Abstract
The development of effective therapy for children with acute lymphoblastic leukemia (ALL) is one of the great successes of clinical oncology, with long-term survival achieved in over 80% of patients. However, cure rates for adults with ALL remain relatively low, with only 40% of patients cured. With an age-unrestricted, biology-based approach, we anticipate a better understanding about why these outcome differences exist, and think that by extending successful pediatric clinical programs to include adult patients with ALL, we can directly compare uniformly treated adults and children in terms of response to therapy, toxicity and underlying biology.
“The child is the father of the man”…
William Wordsworth
Background
Within childhood acute lymphoblastic leukemia (ALL) populations, older children have had inferior outcomes1 and within adult ALL populations, younger adults have had superior outcomes.2,3 In recent reports, “overlapping” populations of older children and young adults have been compared for the likelihood of inducing a complete remission and long-term event-free survival (Table 1 ).4–8
None of the clinical trials listed in Table 1 was designed to be compared to its age-overlapping pediatric or adult counterpart; thus, the comparisons were all retrospective. Although well-recognized age-related dissimilarities in the biology of ALL and event-free survival exist (Tables 2 and 3 ), the more favorable outcomes for older adolescents treated on pediatric regimens in Table 1 cannot be explained by differences in patient characteristics. Nor would one expect other potential “adult vs. pediatric” differences (for example, co-morbidities associated with smoking, or host pharmacokinetic differences based on age) to play a substantial role in the findings summarized in Table 1 .
There are clear differences between current adult and pediatric therapeutic approaches. For example, bone marrow transplantation in first remission is more common for adults (in part, because of the higher incidence of Philadelphia chromosome–positive ALL), and pediatric regimens generally include greater dose density of many chemotherapeutic agents (such as asparaginase, vincristine, cortico-steroids and methotrexate).
What are some plausible explanations for these differences between pediatric and adult outcomes? In a thoughtful, provocative and prescient editorial, Dr. Charles Schiffer reflected on biological differences among the hosts and diseases, and also raised issues pertaining to clinical practice, such as the frequency of and familiarity with ALL protocols and care of such patients.9 In addition to recognizing that ALL is the most common malignancy in children and relatively rare in adult oncology populations, he also noted that most children, but not most adults, with ALL were treated in the context of clinical trials, by experienced support teams, and that many adults were not treated at academic medical centers. The cultural differences between care of pediatric and adult ALL patients, described as “disparities in treating attitudes” by Boissel and co-workers,5 was highlighted by Schiffer’s colorful and accurate description of pediatricians’ tight adherence to complex treatment protocols “…with a military precision on the basis of near-religious conviction about the necessity of maintaining prescribed dose and schedule come hell, high water, birthdays, Bastille Day, or Christmas.” Although the necessity of such vigor has not been formally tested, the proof of the pudding is in the tasting.
Obstacles to Age-Unrestricted, Biology-based Treatment
Why have adults with ALL not received the same chemotherapeutic regimens as children?
There are two principal hurdles: 1) systems of care, and 2) regulatory impediments.
“Systems of care” and related differences
Referral patterns
Commonly held views suggest that pediatricians refer 15- to 20-year-old patients to pediatric academic medical centers where (a) > 90% of patients are < 15 years old, and b) > 90% of patients with ALL are enrolled on clinical trials.
Similarly, internists frequently refer 15- to 20-year-old patients to adult hospitals, mostly not academic medical centers, where a) > 90% of the patients are > 40 years old and b) most patients with ALL are not entered on clinical trials.10
Frequency of and familiarity with ALL
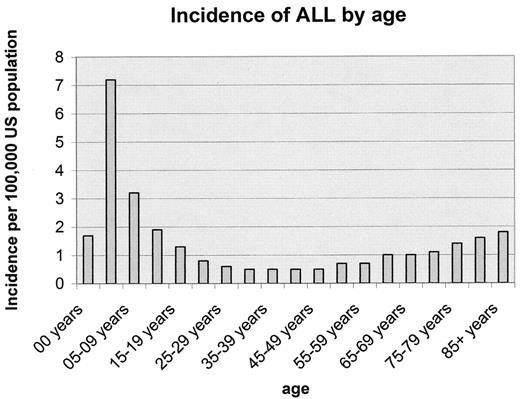
Although there might be as many adults with ALL as there are children with the disease11 (Figure 1 ), the relative frequency of the disease is markedly higher in young children. ALL represents about 15% of all malignancies in 1–15 year olds, 5% in 15–19 year olds, and < 10% of malignancy in > 20 year olds.
Adherence to protocol and mitigating psychosocial factors
Although most of the drugs used for ALL today have existed for over four decades, little is known about their ideal antileukemia dose or the role of individual patient differences in drug metabolism. In pediatric practice, “pushing the dose” by using myelosuppression as the endpoint, assures delivery of the maximum dose for each child with each course of chemotherapy. Although it is difficult to assess whether such rigid adherence to protocols is necessary, Boissel and co-workers reported on one “therapeutic practice” measure. They determined the median number of days from the time of initial complete remission until the time of the first postremission chemotherapy: 2 days in the pediatric study and 7 days in the adult study; P = 0.002.5
Is this time-to-next-treatment measure a medical or psychological problem? Have the older patients fully recovered physiologically, or are there other mitigating features (e.g., “I need some time with my family”) resulting in delays in therapy?
In the pediatric setting, in addition to the meticulous attention to detail by the pediatricians, a primary driver of subsequent therapy is the caretaker, usually the mother. (“What are we waiting for? The leukemia is growing back. Let’s get going!”)
Difficulties in comparisons
“Simple” apples-to-apples comparisons in a heterogenous disease such as ALL can be difficult, and occult differences in practices among internists and pediatricians might not be appreciated.
For example, Table 1 demonstrates comparisons in the percentage of complete remissions between older children and adults. Not shown is the time to enter complete remission. All pediatric trials report complete remission at the end of approximately 1 month of multiagent chemotherapy. However, many adult trials report on the complete remission rates after 2 months of treatment (Table 4 ). Although the time to attain a complete remission was not of prognostic importance in at least one adult trial,12 we found that in childhood ALL, the time to enter a complete remission had significant implications, and the difference between a complete remission in 1 month or 2 months was a matter of life or death for the majority of slow responders.15
Regulatory obstacles
No diseases begin or end at age 18 years. But current review systems of clinical trials (e.g., Institutional Review Boards) and funding sources (e.g., cooperative groups and pharmaceutical company-sponsored trials) frequently establish “biologically arbitrary” age limits for protocol eligibility and evaluation. Thus, diseases such as ALL, whose biology extends across a broad age spectrum, are usually approached as two diseases: childhood ALL (patients < 18 years old), and adult ALL (patients ≥ 18 years old). Such “age-restricted” criteria have played an integral role in our current understanding of the disease and its treatment. However, this approach, based on the single differentiating measure—patient age—has until recently hampered clinical research across a broader spectrum of the disease. Although likely a meaningful host variable, age, per se, is highly unlikely to trump leukemia biology or the impact of an individual treatment regimen. Overcoming such well-institutionalized barriers to research remains a formidable challenge.
Recently, the Dana-Farber Pediatric ALL Consortium, a group of nine collaborating pediatric centers with a successful record of ALL research and treatment,16 joined together with nine adult institutions to form a Dana-Farber Combined Adult/Pediatric ALL Consortium.
In anticipation of a common approach to the over 18-year-old patients, we evaluated our oldest pediatric ALL population—those aged 15–18 years old.17 Among 844 patients treated on two consecutive protocols (Dana-Farber 91-01 and 95-01) between 1991–2000, we found 51 (6%) were in the 15- to 18-year-old population. Statistically significant biological characteristics at the time of diagnosis included a higher proportion with T-cell ALL and a lower proportion with hyperdiploidy or the TEL/AML1 translocation compared to younger patients. Except for a lower incidence of complete remissions (1–14 year olds, 98% vs. 15–18 year olds, 94%; P = 0.01), there were no other statistically significant differences with regard to 5-year event-free survival (> 1–10 years old, 85%; > 10–15 years old, 77%; and >15–18 years old, 78%; P = 0.10). Patients 10 years old and older had more asparaginase-related pancreatitis and thrombosis, but there were no differences between the 10- to 14-year-old and 15- to 18-year-old populations. An updated analysis of the efficacy of our pediatric regimen for adult patients, 18–50 years old, appears as a 2006 American Society of Hematology abstract (DeAngelo et al).
We are now poised to investigate the biology of ALL and its treatment across a broad age spectrum, and to have common treatment regimens for common disease among patients ages 1–50 years old.
From our DFCI Combined Adult/Pediatric ALL Consortium, many myths have surfaced, and many lessons have been (and are being) learned.
Myths and Lessons
Myth #1.
It cannot be done!
You cannot have a common approach to therapy for children and adults. The disease biology, medical practices, regulations and cultures are incompatible with a uniform approach to clinical research.
Lesson #1.
It can be done!
(But it isn’t easy.) Finding common ground requires time, communication and compromise. We are confident that our work within the DFCI Combined Adult/Pediatric ALL Consortium, as well as similar efforts by others who are striving toward the same goals—improved outcome for all, diminished toxicity and a better understanding of leukemia biology—will be rewarding and result in lives saved and quality years of life added.
Myth #2.
Adults do not tolerate asparaginase.
It is generally assumed that adults are less tolerant to the acute and cumulative side effects of antileukemia drugs, and asparaginase, a drug used in all pediatric ALL regimens, is often cited as a prime example.
Lesson #2.
Our initial adult protocol, a feasibility trial using one arm of our pediatric ALL regimen, includes 30 weeks of high-dose asparaginase therapy. Adults ages 18–50 years old and children ages 10–18 years old tolerate multiple weekly asparaginase doses (≥12,500 IU/m2) with similar incidences of allergic reactions, pancreatitis and thrombosis.
Myth #3.
Adults have a high rate of therapy-related deaths. Some would argue against initiating combined adult/pediatric clinical trials because the remission mortality in children (now in the 1–2% range) is far less than in adults.
Lesson #3.
In fact, the experience of Dutch6 and British7 investigators suggests that it is the practice of bone marrow transplantation in first remission for non-Philadelphia chromosome–positive ALL that accounts for the increased incidence of therapy-related deaths. Given the paucity of data supporting the superiority of bone marrow transplantation in first remission, many now agree that use of chemotherapy alone would result in both improved outcomes and in a lower incidence of therapy-related deaths. Our DFCI adult pilot protocol and mature pediatric protocols have ~1% incidence of therapy-related deaths.
Myth #4.
Adults don’t have asymptomatic central nervous system (CNS) leukemia at diagnosis.
And because they don’t, the practice of routine sampling of CSF at the time of diagnosis in asymptomatic patients is variable among adult treatment regimens.
Lesson #4.
ALL is always a systemic disease. About 2–3% of children and adults have symptomatic CNS disease at diagnosis and an additional 5–10% of asymptomatic children and 5% of adults have lymphoblasts in their CSF disease at time of diagnosis. Routine lumbar puncture at the time of diagnosis and prior to initiating systemic chemotherapy, as well as routine CNS treatment, should be part of ALL management for all patients. An argument against this practice, that one might inadvertently introduce blasts into the CSF, can be readily mitigated by the routine use of intrathecal chemotherapy at the time of the initial diagnostic lumbar puncture.
Myth #5.
Who needs a parent or caregiver?
The patient and his or her physician are sufficient for optimal care and outcomes.
Lesson #5.
Never underestimate the value of a caregiver! The best motivated patient and physician can have their well-intended treatments and outcomes enhanced by surrounding themselves with “a mother figure”; in fact, parental surrogates, committed family members and loved ones, as well as an extended supportive team of professionals (especially nurses), are likely to assure optimal adherence to protocol, emotional support, and (hopefully) better outcomes.
Conclusion
Retrospective analyses clearly demonstrate that current pediatric therapeutic regimens are more effective than adult regimens for 16- to 21-year-old patients. Current and future clinical trials will focus on “the ages of uncertainty”: the 21- to 50-year-old population. Age-unrestricted, biology-based therapy should be the standard of all patients with ALL. Pediatricians and internists treating patients with ALL have much to learn from one another. Such efforts are likely to result in more cures and a higher quality of life among the survivors.
Older child/young adult acute lymphoblastic leukemia (ALL) outcomes.
| Country . | Nat’l Trial . | Age in Years . | # Pts . | CR (%) . | 5-Yr EFS (%) . | Ref. . |
|---|---|---|---|---|---|---|
| Abbreviations: CR, complete remission; EFS, event-free survival; P, pediatric protocol; A, adult protocol | ||||||
| * 6-yr EFS | ||||||
| ** 2-year overall survival | ||||||
| US: | CCG (P) | 16–21 | 196 | 96 | 64* | 4 |
| CALGB (A) | 103 | 93 | 38* | |||
| France: | FRALLE 93 (P) | 15–20 | 77 | 94 | 67 | 5 |
| LALA94 (A) | 100 | 83 | 41 | |||
| Holland: | DCOG (P) | 15–18 | 47 | 98 | 69 | 6 |
| HVON (A) | 44 | 91 | 34 | |||
| UK: | ALL97 (P) | 15–17 | 61 | 98 | 65 | 7 |
| UKALLXII (A) | 67 | 94 | 49 | |||
| Italy: | AIEOP (P) | 14–18 | 150 | 94 | 80** | 8 |
| Gimema (A) | 95 | 89 | 71** | |||
| Country . | Nat’l Trial . | Age in Years . | # Pts . | CR (%) . | 5-Yr EFS (%) . | Ref. . |
|---|---|---|---|---|---|---|
| Abbreviations: CR, complete remission; EFS, event-free survival; P, pediatric protocol; A, adult protocol | ||||||
| * 6-yr EFS | ||||||
| ** 2-year overall survival | ||||||
| US: | CCG (P) | 16–21 | 196 | 96 | 64* | 4 |
| CALGB (A) | 103 | 93 | 38* | |||
| France: | FRALLE 93 (P) | 15–20 | 77 | 94 | 67 | 5 |
| LALA94 (A) | 100 | 83 | 41 | |||
| Holland: | DCOG (P) | 15–18 | 47 | 98 | 69 | 6 |
| HVON (A) | 44 | 91 | 34 | |||
| UK: | ALL97 (P) | 15–17 | 61 | 98 | 65 | 7 |
| UKALLXII (A) | 67 | 94 | 49 | |||
| Italy: | AIEOP (P) | 14–18 | 150 | 94 | 80** | 8 |
| Gimema (A) | 95 | 89 | 71** | |||
Acute lymphoblastic leukemia (ALL): incidence & biological differences.
| . | Children . | Adults . |
|---|---|---|
| Peak incidence | 5 years | 50 years |
| % of Leukemias | 80–85% | 15% |
| Chromosomes | ||
| Ph+ | 3% | 30% |
| MLL | 1–2% | 7% |
| TEL/AML1 | 20% | 2% |
| Hyperdiploid | 25% | 5% |
| T-cell | 10–15% | 20–25% |
| Mature B | 1–2% | 3–5% |
| . | Children . | Adults . |
|---|---|---|
| Peak incidence | 5 years | 50 years |
| % of Leukemias | 80–85% | 15% |
| Chromosomes | ||
| Ph+ | 3% | 30% |
| MLL | 1–2% | 7% |
| TEL/AML1 | 20% | 2% |
| Hyperdiploid | 25% | 5% |
| T-cell | 10–15% | 20–25% |
| Mature B | 1–2% | 3–5% |
Five-year event-free survival.
| . | Children . | Adults . |
|---|---|---|
| Pre-B | > 80% | 30–40% |
| T-cell | 75–80% | 45–55% |
| Ph+ | 20–25% | < 10% |
| MLL | 40–50% | 20% |
| TEL/AML1 | 90% | N/A |
| . | Children . | Adults . |
|---|---|---|
| Pre-B | > 80% | 30–40% |
| T-cell | 75–80% | 45–55% |
| Ph+ | 20–25% | < 10% |
| MLL | 40–50% | 20% |
| TEL/AML1 | 90% | N/A |
Time to complete remission in adult acute lymphoblastic leukemia (ALL) trials.
| Study . | % CR after One Cycle of Chemo . | % CR after Two Cycles of Chemo . |
|---|---|---|
| UKALLXII/ECOG12 | 51% | 91% |
| CALGB 88113 | 62% | 86% |
| LALA-9413 | 72% | 84% |
| Hyper-CVAD2 | 81% | 92% |
| DFCI pilot14 | 82% | N/A |
Incidence of acute lymphoblastic leukemia (ALL) by age, SEER 1992–1999.
Division of Hematology/Oncology, Children’s Hospital and Department of Pediatrics, Harvard Medical School, Boston, Massachusetts
Supported in part by grant CA 68484 from the National Cancer Institute, National Institutes of Health, Bethesda, Maryland