, in particular CT and bone marrow biopsy (BMB). This results in a modification of disease stage (usually upstaging) in about 15–20% of patients with an impact on management in about 5–15%.4–12 However, one drawback of these studies is that they did not provide histopathological verification of all or, at least most, of the additional PET findings not shown by conventional staging methods. Reports of up- or downstaging patients by PET were mostly based on theoretical considerations that assumed that the additional PET findings truly represented lymphoma.8,9,10–14 Some studies used the disappearance of abnormal PET up-take following treatment as “proof” of the lymphomatous nature of these additional PET findings, a somewhat problematic approach considering that PET-positive benign entities such as fractures or inflammation may resolve with time irrespective of therapy.8,9,10–14 Nevertheless, several conclusions can still be drawn, particularly based on the studies in which reported modifications of disease stage or management were based on PET findings verified by biopsy, additional imaging (e.g., MRI) or by posttreatment morphologic regression (by CT) of PET-positive lymph nodes presumed to be normal on pretherapy CT.4–7,10 First, PET is able to detect focal or multifocal bone/bone marrow involvement in HL patients with negative iliac crest BMB, which could frequently be verified by guided biopsy of the PET-positive sites or by MRI.5–7,12,15–17 On the other hand, diffusely increased bone marrow uptake on PET may be due to reactive myeloid hyperplasia characteristic of some HL patients rather than lymphoma.16 In general, even focal/multifocal PET findings in the bone/marrow in the face of negative BMB should be confirmed whenever a change in treatment is contemplated based on PET. Second, PET alone is unreliable in detecting bone marrow involvement, particularly of limited degree (i.e., ≤10–20% of marrow space); an estimate of PET sensitivity for detecting marrow infiltration in HL based on a recently reported meta-analysis was 76% (95% CI 47–92).17,18 PET cannot, therefore, replace BMB in the staging of HL, at least based on currently available data. Third, PET and CT lead to concordant clinical stage in the vast majority of HL patients (60–80%).7–13 However, several reports suggest that discordant findings occur in both directions: PET demonstrates abnormalities undetectable by CT resulting, on average, in altering stage in 15–20% of patients and CT shows abnormalities undetectable by PET with an impact of a similar magnitude (i.e., 10–20%) on stage assignment.7–13 Abnormal findings seen only by PET with the potential for modifying disease stage or management include lymphomatous involvement of normal-sized lymph nodes by CT criteria (i.e., ≤ 1 cm in short axis), splenic and hepatic infiltration.4–14 Although most studies show that PET-negative/CT-positive findings are less common than the reverse, it is clear from a number of investigations that PET alone cannot replace CT for pretreatment staging of HL.7,8,10,12 Rather, the modalities are complementary. Fourth, based on the complementary information from PET and CT, it appears likely that a PET/CT scan, which combines a PET and a CT scan in a single study, performed in the staging setting using i.v. contrast, will provide at least equal information to that provided by PET and a separately obtained i.v. contrast-enhanced CT (CECT) acquired using a dedicated CT scanner. The use of i.v. contrast when performing CT, regardless of whether it is performed as a separate study or as part of PET/CT, enables more accurate CT assessment of the liver and spleen. The adequacy of PET/CT performed under these conditions is supported by mostly retrospective or small trials showing that PET/CT performed even without i.v. contrast provides similar information to that provided by PET and a separately performed CECT.14,19 Thus, a pretreatment PET/CT with i.v. contrast may represent a reasonable choice of a modality providing an integrated functional/ anatomical assessment of HL with the added advantage of facilitating the interpretation of posttherapy PET or PET/ CT scans that are often obtained in patients with HL. However, pretreatment PET (PET/CT) is clearly not mandatory for staging and, while useful for a more reliable interpretation of posttherapy PET, is only fully justified outside of clinical trial setting if its results will alter patient management. Given the increasing reliance on systemic treatment with chemotherapy either alone or combined with radiation therapy (RT) depending on disease stage and certain risk factors (e.g., B-symptoms or bulky disease) PET is increasingly unlikely to result in a change in treatment approach if it demonstrates additional sites of disease or even alters the disease stage.8,10 In this context, it is noteworthy that Meyer et al demonstrated that in patients with nonbulky limited stage HL (clinical stage I and II A) there was no difference in overall survival between patients randomly assigned to receive treatment that includes RT or ABVD alone, making the latter a reasonable and often-utilized treatment option in such patients similar to the treatment utilized in patients with nonbulky advanced stage HL.20 Hence, PET upstaging of limited stage patients treated with ABVD alone would, in most instances, not have any impact on management. Interestingly, however, > 2 or 3 involved nodal regions are considered risk factors in limited stage HL according to the German Hodgkin Lymphoma Study Group (GHSG) and European Organization for Research and Treatment of Cancer (EORTC), respectively, and any extranodal disease is also considered a risk factor by the GHSG. According to treatment schemes recommended by these organizations, these risk factors warrant a more intensive treatment (i.e., a greater number of chemotherapy cycles combined with RT) in patients with nonbulky limited stage HL even without altering the disease stage.12,21 Based on such treatment schemes, PET detection of a greater number of nodal sites or extranodal disease would indeed result in a management change even without changing the disease stage.12
In summary, considering the relatively small impact of pretreatment PET on management of HL found in most carefully conducted studies, it should only be employed clinically if its results will clearly alter management.
PET for Restaging
As stated earlier, restaging PET may be performed to determine the extent of known or suspected recurrence or to assess response after completion of therapy. Published data and clinical experience suggest that the accuracy of PET for assessment of patients with known relapse is similar to pretreatment staging.8,12,22 The accuracy of PET in patients with suspected relapse based on vague or nonspecific symptoms is probably lower because of the potential for false-positive findings caused by benign processes, such as infection or inflammation, which may also explain the patient’s symptoms.
Response assessment after completion of therapy is currently the most widely utilized application of restaging PET in HL. In this setting, PET has consistently been shown to have a very high negative predictive value or NPV (a measure of the ability of a negative PET scan to exclude persistent disease or future relapse) averaging about 90% and exceeding 80% in virtually all reported studies.23–33 The 10–20% false-negative rate with PET is mostly related to its inability to detect microscopic disease resulting in future relapse, a feature common to PET and all other conventional imaging methods, such as CT or MRI. The positive predictive value (PPV) of PET (a measure of the ability of a positive PET scan to predict persistent disease or future relapse) is substantially lower and considerably more variable averaging approximately 65% with most studies reporting values exceeding 50%.23–33 Still, the PPV of PET is substantially higher than that of CT with a reported PPV in HL of about 20%; the NPV of CT is similar to that of PET. 23–33 The overall result is a considerably higher accuracy of PET for response assessment compared with CT (85% vs. 40%, respectively). This higher accuracy is largely related to the clear superiority of PET in distinguishing between viable tumor and necrosis or fibrosis in residual mass(es) (RM) demonstrated by CT, which is generally unable to differentiate between these entities.1,2,34 This is particularly relevant in patients with HL where about two-thirds of patients have RMs by CT without any other clinical or biochemical evidence of disease; only a small fraction of these patients (i.e., 10–15%) have residual HL explaining the very poor PPV of CT.23–33,34 Overall, more than two-thirds of RMs by CT are interpreted as negative by PET with relapse occurring in < 10% of these patients and these patients can, therefore, be safely observed. The other one-third of patients with a positive PET are clearly at a higher risk of progression or relapse, which occur in about 60–70% of patients (Figure 2; see Color Figures, page 510). However, 30–40% of patients with PET-positive RMs do not progress or relapse, emphasizing the importance of histopathologic confirmation of the positive PET findings prior to administering salvage treatment.
Interestingly, the PPV of PET in patients with HL is substantially lower than in patients with aggressive NHL with a reported PPV averaging about 85%.33 This is presumably related to the substantial fraction of HL patients who received radiation therapy (RT), either alone or combined with chemotherapy, prior to undergoing PET resulting in frequent occurrence of postradiation inflammatory changes often leading to false-positive PET scan. Another reason for the higher rate of false-positive findings in HL is the more frequent occurrence of thymic hyperplasia in the generally younger HL patients (Figure 3; see Color Figures, page 511). Table 1 lists potential causes of false-positive findings on postreatment PET in HL patients.
To minimize the frequency of false-positive PET, particularly at the site of RMs, an International Harmonization Project (IHP), which was convened to discuss harmonization of clinical trial parameters in lymphoma, recommends that PET not be performed prior to at least 3 weeks following chemotherapy and preferably 8–12 weeks after completion of RT.35 Another important issue recognized by the IHP was the need for a standardized definition for PET-positivity of a RM, the current lack of which may have contributed to the wide variability in the reported PPV of PET in response assessment of lymphoma. The IHP formulated a standardized definition for a PET-positive RM: RMs ≥ 2 cm in greatest transverse diameter (GTD) with FDG activity visually exceeding that of mediastinal blood pool are considered PET-positive, whereas RMs 1.1–1.9 cm are positive if their activity exceeds surrounding background activity. The IHP consensus was also that visual assessment alone is adequate for determining whether PET is positive or negative for response assessment at the conclusion of therapy and that quantitative or semiquantitative approaches, for example using the standardized uptake value (SUV), are not necessary in this setting.35 Using this definition, Juweid et al recently evaluated 50 consecutive patients with HL (n = 26) or aggressive NHL (n = 24) who underwent PET/CT within 3–12 weeks posttherapy and had at least 1 year of follow-up post treatment. A total of 55 RMs were found in 29 patients (58%), 31 ≥ 2 cm in GTD and 24 1.1–1.9 cm in GTD. The investigators found that the proposed IHP interpretation results in high predictive value in posttreatment evaluation of RMs in both patients with HL and aggressive NHL.36 The 1-year event-free survival (EFS) in patients with PET-positive RMs by the IHP definitions was 0% compared to 96% in patients with PET-negative RMs and 90% in patients without RMs (Figure 4; see Color Figures, page 511).36
The higher accuracy of PET compared with CT in assessment of response in HL and aggressive NHL recently resulted in a revision of the primarily CT-based International Workshop Criteria (IWC) that are widely used for response assessment of NHL and increasingly also HL. For routinely FDG-avid lymphomas, such as HL and DLBCL, PET will now be incorporated into the definitions of the various response designations, such as complete response (CR), partial response (PR), stable disease (SD), unconfirmed complete (CRu) and progressive disease (PD).36 Most notably, the CR designation will now be primarily based on a negative PET in these patients: a posttherapy residual mass of any size is permitted as long as it is PET-negative. A PR still requires ≥ 50% decrease in sum of the product of the perpendicular diameters (SPD) of up to 6 of the largest dominant nodes or nodal masses with no new lesions, but to be considered PR at least 1 of the residual lesions must now be PET-positive; otherwise, the patient is considered in CR. An interesting feature of the new criteria is that the CRu designation will now be eliminated; those responses will now be designated as CR if the RM is PET-negative or PR if it is PET-positive. Using criteria very similar to those outlined here, Juweid et al showed in a retrospective study of 54 patients with aggressive NHL (mostly DLBCL) who had been treated with an anthracycline-based regimen that PET not only doubled the number of CRs and eliminated the CRu’s but significantly enhanced the difference in progression-free survival between CR and PR.38 These findings provided the rationale for incorporating PET into revised criteria for routinely FDG-avid lymphomas, such as DLBCL and HL.37
In conclusion, based on a large body of evidence, PET can now be considered standard of care for posttreatment assessment of patients with HL.
Postchemotherapy PET and Use of Radiation in Patients with Bulky HL
One intriguing issue is whether posttherapy PET can be used to determine whether patients with bulky disease at diagnosis should receive radiation or not. For this use, PET must be done relatively soon after the completion of chemotherapy but no sooner than after 3 weeks to minimize the chance of posttherapy inflammatory changes resulting in a false-positive PET.35 Several reports show that at least two-thirds of HL patients with a residual mass following treatment will have a negative PET scan at that time. Unfortunately, no data have been reported on the likelihood of relapse in PET-negative residual masses in patients with bulky vs. nonbulky HL at diagnosis; hence, it cannot be ascertained that the relapse rate in these two groups of patients will be similar. However, data in patients with aggressive NHL, of whom about one-third develop residual masses following treatment, particularly if they have bulky disease at diagnosis, indicate that the likelihood of residual disease at the site of residual mass in patients who are otherwise in clinical CR (i.e., without clinical or biochemical evidence of disease) is only about 10–20% and is similar in patients with bulky and nonbulky disease at diagnosis.39,40 Overall, these patients also appear to have a similar relapse rate to that in patients with CR by CT.41 Since the true nature of residual mass in the vast majority of such patients has been identified as necrosis/fibrosis, the nature of such masses also holds true in patients with HL, it is reasonable to assume that the majority of HL patients with bulky disease at baseline who have a residual mass post therapy but are otherwise in clinical CR will do well without RT, analogous to the experience reported in aggressive NHL lymphoma patients with bulky masses at diagnosis who did not receive RT.34,39 PET scanning is likely to identify a substantial fraction of those patients who have histopathologic evidence of residual disease despite clinical CRs; these patients can then undergo RT. Those patients who are PET-negative are then likely to have a substantially reduced risk of relapse compared to the overall group, probably justifying close observation as an alternative to RT in these patients. However, this hypothesis should be experimentally tested before changing current management approach in such patients, preferably by a randomized trial in which patients with bulky HL at diagnosis who are PET-negative 3–4 weeks following completion of chemotherapy are allocated to receiving RT vs. observation. If prospective well-controlled clinical trials prove PET to be a reliable tool in this setting, many patients with bulky disease at diagnosis could be spared radiation with an expectedly substantial impact on patient event-free and overall survival which is in large part determined by the adverse effects of RT.20
PET for Therapy Monitoring
The role of PET for therapy monitoring, as defined above, is still evolving. It may ultimately prove to be the most exciting application of the technique, with potentially high impact on patient management and outcome. It seems logical that PET scanning in this setting is justified in patients with HL (and aggressive NHL) if the purpose is to provide an early and accurate assessment of response with the intent of tailoring therapy according to the information provided by the scan. Several studies have demonstrated a correlation between a rapid decline or normalization of FDG uptake or SUV as early as after 1 to 4 cycles of chemo-or chemoimmunotherapy and patient outcome.42–47 For example, in a retrospective evaluation of 85 patients with HL who underwent PET scanning after 2 or 3 cycles of first-line chemotherapy, Hutchings et al showed that 94% (68/ 72) of patients with a “completely” negative PET (no uptake above background) or only minimal residual uptake (similar or only slightly higher than normal liver uptake) were progression free after a median follow-up of 3.3 years compared to 39% (5/13) of patients with a clearly positive PET scan.45 Interestingly, a more stringent definition of PET-negativity during therapy did not enhance the difference in PFS between positive and negative studies; the opposite was true: 95% (60/63) of patients with a completely negative PET remained progression free compared to 59% (14/ 22) of patients with any residual uptake above background.45 Using the more liberal criteria for defining PET negativity during therapy, “midtherapy” PET also appears to be at least as accurate for predicting patient outcome as end of therapy PET (Table 2 ).45 Similar findings were subsequently reported in a prospective study by the same author.46 This suggests that a liberal interpretation of PET negativity of the midtherapy PET scan is appropriate and, in fact, more accurate in patients with HL. This may be related to the fact that these scans were only performed 1–2 weeks after the preceding cycle of chemotherapy, and may reflect the tendency for HLs to have a more pronounced inflammatory response early following treatment. This in turn may explain the higher incidence of fibrotic/necrotic RMs in patients with HL compared with NHL. Overall, it appears that a dichotomous interpretation of PET scans based on visual assessment alone (i.e., positive vs. negative) may not be sufficiently reliable in distinguishing between patients with a more favorable and those with less favorable outcome, largely because of an apparent variability in visual scan interpretation between various PET readers, with some more focused on the residual posttherapy uptake in the tumor region while others are more focused on the change in uptake from baseline when rendering their qualitative interpretation. It is therefore possible that semi-quantitative assessment, for example using the SUV, may prove to be necessary for a more uniform and potentially more accurate assessment of midtherapy PET studies. If the SUV method is used for this purpose, a standardized approach for SUV determination is critical, including strict adherence to predefined reconstruction algorithms and timing of PET imaging following FDG injection. Only such standardization will ensure comparability among various studies.
Interestingly, in current clinical practice, PET for therapy monitoring appears to be primarily performed because of the prognostic information provided and there are no published reports to date demonstrating that PET in this setting successfully altered treatment of patients by improving outcome or reducing the number of cycles of therapy without adversely affecting outcome. However, this issue is being activity investigated in a number of trials, including those by US and European Cooperative groups in patients with HL and aggressive NHL and it appears certain that the role of PET for therapy monitoring will dramatically increase if clinical trials demonstrate that the information provided by PET affects ultimate patient outcome. In this context, it is noteworthy that midtherapy CT performed after 2 cycles of chemotherapy was used by Meyer et al in the above-mentioned trial in order to allocate non-bulky limited-stage HL patients to receive a total of 4 or 6 cycles of chemotherapy (ABVD).20 Those achieving a CR or CRu after 2 ABVD cycles received a total of 4 cycles; those not achieving this endpoint after their second cycle received a total of 6 cycles. The investigators found that freedom from disease progression was superior in patients achieving a CR or CRu after 2 cycles of therapy (P = 0.007; 5-year survival estimates 95% vs. 81%) despite receiving a lower total number of chemotherapy cycles. However, only 69 of 196 patients (35%) allocated to receive ABVD achieved a CR/CRu by CT after 2 cycles. It is conceivable that had PET been used instead of CT at this timepoint, more than two-thirds of patients would be in a “metabolic CR” with an outcome presumably similar to patients with an anatomic CR/CRu (by CT) while the remaining fraction of patients with a positive PET (metabolic PR) will almost certainly have an inferior progression-free survival. In fact, data reported by Hutchings et al in patients with early-stage HL indicate that about 88% (50/57) of patients achieved a metabolic CR by PET after 2–3 cycles of chemotherapy. After a median follow-up of about 3 years, 98% (49/50) of these patients were progression free compared to 71% (5/7) of patients without a metabolic CR.45 These and similar findings reported in studies with shorter follow-up period provide the rationale for conducting clinical trials of risk-adapted treatment based on the midtherapy PET scan.46 However, outside of clinical trial setting, midtherapy PET cannot currently be recommended as standard of care.
PET for Posttherapy Surveillance
PET scanning for surveillance is performed following treatment in the absence of clinical, biochemical or radiographic evidence of recurrent disease with the aim of early detection of recurrence. This PET application remains controversial, primarily because of the potential for a disproportionate fraction of false-positive findings, potentially resulting in increasing cost without proven benefit from earlier PET detection of disease compared to standard surveillance methods.48 For example, Jerusalem et al performed PET imaging in 36 HL patients at the completion of therapy and every 4–6 months thereafter for 2–3 years. One patient with persistent tumor and four relapses were identified a few months prior to clinical, laboratory or CT evidence of disease. However, there were also 6 patients with false-positive PET studies requiring additional restaging procedures for further clarification, including subsequent PET scans performed several months later, all of which were then negative.48 This negative experience, albeit somewhat limited, clearly suggests that large prospective trials are needed to determine whether posttherapy surveillance by PET is cost-effective and whether it results in meaningful changes in patient management and/or outcome. In the absence of such trials, it cannot be recommended as standard of care.
Potential reasons for false-positive findings on post-therapy positron emission tomography (PET) Hodgkin lymphoma.
|
|
Mid (post 2–3 cycles) vs. end of TX PET positron emission tomography therapy (TX PET) in Hodgkin lymphoma using liberal criteria for defining PET negativity at mid-TX. Data from Hutchings M, Mikhaeel NG, Fields PA, Nunan T, Timothy AR. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol. 2005;16:1160–8. N = 42 patients
| . | NPV . | PPV . | Accuracy . |
|---|---|---|---|
| * P = NS | |||
| Abbreviations: NPV, negative predictive value; PPV, positive predictive value | |||
| Mid | 97% (34/35) | 71% (5/7) | 93% (39/42) |
| End | 94% (33/35)* | 57% (4/7)* | 88% (37/42)* |
| . | NPV . | PPV . | Accuracy . |
|---|---|---|---|
| * P = NS | |||
| Abbreviations: NPV, negative predictive value; PPV, positive predictive value | |||
| Mid | 97% (34/35) | 71% (5/7) | 93% (39/42) |
| End | 94% (33/35)* | 57% (4/7)* | 88% (37/42)* |
Pretreatment PET scan in a patient with Hodgkin lymphoma showing widespread cervical, axillary, mediastinal, hilar, abdominal and pelvic lymphadenopathy in addition to diffuse involvement of the spleen and focal liver involvement.
Pretreatment PET scan in a patient with Hodgkin lymphoma showing widespread cervical, axillary, mediastinal, hilar, abdominal and pelvic lymphadenopathy in addition to diffuse involvement of the spleen and focal liver involvement.
From the Department of Radiology and the Holden Comprehensive Cancer Center, University of Iowa, Iowa City, IA