Abstract
The analysis of specific gene defects in disorders of phagocyte function has shed light on important aspects of the innate immune response. Each disorder has distinctive features in the clinical presentation and characteristic microbial pathogens. Chronic granulomatous disease has been extensively studied both in patient series and in mouse models. New insights continue to be obtained regarding the role of the nicotinamide dinucleotide phosphate (NADPH) oxidase and related enzymes in host defense and other aspects of the inflammatory response, as well as optimal management of this disorder. Approaches based on hematopoietic stem cell transplantation and gene therapy offer promise for the future, but are still under investigation. Also briefly summarized are updates on newly described leukocyte adhesion defects and on inherited susceptibility to mycobacterial infection due to defects in interleukin (IL)-12 and interferon-γ pathways.
Phagocytic leukocytes are an essential component of the innate immune system that has evolved to respond rapidly to the presence of invading bacteria, fungi, and parasites. Hence, patients with defects in phagocyte function typically present in infancy or childhood with recurrent, unusual, and/or difficult to clear bacterial infections. Infections commonly seen include those of skin or mucosa, lung, lymph node, deep tissue abscesses, or childhood periodontitis. Bacterial sepsis is an unusual initial symptom and usually reflects dissemination from an infected site. Many of these disorders have distinctive clinical and microbiologic features related to the particular functional defect. This review will provide a selected update on chronic granulomatous disease and several other inherited disorders of neutrophils and mononuclear phagocytes.
Chronic Granulomatous Disease
Chronic granulomatous disease (CGD) is caused by defects in the phagocyte nicotinamide dinucleotide phosphate (NADPH) oxidase (also referred to as the respiratory burst oxidase).1 Superoxide generated during the phagocyte respiratory burst is the precursor to numerous microbicidal oxidants, including hydrogen peroxide and myeloperoxidase-catalyzed formation of hypochlorous acid. Respiratory burst-derived oxidants are an important component of the innate immune response, and their absence results in recurrent, often life-threatening bacterial and fungal infections and is also associated with formation of inflammatory granulomas, a distinctive feature of CGD.
NADPH oxidase and molecular genetics of CGD
The NADPH oxidase is a phagosomal and plasma membrane-associated enzyme complex that is dormant in resting neutrophils and rapidly assembled when cells are activated by a variety of inflammatory stimuli.1,2 Four polypeptide subunits are essential for NADPH oxidase activity and mutations in the corresponding genes are responsible for the four different genetic subgroups of CGD (Figure 1 ). The oxidase subunits are referred to by their apparent molecular mass (kDa) and have been given the designation phox, for phagocyte oxidase. Overall, CGD has a minimum estimated incidence of between 1/200,000 and 1/250,000 live births.3
Approximately two-thirds of CGD cases result from defects in the X-linked gene encoding the gp91phox subunit of flavocytochrome b558, a membrane-bound heterodimer that is the redox center of the oxidase. The X-linked form of CGD is sometimes denoted as the X91 subgroup. The flavocytochrome is present in the plasma membrane, and, in neutrophils, much resides in the specific granules, which fuse with either the phagolysosome or plasma membrane upon cellular activation. The gp91phox subunit contains both flavoprotein and heme-binding domains responsible for electron transport. A rare autosomal recessive form of CGD (A22 CGD) is caused by mutations in the gene encoding p22phox, the smaller subunit of flavocytochrome b558, which contains a docking site for p47phox. The remaining cases of autosomal recessive CGD (A47 and A67 CGD) involve genetic defects in either p47phox or p67phox, two regulatory proteins that are associated with each other in the cytosol of unstimulated cells. When neutrophils or macrophages are exposed to inflammatory or phagocytic stimuli, p47phoxand p67phox rapidly move to the membrane to activate flavocytochrome b558 and induce superoxide formation.
Mutations identified in the genes encoding gp91phox, p22phox, and 67phox are very heterogenous.1 In contrast, virtually all A47 patients are either homozygotes or compound heterozygotes for a mutant allele with a GT deletion at the beginning of exon 2 that predicts a premature stop codon. The high frequency of the p47phox GT deletion mutation appears to reflect the existence of highly conserved and closely linked p47phox pseudogenes, which leads to recombination events with the wild-type gene.1
Other regulatory subunits of the NADPH oxidase.
Regulation of superoxide formation also involves the small GTP-binding protein Rac and possibly Rap1a, which act as molecular switches through conformational changes between the GDP and GTP-bound forms. Rac-GTP binds to the membrane and to p67phox, and is required for NADPH oxidase activity. A fifth phox protein, p40phox, is also bound to p67phox and may additionally help to regulate NADPH oxidase activity. Genetic defects in Rap1a, Rac, or p40phoxhave not been reported in CGD. However, a dominant-negative mutation in the hematopoietic-specific Rac2 GTPase was identified in an infant with a clinical picture more similar to leukocyte adhesion deficiency (see below). Neutrophils showed normal leukocyte β2 integrin expression but severe defects in adhesion and motility, along with partial NADPH oxidase defects.4,5
NADPH oxidase, proteases and microbial killing
The generation of respiratory burst oxidants is essential for normal killing within the phagosome, acting in concert with proteases, defensins, and other compounds released into the phagosome by fusion of different granule populations. Activation of the NADPH oxidase also results in changes in intraphagosomal pH that facilitate microbial killing.6 The importance of granule compounds for intact innate immunity is well illustrated by the rare neutrophil function defect, specific granule deficiency. In this disorder, which is due in at least some cases to mutations in the myeloid transcription factor C/EBPε, patients lack primary granule defensins, gelatinase, and all secondary granule proteins, and consequently suffer from a variety of bacterial infections.1 Of note, studies in knock-out mice genetically deficient in neutrophil elastase show enhanced susceptibility to gram-negative organisms such as Klebsiella and Escherichia coli, and mice deficient in cathepsin G and/or elastase appear to be more susceptible to Aspergillus.7 The optimal release of granule matrix-associated proteases and other proteins into the phagosome lumen may depend on ion fluxes set into motion by changes in membrane potential resulting from NADPH oxidase-mediated electron transport across the membrane.8 However, it is considered unlikely that the main function of the NADPH oxidase is to promote granule protein activation. A large body of evidence supports the view that respiratory burst oxidants have direct microbicidal effects.7,9 The specific ions transported in response to the electrogenic effects of the NADPH oxidase also remain a topic of active investigation (see 6,9,10).
Clinical features of CGD
The clinical manifestations of CGD typically begin in infancy or early childhood. CGD patients are particularly susceptible to Staphylococcus aureus, Aspergillus species, Nocardia species, and a variety of gram-negative enteric bacilli including Serratia marcescens, Salmonella species and Burkholderia cepacia. Many of these organisms contain catalase, which prevents CGD phagocytes from utilizing microbe-generated hydrogen peroxide to promote killing of ingested organisms. Frequent sites of infection include skin and its draining lymph nodes, lungs, bone and gastrointestinal tract, including Staphylococcal liver abscesses, which are almost unique to CGD.1,3 In contrast to infections with bacteria and Nocardia, Aspergillus species infections can often be indolent with absence of fever or leukocytosis, and few if any symptoms.11 Although S aureus is the most frequently isolated organism overall, the most common causes of death reported in a recent series were pneumonia and/or sepsis due to Aspergillus or B cepacia.3
Many CGD patients also develop chronic inflammatory granulomas, which are a distinctive hallmark of this disorder. Symptomatic disease can include colitis/enteritis or granulomatous obstruction of either the gastric outlet or urinary tract. A recent analysis of 140 patients with CGD followed at the National Institutes of Health revealed inflammatory involvement of the gastrointestinal tract in 32.8% of patients, 89% of whom had X-linked inheritance.12 In some cases, granuloma formation is a response to active infection, but in many cases it is believed to reflect a dysregulated inflammatory response and/or inefficient degradation of inflammatory mediators and debris in the absence of respiratory burst-derived oxidants.13,14 Production of oxidants appears to be an important trigger of neutrophil apoptosis at sites of inflammation, which is also important for resolution of the inflammatory response. Recent studies have shown that apoptosis is delayed in CGD neutrophils.15–17
Correlation between genetic defect and clinical course.
As a group, patients with X-linked CGD, A22 CGD, and A67 CGD tend to have a more severe clinical course compared to patients with A47 CGD.1,3 This may reflect residual superoxide formation by p47phox-deficient neutrophils, which can be detected using sensitive fluorescent probes. Some X91 patients have a partially functional gp91phox, associated in some cases with a milder clinical course.1 However, despite the fact that more than 90% of patients with non-p47phox-deficient forms of CGD have undetectable levels of O2− production, there is a surprising clinical heterogeneity.1,3 At one end of the spectrum are patients who develop severe and recurrent bacterial and fungal infections beginning during infancy. At the other end of the spectrum are patients who are well for many years and then unexpectedly develop a serious infection typical of CGD. Polymorphisms in oxygen-independent antimicrobial systems or other components regulating the innate immune response are likely to play an important role in modifying disease severity. These remain to be fully defined, although specific polymorphisms in the myeloperoxidase, mannose binding lectin, and FcγRIIa genes are associated with a higher risk for granulomatous or autoimmune/rheumatologic complications in CGD.18
Mouse models of CGD
Gene targeting has been used to develop mouse models for both X-linked (gp91phox-/-) and an autosomal recessive (p47phox-/-) form of CGD.19,20 CGD mice have abnormalities in both host defense and inflammation that are similar to their human counterpart, confirming the importance of the respiratory burst in innate immunity. CGD mice exhibit a marked increase in susceptibility to the opportunistic pathogens, B cepacia and Aspergillus species,13,19,21,22 two organisms that are particularly problematic in CGD patients. Other organisms showing increased virulence in CGD patients and, similarly, in CGD mice include S aureus,19Salmonella typhimurium, Mycobacterium tuberculosis, and Candida.7,19 The generation of reactive nitrogen intermediates via inducible nitric oxide synthase (NOS2) is a second oxygen-dependent phagocyte antimicrobial system that plays an important role in host defense.7 Mice with a double deficiency in both the NADPH oxidase (gp91phox-/-) and NOS2 have a high rate of spontaneous infection with commensal organisms, mostly enteric bacteria, whereas mice with a single enzyme deficiency rarely exhibit spontaneous infections when raised in specific pathogen-free conditions.23 Superoxide production from xanthine oxidase, which is expressed in endothelial cells and other tissues, may be another source of antioxidants that can function as a back-up to the phagocyte NADPH oxidase.7
Both gp91phox- and p47phox-deficient CGD mice also have abnormalities in the inflammatory response. These include a marked increase in exudate neutrophils compared to wild-type mice in response to peritoneal instillation of thioglycollate,19,20 which may be related to impaired degradation of leukotriene B4.14 gp91phox-/- mice exhibit an exaggerated acute inflammatory response after instillation of sterilized hyphae into the lungs or upon intradermal injection, which evolves into a chronic granulomatous infiltrate.13,24 In models of experimental arthritis, both gp91phox- and p47phox-deficient CGD mice had more severe joint inflammation that developed into a granulomatous synovitis.25 These studies support the concept that respiratory burst products play an important role in inflammation outside of their function in microbial killing.
Whether absence of phagocyte NADPH oxidase activity has a protective effect in diseases with an inflammatory component has also been investigated using knockout CGD mice. Decreased injury has been reported in some models including stroke due to transient occlusion of the carotid artery, whereas protection against atherosclerotic lesions has generally not been observed (see 26).
Treatment of CGD
Current approaches.
The use of prophylactic antibiotics and interferon-γ (IFN-γ), coupled with aggressive treatment of acute infections and prolonged courses of antimicrobial treatment, has markedly improved the clinical course of patients with CGD. Trimethoprim/sulfamethoxazole (or, in sulfa-allergic patients, dicloxacillin) is ordinarily used for antibiotic prophylaxis. Prophylactic IFN-γ is another mainstay of current management, although its use is not accompanied by any measurable improvement in phagocyte NADPH oxidase activity in the majority of CGD patients. The clinical benefit of IFN-γ is probably related to enhanced phagocyte function and killing by non-oxidative mechanisms and perhaps the NOS2 and xanthine oxidase pathways. A large, multicenter trial initially established that recipients of IFN-γ had 70% fewer and less severe infections.27 A recent update reinforced these findings, reporting only 0.30 serious bacterial infections and 0.12 serious fungal infections observed per patient-year, in patients observed for up to 9 years, with a total observation period of 328.4 patient-years.28 The most common side effects were fever and flu-like symptoms. Importantly, there was no increase in the incidence of chronic inflammatory complications of CGD in patients receiving IFN-γ. A new study showed that itraconazole is an effective agent for prophylaxis for fungal infections in CGD, as evaluated in a randomized, double blind, placebo-controlled study conducted by the National Institutes of Health.29 Hence, for all patients with CGD, regardless of genetic subgroup, the current recommendation is to use prophylaxis with trimethoprim/sulfamethoxazole, itraconazole, and IFN-γ.
Corticosteroids are used to treat clinically significant granulomatous complications of CGD, although with caution, given the underlying microbial killing defect.
The prognosis of CGD has improved dramatically in the past two decades with the advent of prophylactic antimicrobials and IFN-γ.1 The overall mortality rate in the study on long-term IFN-γ therapy cited above was 1.5% per patient-year,28 and may be even lower in newly diagnosed children managed with optimal care, including the use of anti-fungal prophylaxis. It will be important to continue to monitor the changing outlook for patients with this disease, in order to weigh the risks and benefits of approaches using stem cell transplantation.
Stem cell transplantation in CGD.
Allogeneic bone marrow transplantation can be used to treat CGD and has been employed successfully.1 However, because of the risks associated with this procedure and the frequent lack of an HLA-matched sibling, conventional marrow transplantation has generally been considered only for those patients who have frequent and severe infections despite aggressive medical management. Some success has also been achieved in transplants done in the setting of active infection.30 The development of reduced intensity conditioning regimens for allogeneic transplantation may be an emerging approach for stem cell transplantation in CGD. A “mini-transplant” regimen was used successfully in a small number of children, but graft-versus-host disease was a significant problem in the adult patients in this study.31
Gene therapy of CGD.
CGD is also a candidate disease for gene therapy targeted at hematopoietic stem cells (HSCs).32 Observations on female carriers of X-linked CGD, variant X-linked CGD patients with residual enzyme activity, and preclinical studies in murine CGD models suggest that complete correction of respiratory burst activity in ~10% of circulating neutrophils would lead to clinically relevant improvements in host defense, particularly against Aspergillus.32 However, correction of > 20% of neutrophils will likely provide broadest protection against bacterial infection and granulomatous complications.22,33 The relative level of superoxide within individual neutrophils may also be an important factor, and only partial correction of cellular NADPH oxidase activity may not restore full antimicrobial activity.22
The majority of preclinical studies in CGD to date have used gamma-retroviral vectors, although lentiviral vectors have attracted increasing attention. Lentiviral vectors can transduce quiescent cells, which is an advantage for future use in human hematopoietic stem cells. “Self-inactivating” lentiviral vectors with deleted viral enhancer sequences may also provide safety advantages over gamma-retroviral vectors, because the former tend to insert into the body of the gene rather than near gene promoters, with less chance of activating expression of neighboring genes.34
Several phase I CGD gene therapy clinical trials using cytokine-mobilized peripheral blood CD34+ cells have either been completed or are ongoing. In initial trials by Malech and colleagues at the National Institutes of Health35 and by our group at the Indiana University School of Medicine, retrovirally transduced autologous peripheral blood CD34+ cells were reinfused into patients without any bone marrow conditioning. Oxidase-positive peripheral blood neutrophils were observed over the following months, although numbers were very low (at most, 0.2% of neutrophils) and disappeared altogether within a year. These studies suggested that, not surprisingly, marrow conditioning prior to reinfusion of transduced cells was going to be important in order to achieve higher-level engraftment of corrected stem/progenitor cells.
A new trial being conducted in Europe for X-linked CGD patients utilizes moderate dose busulfan as conditioning prior to infusion of CD34+ cells transduced with a gamma-retroviral vector for gp91phox expression.36 In the two patients reported thus far, both adults, higher than expected numbers of oxidase-positive granulocytes were seen post-transplant, which then increased in the months following reinfusion, reaching over 35% in one patient and ~15% in the second. Both patients are currently well, with normal peripheral blood counts, and chronic infections associated with CGD have resolved. Analysis of peripheral blood granulocyte DNA showed that the majority of proviral insertions were non-random, and the authors postulated that vector-mediated activation of genes at the insertion sites contributed to the increase in oxidase-positive neutrophils.37 A recent study using the mouse transplant model also suggested that retroviral integration might trigger non-malignant expansion in murine hematopoiesis due to transcriptional activation of neighboring genes.38 The long-term significance of these findings is currently unknown.
NADPH oxidases: beyond phagocytic leukocytes
Although beyond the scope of this brief review, numerous studies have now reported detecting the expression of gp91phox, p22phox, p67phox, and p47phox in vascular endothelial cells and/or smooth muscle cells.39 Expression of these components as well as oxidant production appears to be much lower than in phagocytic leukocytes. The physiologic significance of phox expression in non-phagocytic cells remains to be resolved, but it has been speculated that derivative reactive oxidants may play a role in intracellular signaling. Other potential sources of reactive oxidants, e.g., from xanthine oxidase, mitochondria, or other NAD(P)H oxidases, has made it difficult to ascribe any specific functions to vascular phox protein expression. However, studies in CGD knockout mice have provided some support that endothelial cell expression of the phagocyte NADPH oxidase plays a role in inflammation. For example, gp91phox-deficient mice transplanted with β-S sickle cell marrow had decreased leukocyte and platelet adhesion in the cerebral microvasculature.40
Mammalian homologs of the gp91phox flavocytochrome have also been recently discovered.41,42 This family is known as the NOX (NAD(P)H oxidase) proteins, of which there are 5 members, including gp91phox, which is designated as NOX2 in this classification. All NOX proteins share a similar protein structure, with multiple trans-membrane domains for heme binding and an intracellular flavoprotein domain. The other NOX proteins are expressed in various cell types, including the epithelium, smooth-muscle cells, gut and the endothelium, and appear capable of generating superoxide, generally at much lower levels than the phagocyte enzyme, and other reactive oxygen species, including hydrogen peroxide. The regulation of oxidant production by these enzymes and their biologic roles are currently under intense investigation. At least some of the other NOX flavocytochromes appear to be regulated by homologs of p67phox and p47phox. The derivative oxidants are proposed to mediate signal transduction and, in the gut, may function in innate immunity. The phagocyte NADPH oxidase can thus be viewed as a specialized, high output system for producing superoxide, and it will be fascinating to compare this well-studied enzyme and its associated pathophysiology with emerging findings on the other NOX members.
Other Disorders of Phagocyte Function
New disorders of phagocytic leukocyte function continue to be described. Although all share a common theme of recurrent microbial infections, these disorders were recognized as specific entities due to distinctive clinical features, the failure to fit into known diagnostic categories, and continued improvements in our understanding of signaling networks that regulate the functional responses of phagocytic leukocytes.
Adhesion of neutrophils to the endothelium, tissue matrix, and microbes is essential for their ability to emigrate into sites of infection and eliminate pathogens. Many of these adhesive interactions are mediated by the integrin family of cell surface glycoproteins. Leukocyte adhesion deficiency type-1 (LAD-1) is a well-known autosomal recessive disorder caused by genetic defects in CD18, the common chain of the β2 integrin family.1 LAD-1 neutrophils have profound adhesive and motility defects. Patients present with recurrent severe infections, including deep tissue abscesses caused by S aureus or gram-negative enteric organisms, and also have neutrophilia but impaired formation of pus. New variants of leukocyte adhesion deficiency include leukocyte adhesion deficiency type 2 (LAD-2) and type 3 (LAD-3), both of which have been described in only a handful of patients.43 LAD-2 results from mutations in the Golgi GDP-fucose membrane transporter,1,43 which results in a generalized loss of expression of fucosylated glycans on the cell surface of cells. Thus, LAD neutrophils are unable to bind to E- and P-selectin receptors on endothelium. Infections are less severe than LAD-1, but patients also have developmental delay as well as manifesting the Bombay (hh) erythrocyte phenotype due to lack of fucosylation of RBC membrane proteins. LAD-3 has been described in four patients and appears to be an autosomal recessive disorder characterized by functional defects in the activation of multiple classes of integrins in response to G protein-coupled chemokine receptor stimulation, possibly due to impaired activation of the Rap1 GTPase.43 These patients had severe recurrent infections and leukocytosis, similar to LAD-1, and a bleeding tendency.
A neutrophil defect resulting from a dominant-negative mutation in the Rac2 GTPase has also been described in one patient and is associated with poor neutrophil adhesion and motility, along with impaired NADPH oxidase activation and degranulation in response to chemo-attractants.4,5 The presentation was similar to LAD, with neutrophilia, absent pus at sites of infection, and deep-seated soft tissue abscesses. The molecular basis of the dominant-negative Rac2 mutation is incompletely understood but presumably results from interference in Rac-regulated signaling pathways downstream of integrin and chemoattractant receptors. Of interest, this phenotype is similar to that reported for Rac2 knockout mice.44,45
A recently described group of inherited phagocyte functional disorders are due to genetic defects in macrophage IL-12 production or the IFN-γ receptor axis, which present with recurrent and severe atypical mycobacterial infections, including multifocal osteomyelitis.46 Ingestion of mycobacteria normally leads to macrophage production of IL-12, which in turn stimulates T cells and natural killer cells to produce IFN-γ, which then activates macrophages via downstream signals carried by JAK kinases and the STAT1 transcription factor.46,47 A variety of autosomal recessive as well as autosomal dominant mutations have been described in the IFN-γ receptor complex, the IL-12 receptor, and STAT1 that lead to susceptibility to nontuberculous mycobacterial infection and BCG. These disorders have been grouped as Mendelian susceptibility to mycobacterial disease (MSMD), and more than 100 patients have been described overall.47 Patients with more severe forms of this disorder, with complete IFN-γ receptor defects, are also susceptible to Salmonella and certain viral infections. Management of patients depends on the specific molecular defect. Patients with autosomal dominant defects in the IFN-γ receptor (which commonly involves a truncated form of the IFN-γ receptor) or IL-12–related defects can be treated with subcutaneous IFN-γ, whereas this is ineffective in complete IFN-γ receptor defects. Long-term prophylaxis against mycobacterial infections with azithromycin or clarithromycin is recommended.
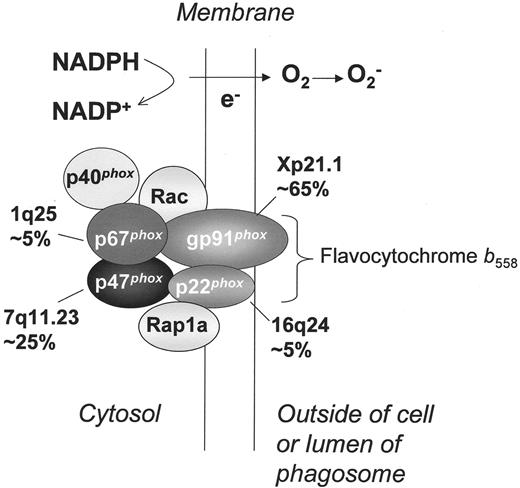
NADPH oxidase and molecular genetics of chronic granulomatous disease. Shown is the assembled form of the enzymatically active NADPH oxidase, along with subunits affected in the four different genetic subgroups of CGD, the approximate incidence, and the chromosomal location of the corresponding gene. Flavocytochrome b558 is the redox center of the enzyme, and is located in plasma, specific granule, and phagolysosomal membranes. This heterodimer is comprised of the gp91phox and p22phox subunits of the NADPH oxidase, which are affected in X-linked and an autosomal recessive form of CGD, respectively. The soluble regulatory proteins p47phox, p67phox, and p40phox are found in the cytosol until phagocyte activation by soluble or particulate inflammatory stimuli, upon which they move to the membrane where p47phox and p67phox bind flavocytochrome b558. Mutations in the genes encoding p47phox and p67phox account for two autosomal recessive forms of CGD. Another essential regulatory component of the NADPH oxidase is the small GTPase, Rac, which in its active GTP-bound state, becomes membrane-bound and associates with the oxidase. By a mechanism that is not fully understood, binding of these multiple regulatory subunits activates the flavocytochrome to catalyze the transfer of electrons from cytosolic NADPH across the membrane via the FAD and heme redox centers to molecular oxygen, thereby forming superoxide in the extracellular or intraphagosomal compartment. The Rap1a GTPase co-purifies with flavocytochrome b558, but its function in superoxide production is unknown. No cases of CGD have been described due to mutations in p40phox, Rac, or Rap1a.
NADPH oxidase and molecular genetics of chronic granulomatous disease. Shown is the assembled form of the enzymatically active NADPH oxidase, along with subunits affected in the four different genetic subgroups of CGD, the approximate incidence, and the chromosomal location of the corresponding gene. Flavocytochrome b558 is the redox center of the enzyme, and is located in plasma, specific granule, and phagolysosomal membranes. This heterodimer is comprised of the gp91phox and p22phox subunits of the NADPH oxidase, which are affected in X-linked and an autosomal recessive form of CGD, respectively. The soluble regulatory proteins p47phox, p67phox, and p40phox are found in the cytosol until phagocyte activation by soluble or particulate inflammatory stimuli, upon which they move to the membrane where p47phox and p67phox bind flavocytochrome b558. Mutations in the genes encoding p47phox and p67phox account for two autosomal recessive forms of CGD. Another essential regulatory component of the NADPH oxidase is the small GTPase, Rac, which in its active GTP-bound state, becomes membrane-bound and associates with the oxidase. By a mechanism that is not fully understood, binding of these multiple regulatory subunits activates the flavocytochrome to catalyze the transfer of electrons from cytosolic NADPH across the membrane via the FAD and heme redox centers to molecular oxygen, thereby forming superoxide in the extracellular or intraphagosomal compartment. The Rap1a GTPase co-purifies with flavocytochrome b558, but its function in superoxide production is unknown. No cases of CGD have been described due to mutations in p40phox, Rac, or Rap1a.