Abstract
The development of new therapeutic strategies for myelodysplastic syndromes (MDS) has gained new momentum fueled by improved characterization of the disease’s natural history and biology and by the recent US Food and Drug Administration (FDA) approval of the first agent with an indication for MDS. By integrating morphologic and cytogenetic features with greater discriminatory power, the World Health Organization (WHO) has refined the classification of these stem cell malignancies and enhanced its prognostic utility. Recognition that the malignant phenotype, which characterizes MDS, may arise from mechanistically diverse biological processes has raised new awareness that treatment strategies must be tailored to the pathobiology of the disease. Therapeutics targeting chromatin structure, angiogenesis and the microenvironment that nurtures the MDS phenotype have demonstrated remarkable activity and offer an opportunity to alter the natural history of the disease. This chapter provides an overview of recent developments in the characterization of MDS from the microscope to the laboratory and the translation of these findings into promising therapeutics.
In Section I, Dr. James Vardiman reviews the cytogenetic abnormalities that characterize MDS, their clinical and pathologic significance, and the application of the WHO classification. In Section II, Dr. Alan List reviews treatment goals driven by prognostic variables and biological features of the disease that have led to promising small molecule, selective therapeutics. In Section III, Dr. Jean-Pierre Issa provides an overview of epigenetic events regulating gene expression, which may be exploited therapeutically by chromatin remodeling agents. In Section IV, Dr. Theo DeWitte discusses new developments in hematopoietic stem cell transplantation, including reduced-intensity and myeloablative approaches.
I. Characterization and Classification of Myelodysplastic Syndromes— From Morphology to Cytogenetics
James Vardiman, MD*
University of Chicago, 5841 S. Maryland Ave., MC0008, Chicago IL 60657 Acknowledgments: The author thanks Dr. Anna Porwit-MacDonald, Professor, Department of Pathology, Karonlinska University Hospitals, for her contribution of flow cytometry histograms.
Although the myelodysplastic syndromes (MDS) were initially considered by many to be synonymous with “preleukemia,” this notion has given way to the realization that MDS is a heterogeneous spectrum of stem cell malignancies, with the majority of patients succumbing to complications of bone marrow failure rather than acute leukemia. In 1982 the French-American-British (FAB) cooperative group proposed morphologic guidelines for the diagnosis and classification of MDS that provided a framework against which clinical, biologic and genetic studies could be universally compared.1 Since then nearly 20,000 publications have characterized various features of MDS. Despite such active investigation, a biologic marker that reliably identifies MDS remains elusive. Therefore, morphology remains the cornerstone of diagnosis and an important tool that complements cytogenetic findings for prognostic discrimination.2 Nevertheless, particularly in “low grade” disease, the morphologic recognition of MDS can be difficult, creating diagnostic indecision. For such cases, appreciation of the basic guidelines for the interpretation of morphology and its correlation with clinical and cytogenetic findings is essential for patient management.
Morphologic Characterization of MDS— Guidelines, Problems and Pitfalls
General guidelines
Although most hematologists and pathologists can recite the morphologic features of myelodysplasia, in practice inter-observer reproducibility for recognition of dysplasia is poor, particularly in low-grade MDS. In one study, inter-observer agreement among five expert morphologists was reasonably good for recognition of blasts and ringed sideroblasts, but poor for dyserythropoiesis (R = 0.27) and not much better for dysgranulopoiesis (R = 0.45).3 A few simple guidelines can minimize such problems and improve recognition of MDS.
Prerequisites for evaluation of morphologic features in MDS include the availability of well-prepared peripheral blood and bone marrow aspirate smears. They should be stained with Wright-Giemsa or May-Grunwald-Giemsa because Wright’s stain alone may not adequately demonstrate cytoplasmic granules.4 Iron stains of the marrow aspirate are essential to detect ringed sideroblasts. Blood and marrow smears should be examined for dyplasia, the percentage of blasts and monocytes (nonspecific esterase stains may be helpful to detect monocytes in the marrow), and ringed sideroblasts. The enumeration of blasts is important for diagnosis, classification and prediction of prognosis.1,2 In myeloid neoplasms, myeloblasts, monoblasts, and megakaryoblasts are included in the blast calculation. Small, dysplastic megakaryocytes are not blasts and should not be counted as such. Erythroid precursors are also not counted as blasts, except in rare cases of “pure” erythroleukemia in which primitive erythroblasts account for the majority of cells. In myelomonocytic proliferations, promonocytes are included as “blast equivalents.”4,5 Substitution of the percent of CD34+ cells determined by flow cytometry for a visual blast count is discouraged. Although hematopoietic cells that express CD34 are blasts, not all blasts express CD34. In addition, dilution of the marrow sample by peripheral blood during aspiration and processing of the sample for flow cytometry analysis complicates comparison between the visual count and the CD34 value.
Although a bone marrow biopsy specimen is not always necessary to establish a diagnosis of MDS, it offers valuable diagnostic and prognostic information.4– 6 Dysplasia, particularly of megakaryocytes, can be appreciated in well-prepared biopsies, and evidence of disruption of the normal marrow architecture, such as abnormal localization of immature precursors (ALIP), lends further support for the diagnosis of MDS. Moreover, the biopsy provides confirmation of the blast percentage and distribution, and serves as a source for immunohistochemical studies that may have diagnostic and prognostic value. An underappreciated role of the biopsy is that it may provide evidence for another disease that can mimic MDS clinically, such as lymphoma or metastatic tumor. In cases of MDS that are hypocellular or associated with fibrosis, the biopsy is essential for diagnosis.
Morphologic problems and pitfalls
One of the most difficult diagnostic challenges in MDS is that morphologic dysplasia is not specific for MDS but can be seen in other conditions, including megaloblastic anemia, congenital dyserythropoietic anemia, exposure to toxins such as arsenic and alcohol, after cytotoxic and growth-factor therapy, and in HIV or parvovirus B19 infections—to name a few. Modest dyserythropoiesis is also not uncommon when there is brisk erythroid hyperplasia or regeneration, i.e., “stress erythropoiesis.” This “secondary” dysplasia is most problematic when only one (usually the erythroid ) lineage is involved, but multilineage dysplasia can also be a transient, reactive change. Causes of secondary dysplasia must be considered and excluded by appropriate clinical and laboratory studies prior to rendering a diagnosis of MDS. Furthermore, a small number of dysplastic erythroid, granulocytic or megakaryocytic cells can be seen in marrow specimens from normal individuals.7 Hence, the guideline that 10% of the cells in a lineage should be dysplastic to consider the lineage as dysplastic and as evidence for MDS is a reasonable rule of thumb.6
The quality of the specimen is a common obstacle in the accurate diagnosis of MDS. For example, hypogranularity of the cytoplasm of neutrophils is a well-accepted feature of dysplasia, but visualization of neutrophil granules is critically dependent on an optimal stain. The diagnosis of MDS should never be based on “pale granulocytes” without other features to substantiate the diagnosis.6 Biopsies should be of adequate size for evaluation (at least 1–2 cm) and should extend into the marrow well past the cortical bone. The marrow immediately under the cortical bone is normally less cellular than deeper marrow. In a case of MDS, the combination of cytopenias in the blood and a superficial, apparently hypocellular biopsy specimen might result in an erroneous diagnosis of aplastic anemia.
Even when these guidelines are carefully followed, the diagnosis of MDS may remain problematic because of morphologic features that are not clear-cut. Cytogenetic studies may lend valuable support for the diagnosis in such cases. In addition, characterization by flow cytometry may provide evidence for abnormal maturation of the myeloid lineages. Although no specific surface antigenic pattern is unique to MDS, the finding of aberrant expression of antigens normally associated with different stages of maturation of myeloid cells provides additive information.8 In addition, abnormally granulated neutrophils may demonstrate abnormal light scatter properties on the flow cytometer (Figure 1; see Color Figures, page 518). However, these abnormalities must be carefully interpreted in the light of other morphological and clinical features because of their limited specificity.
Cytogenetic Characterization of MDS
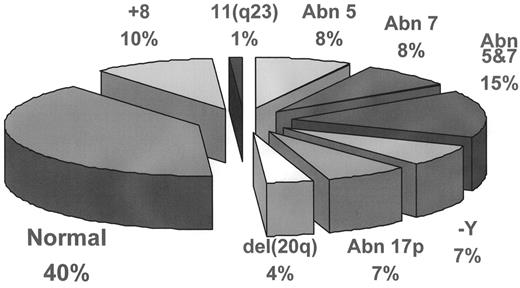
Cytogenetic studies play a major role in confirmation of diagnosis and prediction of clinical outcome in MDS, and have contributed to the understanding of its pathogenesis. Clonal chromosomal abnormalities are detected by routine karyotyping techniques in 40%–70% of cases of de novo MDS, and 95% of cases of therapy-related MDS (t-MDS).9 The relative frequency of the most common cytogenetic abnormalities in de novo MDS are depicted in Figure 2 . In t-MDS, deletion of part or all of chromosomes 5 and/or 7, and complex chromosomal abnormalities account for 90% of the karyotypic changes. If the morphology of a case is strongly suspicious but not entirely convincing for MDS, discovery of a recurring chromosomal abnormality can lend strong support to the diagnosis. Occasionally, recurring cytogenetic abnormalities are detected in cases suspected clinically to be MDS because of unexplained cytopenia, yet there is no morphologic dysplasia. In a recent study of patients with these latter findings, Steensma and associates reported that the cytopenia and chromosomal abnormalities usually persist.10 They suggest that in such cases the karyotypic abnormalities may be evidence of a “form fruste” of MDS and, in some cases, morphologic evidence of MDS will appear after variable length of follow-up.
The importance of cytogenetic abnormalities in the prediction of survival and in assessing the risk of transformation of MDS to acute leukemia is well known, and cytogenetic studies have been included in most of the predictive scoring systems developed for MDS.2,9 No cytogenetic abnormality is specific for MDS or for a specific morphologic subgroup of MDS. However, some unique cytogenetic/morphologic correlations exist, the most common of which is the “5q- syndrome,” described in the classification section below.
Although it might be expected that chromosomal abnormalities would ultimately lead to the discovery of the genetic lesions important in the pathogenesis of MDS, progress in this area has been slow. The pathogenesis of MDS is likely a multi-step process that involves a number of insults to the genome of a marrow stem cell, many of which are cytogenetically silent. The search for genes critical to disease pathogenesis is further complicated because the characteristic chromosomal abnormalities in MDS involve loss of genetic material. Currently it is not clear whether the loss of genetic material involves the total loss of function of a tumor suppressor gene, a tumor suppressor gene that acts by haploinsufficiency, or through some other associated defect that has not yet been discovered. Gene expression profiling of cDNA from patients with MDS has recently been reported. Genes reportedly upregulated include those involved in cell proliferation, including members of the Ras gene family, and some that are downregulated, reportedly including genes encoding anti-apoptotic proteins.11
The WHO Classification of MDS
The FAB classification of MDS has been widely used since its introduction in 1982, and its clinical relevance has been demonstrated in numerous studies. However, each FAB subgroup is heterogeneous and includes cases with variable lineage involvement, various cytogenetic abnormalities, and widely variable clinical outcomes. In 2001, the World Health Organization (WHO) published new classification schemes for neoplasms of the hematopoietic and lymphoid tissues.12 For MDS, the WHO relied on data accumulated over the past two decades to propose modifications to improve the prognostic value of MDS classification. The major changes include (1) lowering the threshold for defining acute myeloid leukemia (AML) from 30% to 20% blasts in the bone marrow or peripheral blood with elimination of the FAB category of refractory anemia with excess blasts in transition (RAEBT); (2) division of the low-grade categories of refractory anemia (RA) and refractory anemia with ringed sideroblasts (RARS) into 5 separate entities, depending on whether single lineage or multilineage dysplasia is present and on whether an isolated interstitial deletion of chromosome 5q is present; (3) subdividing RAEB into two categories depending on the number of blasts in the blood and marrow; and (4) removing chronic myelomonocytic leukemia (CMML) from the MDS category into a new group of diseases, the Myelodysplastic/Myeloproliferative Diseases. The WHO classification and the criteria for each subgroup are shown in Table 1 . The most controversial changes in the WHO classification proved to be the reduction in the blast threshold for the diagnosis of AML and the refinements in the FAB categories of RA and RARS, which deserve further comment.
Elimination of RAEBT
A detailed discussion of the rationale for reducing the number of blasts required for the diagnosis of AML and the elimination of RAEBT is found in reference 5. In brief, the WHO classification of AML includes a new subcategory, AML with multilineage dysplasia, that is intended to capture cases of AML evolving from MDS or that have MDS-related features. A number of studies have shown clinical, biologic and genetic similarities between the FAB category of RAEBT and the WHO category of MDS-related AML. Furthermore, 50%–60% of patients with RAEBT evolve to overt AML with 30% blasts or more within 6 months of their initial diagnosis.2 An additional argument for the elimination of RAEBT is that some patients with AML who have no dysplastic-related features may have less than 30% blasts on an initial marrow examination. They would therefore be assigned to the poor-risk category of RAEBT even though their leukemia is not MDS-related. Since publication of the WHO classification, reports comparing survival of patients diagnosed as RAEBT by the FAB criteria with survival of patients diagnosed as MDS-related AML and 30% or more blasts have shown no significant differences.13,14 Admittedly, similar median survival times do not necessarily prove synonymy between the two groups.
Refractory anemia, refractory anemia with ringed sideroblasts, refractory cytopenia with multilineage dysplasia, and MDS with isolated del(5q) chromosomal abnormality
The FAB criteria for RA and RARS were vague as to whether these categories should include patients with dysplasia in lineages other than the erythroid lineage and, if so, how much dysplasia should be permitted. In practice, the FAB categories of RA and RARS were heterogeneous and included MDS with unilineage erythroid dysplasia, as well as cases with severe multilineage dysplasia. A number of investigators have reported that in low-grade MDS, multilineage dysplasia does influence overall survival times and the incidence of transformation to acute leukemia.15,16 In the WHO classification, the criteria for RA and RARS were revised to take these studies into account. A new category, refractory cytopenias with multilineage dysplasia (RCMD), was added to incorporate patients with MDS characterized by fewer than 5% blasts in the marrow but dysplasia in 10% or more of the cells of at least two hematopoietic lineages (see Table 1 for details). Additionally, the WHO recognizes another category of low-grade MDS, the “5q- syndrome,” defined as having del(5q) as the sole chromosomal abnormality, < 5% blasts in the marrow, and characteristic megakaryocytes with hypolobated nuclei.
Several studies have now reported that the WHO classification does have improved prognostic value for the low-grade MDS categories.17–,19 In an analysis of 103 patients diagnosed as RA by FAB criteria, Cermak and colleagues applied WHO guidelines (Table 1 ) to reassign 43 (42%) as “pure” RA, 56 (54%) as RCMD, and 4 (4%) as 5q- syndrome.18 For the entire group, median survival was 57.7 months, whereas for those reclassified as “pure RA,” RCMD and 5q-syndrome, median survival times were > 102 months, 27 months, and 53.3 months, respectively. A separate report by Howe and associates showed that in a series of 64 patients with low-risk MDS and < 5% marrow blasts, there was a significant difference in median survival between patients with unilineage dysplasia (51% surviving at 67 mos) and those with multilineage dysplasia (median survival 28.5 mos).19 Recent clinical as well as gene profiling studies have validated the WHO proposal that cases characterized by del(5q) as the sole cytogenetic abnormality comprise a unique group of MDS.11,20
From the data currently available, it is likely the WHO classification will offer improved prognostic information. Still, other categories of MDS are not addressed by this classification, such as hypocellular MDS and MDS with fibrosis. These latter entities remain problematic, and their recognition as well as their place among the other subcategories await further clarification. Perhaps, when it is time to revise the WHO classification, these entities will be better understood and a universally applicable biologic marker for MDS will be available.
II. Novel Therapeutics for an Orphan Disease
Alan List, MD*
H. Lee Moffitt Cancer Center and Research Institute, Malignant Hematology, SRB-4, 12902 Magnolia Drive, Tampa FL 33612-9497
The remarkable hematologic, pathologic, and biological heterogeneity of the myelodysplastic syndromes (MDS) demands that disease-specific features be scrutinized to properly gauge natural history of disease, determine therapeutic goals, and select optimal therapy. Morphologic classification alone is insufficient, deriving prognostic power primarily from thresholds in blast percentage.1,2 Prognostic modeling permits identification of variables with independent power for outcome prediction. The International Prognostic Scoring System (IPSS) represents the first such system to be accepted worldwide for application in routine management decisions and clinical trials.3
The IPSS is derived from the analysis of data from more than 800 patients with de novo MDS and nonproliferative CMML (i.e., WBC ≤ 12,000/μL) managed solely with supportive care. As such, expectations for survival and leukemia evolution reflect the intrinsic natural history of disease that can serve as a benchmark to be surpassed by novel therapeutics and management decisions. This model applies a score that is weighted according to the independent statistical power of each of three prognostic features: bone marrow blast percentage, cytogenetic pattern, and the number of cytopenias (Table 2A ). The cumulative score enables segregation of patients into four subgroups with varying expectations for survival, and risk of and interval to AML progression (Table 2B ). Although age offers further survival discrimination in lower risk patients because of competing causes for death (i.e., Low- and Intermediate-1 risk), it has no correlation with disease-related risks and therefore is not included in the prognostic model. The need to integrate morphologic and biologic features with independent prognostic power into a clinically meaningful classification system that can be universally applied served as the impetus for the WHO’s recent proposals. Although this new schema offers greater prognostic discrimination than FAB, the IPSS complements both classification systems by its incorporation of unfavorable cytogenetic abnormalities and number of lineage deficits.
These recently adopted tools for prognostic discrimination provided the foundation for creation of universal measures of response by an International Working Group (IWG).4 Like most other malignancies, management must be guided by the risks imposed by the disease, the patient’s age and performance status, expectation for treatment tolerance, and quality of life. The therapeutic goals, therefore, should be judged by the natural history of disease and patient preference. Implicit in these recommendations is the notion that patients with Low- or Intermediate-1 IPSS risk categories experience longer survival, and therefore, amelioration of hematologic deficits should represent the principal therapeutic goal and be relatively durable to translate into clinically meaningful benefit. Such improvements therefore must exceed minimally accepted thresholds for at least 2 month’s duration. For higher-risk patients (i.e., IPSS Intermediate-2/High risk categories), extending survival is of immediate priority, necessitating the incorporation of complete pathologic and cytogenetic remission as an early surrogate milestone for survival extension.
Therapeutic Developments
Identification of transforming events integral to maintenance or propagation of the MDS clone has remained illusive. Nonetheless, delineation of biologic features that nurture the neoplastic phenotype has led to the development of novel therapeutics that have shown considerable promise. A partial summary of those agents in clinical development is provided in Table 3 .
Angiogenic molecules generated by the neoplastic clone represent one such lead that has yielded promising new therapeutics for patients with hematologic malignancies. In MDS, in particular, vascular endothelial growth factor-A (VEGF-A) has emerged as an important angiogenic molecule that is implicated not only as a soluble effector of medullary neovascularity but also in the clonal expansion of receptor-competent myeloblasts as well as ineffective hematopoiesis in receptor naïve progenitors.1,5 Paracrine induction of inflammatory cytokines from receptor-competent adventitial cells within the microenvironment potentiates ineffective hematopoiesis by suppressing formation of VEGF receptor-naïve primitive progenitors. Based upon these and other preclinical investigations, small molecule inhibitors of angiogenic cytokines have emerged as a promising class of therapeutics for MDS with the principal impact on erythropoiesis.
Thalidomide (Thalomid™, Celgene Inc, Warren NJ), which has both anti-angiogenic and tumor necrosis factor (TNF)α inhibitory properties, represents the first agent in this class to be investigated in MDS. In a Phase II trial of thalidomide performed at the Rush Presbyterian Cancer Institute,6 15 of 83 (18%) evaluable patients experienced either red blood cell transfusion independence or a > 50% decrease in transfusion burden, whereas improvement in non-erythroid lineages was uncommon. Dose escalation beyond 200 mg daily was limited by cumulative neurological toxicity and is likely unnecessary. An aggressive dose-escalation schema of 200 mg to 1000 mg daily evaluated by the North Central Cancer Treatment Group study was compromised by excessive early attrition due to toxicity at a median interval of ≤ 2.5 months.7 Prolonged drug treatment, when tolerated, appears necessary to maximize hematological benefit. Median interval to erythroid response was 16 weeks in the Rush trial (range, 12–20 weeks), with an erythropoietic response rate of 29% among the 51 patients completing a minimum of 12 weeks of study treatment. The overall clinical benefit of low-dose thalidomide in MDS was evaluated in a national randomized, placebo-controlled Phase III trial completed in the fall of 2003, the results of which should soon be available.
Novel, more potent thalidomide analogues with improved toxicity profiles recently entered clinical investigations.8 Lenalidomide (CC-5013, or Revlimid™: Celgene) is a 4-amino glutarimide derivative of thalidomide that lacks the neurological toxicities of the parent compound. It is a potent modulator of ligand-induced cellular response with biological effects that range from potentiation of antigen-initiated immune response, to modulation of integrin affinity, suppression of trophic response to angiogenic molecules, and promoting progenitor responsiveness to erythropoietin.9 Among 36 evaluable patients with MDS either with symptomatic or transfusion-dependent anemia treated with lenalidomide in a safety and efficacy trial, 24 (67%) experienced an erythroid response according to IWG criteria, with 21 patients experiencing sustained transfusion independence.10 Response rate varied by cytogenetic pattern and was highest among patients with a chromosome 5q31.1 deletion (91%) compared to a normal karyotype (68%) or other chromosome abnormality (17%) [P = 0.009]. Similarly, patients with lower risk IPSS categories experienced a higher frequency of erythroid response compared to patients with higher risk disease (72% vs 25%); however, few patients had Intermediate-2 or High-risk disease (n = 4). Unlike cytokine therapy, cytogenetic remissions were common, with 65% of informative patients experiencing 50% or greater reduction in abnormal metaphases, including 10 (57%) complete cytogenetic remissions. Major cytogenetic response occurred most commonly in patients with a chromosome 5q31.1 interstitial deletion (9 of 11 patients). Perhaps of greater importance, responses appear durable. After a median follow-up of 81 weeks, median duration of transfusion-independence had not been reached (48+; range, 13+ to > 101 weeks) with median sustained hemoglobin of 13.2 g/dL (range, 11.5–15.8 g/dL). Neutropenia (67%) or thrombocytopenia (57%) > grade 3 NCI-CTC was the most common adverse event and was dose dependent, necessitating treatment interruption or dose-reduction in 61% of patients. Lenalidomide has completed multicenter Phase II trials in transfusion-dependent patients with Low- or Intermediate-1 risk MDS and either chromosome 5q31.1 deletion (n = 148) or other karyotypic abnormalities (n = 215). The results of the trial, if sufficiently encouraging, are expected to undergo accelerated review by the US Food and Drug Administration (FDA) and may secure a new position in the management of ineffective erythropoiesis for patients with MDS.
Small molecule inhibitors of the VEGF receptor tyrosine kinases (RTK) have had limited investigation in MDS. SU5416 (Sugen Inc, S. San Francisco, CA), represents the only agent of its class to complete Phase II investigation. Like most RTK antagonists, specificity is relative, with activity extending to other type III receptors such as those for the PDGFβ, FLT3, and c-kit ligands. A multicenter trial involving patients with higher-risk MDS or AML yielded minimal reduction in leukemia burden and a corresponding degree of hematological benefit despite increased apoptotic index in the myeloblast population.11,12 Clinical development of this agent was limited by its insolubility and requirement for twice weekly intravenous administration. Investigation of the orally bioavailable analogue SU11248 in patients with AML ended prematurely owing to limiting nonhematological organ toxicities.13 Despite the disappointing early results of this class of agents in myeloid malignancies, clinical investigation of potent and orally active receptor antagonists continues. The Cancer and Leukemia Group B (CALGB) is investigating PTK787 (Novartis, East Hanover, NJ) in patients with low- and higher-risk MDS using a once daily administration schedule.
Arsenic trioxide (Trisenox™, Cell Therapeutics Inc., Seattle, WA) has broad biological properties that derive from its ability to bind covalently and deplete cellular sulfhydryl-rich proteins such as glutathione, as well as anti-angiogenic properties. Arsenic in its trivalent form inhibits glutathione peroxidase to potentiate peroxide generation, disrupt mitochondrial respiration and mitochondrial membrane integrity, repress anti-apoptotic proteins and initiate caspase-mediated apoptotic response.14 In MDS and AML, the antiproliferative effects of ATO relate in part to its ability to suppress myeloblast elaboration of VEGF-A and its direct cytotoxicity to neovascular endothelium.15 Not surprisingly, bone marrow specimens from patients with MDS, which natively harbor lower glutathione reserves compared to normal hematopoietic progenitors,16 also demonstrate increased apoptotic susceptibility to ATO that is enhanced by granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulation.17
Preliminary results of three clinical trials indicate that ATO has modest activity in both lower- and higher-risk MDS.18– 20 The doses and schedules applied in these studies vary, ranging from monthly cycles of two sequential weekly treatments of 0.25 mg/kg/day for 5 days followed by a 2-week treatment hiatus to a dose-intense induction with 0.30 mg/kg/day for 5 days followed by 0.25 mg/kg/day twice weekly maintenance for 15 weeks. Overall, approximately 20%–25% of patients have experienced hematological improvement, with few complete or partial remissions. Although erythroid responses predominate, hematologic benefit is not limited to the erythroid lineage and responses may be sustained for prolonged periods after treatment cessation. Given the manageable toxicity of ATO, combination trials are in progress, which may build upon the results obtained with monotherapy.
Other anti-angiogenic agents investigated in MDS have demonstrated either a more limited toxicity:benefit profile for extended use or have not as yet completed clinical investigation. Bevacizumab (Avastin™, Genentech, S. San Francisco, CA), a recombinant humanized monoclonal antibody that neutralizes VEGF-A in vivo, is currently completing Phase II investigation in MDS.21
Farnesyl Transferase Inhibitors
Activating point mutations of the RAS proto-oncogene are detected in fewer than 20% of unselected patients with MDS but are common in CMML.1 The RAS gene superfamily encodes guanosine triphosphate hydrolases (GTPase) that serve as critical regulatory elements in signal transduction, cellular proliferation and maintenance of the malignant phenotype. Farnesylation of carboxy-terminal consensus sequences by farnesyl protein transferase (FPT) represents the first and rate limiting post-translational modification of Ras-GTPases that is requisite for membrane association and transforming activity.22
The farnesyl transferase inhibitors (FTI) represent a novel class of potent, oral inhibitors of Ras and other prenylation-dependent proteins. These agents are able to modulate multiple signaling pathways that have been implicated in the pathobiology or progression of CMML and MDS in the absence of salvage isoprenylation pathways. Preliminary results of Phase I/II studies in MDS and CMML indicate promising hematopoietic promoting activity that extends to non-erythroid lineages.23–,25 Tipifarnib (R115777, Zarnestra™; Janssen Pharmaceuticals, Beerse, Belgium, and Spring House, PA) and lonafarnib (SCH66336 or Sarasar™; Schering-Plough Research Institute, Kenilworth, NJ) are the leading non-peptide, heterocyclic oral FTIs that have completed Phase I and II clinical studies in hematological malignancies.23–,25 In Phase I and II trials of tipifarnib performed in patients with MDS or AML, treatment with 300–600 mg bid for 21 days every 4–6 weeks, yielded partial or complete responses in 20%–30% of patients, without relation to RAS mutation status.23,24,26 Interim results of a multicenter Phase II trial involving patients with either advanced MDS or elderly patients with AML who were not candidates for conventional chemotherapy induction showed promising clinical benefit. Among 98 evaluable patients treated with 600 mg bid for 21 days every 4–6 weeks, 21% achieved a complete remission (CR), whereas 44% experienced either a CR, partial remission or hematologic improvement with a median duration or remission approaching 5.5 months.26 Given the favorable treatment-related mortality (7%) compared to induction chemotherapy, this novel class of agents may create a new paradigm for the treatment of advanced MDS and AML in the elderly. Importantly, the activity of this class of agents cannot be ascribed solely to the inhibition of constitutively active RAS proteins.
Efficacy and safety studies of lonafarnib administered in a continuous schedule have shown a comparable frequency of hematologic improvement but a somewhat lower apparent frequency of CR.25 Toxicity profiles also differ with diarrhea and hypokalemia limiting at 300 mg twice daily. In an expanded Phase II trial in 67 patients with MDS or CMML, erythroid responses were reported in 35%, platelet responses in 22% of thrombocytopenic patients, and a 50% or greater reduction in blast percentage was observed in 43% of patients with excess blasts. A Phase III randomized trial is planned to investigate the clinical benefit and frequency of platelet response to lonafarnib in patients with CMML or advanced MDS with severe thrombocytopenia. Interestingly, 3 patients with proliferative CMML (WBC > 12,000/μL) experienced rapid and sustained leukocytosis, which in 2 cases was complicated by pulmonary infiltrates that resolved either after study drug withdrawal or treatment with dexamethasone.27 The latter findings closely resemble the leukemia differentiation syndrome reported with retinoid therapy for APL and may be linked to the unique ability of lonafarnib and perhaps other FPT inhibitors to activate β-1 and β-2 integrins and promote both heterotypic and homotypic adhesion of CMML cells.28 Overall, the frequency of leukemoid response to lonafarnib treatment was higher in patients with proliferative (≥ 12,000/μL) compared to nonproliferative CMML (54% versus 11%; P = 0.025). Close clinical monitoring of patients with proliferative variants receiving FTI treatment may be warranted, with consideration for early introduction of cytoreductive therapy.
Imatinib (Gleevec™)
Constitutive Ras/mitogen-activated protein kinase (MAPK) activation is demonstrable in 40%–60% of CMML cases, resulting either from mutations within RAS alleles or from reciprocal translocations deregulating RTKs.22,29 In the absence of mutations, sustained activation of the Ras/MAPK cascade may occur through a constitutive upstream signal. Perhaps the most important therapeutic discovery in the management of CMML in recent years is the activity of imatinib in patients harboring a reciprocal chromosome translocation involving chromosome 5q33. Although a number of chromosomes and genes may partner in the gene rearrangements, the clinical phenotype is distinct, recognized by the WHO classification as CMML with eosinophilia (CMML–Eos), but arising from the generation of novel fusion genes involving the PDGFβ receptor with constitutive RTK signaling.1,30,31 Transgenic mouse models have shown that these novel RTK fusion genes are singularly responsible for the generation of these myeloproliferative disorders and are selectively responsive to PDGFβ kinase inhibitors.30 Imatinib binds to the ATP-binding pocket of the PDGFβ receptor analogous to its interaction with BCR/ABL to act as a potent inhibitor of receptor kinase activity. Among 5 patients reported to date, each achieved rapid hematological control and sustained complete cytogenetic remission with imatinib monotherapy.31
Pharmacologic Differentiators
The development of pharmacologic inducers of hematopoietic differentiation in MDS has been limited by the challenge of identifying relevant cellular targets whose function can be modified by synthetic small molecules. TLK199 (Telintra™, Telik, San Francisco, CA), a novel liposomal glutathione derivative that promotes granulopoiesis both in vitro and in animal models, has recently entered investigations in MDS. TLK199 is the tripeptide diethylester, gamma-glutamyl ethyl ester (S-benzyl)cysteinyl-R(-)-phenylglycyl ethyl ester hydrochloride and a selective inhibitor of glutathione S-transferase P1-1 (GST P1-1), a member of a family of enzymes that until recently were believed to exclusively function in cellular defense and drug detoxification.32,33 Recent investigations indicate that GST P1-1 is a negative growth regulator, inhibition of which promotes the proliferation and differentiation of myeloid precursors. TLK199 undergoes intracellular de-esterification to the active diacid form, TLK117, and is released to inhibit GST P1-1 and activate the MAPK pathway. This inhibition is believed to be responsible for its differentiation-promoting activity. Indeed, in animal models, TLK199 accelerates myeloid recovery from chemotherapy-induced neutropenia as well as the myeloid growth factor G-CSF. Preliminary results of a Phase I/II trial in MDS have shown hematologic improvement in two or more lineages in 5 of 16 evaluable patients.34 While investigations with this agent continue, development of orally bio-available analogs is being explored.
III. Chromatin Remodeling and Epigenetic Therapy in the Myelodysplastic Syndrome
Jean-Pierre J. Issa, MD*
University of Texas M.D. Anderson Cancer Center, Dept. of Leukemia, 1515 Holcombe Blvd., Box 428, Houston TX 77030 The author wishes to thank Drs. Guillermo Garcia-Manero and Hagop M. Kantarjian for their significant contributions to this paper.
Chromatin remodeling is a powerful mechanism of regulating gene expression and protein function. In extreme states, chromatin remodeling can permanently repress expression of a gene, a situation termed epigenetic silencing. Such silencing is exploited by cancers to fully express the malignant phenotype. Reversal of silencing (epigenetic therapy) is an achievable goal in the clinic, and a promising new modality of treatment in MDS and other hematologic malignancies.
Chromatin Remodeling and the Power of Epigenetics
We are what our genes say we are. This is, for the most part, true. However, an added level of complexity to the physiologic functioning of multicellular organisms resides in chromatin control—specifically epigenetics.1,2 DNA normally exists in a complex configuration with proteins such as histones. These protein-DNA interactions mediate packaging of DNA from ultra compact (the visible chromosomes during mitosis) to most relaxed (the fine chromatin observed under the microscope in immature cells). The level of packaging helps determine the expression status of DNA.
Epigenetics refers to stable changes in gene expression that are mitotically stable and reversed only under special situations such as embryogenesis.1 Epigenetic silencing, then, refers to nearly irreversible loss of gene expression. This drastic mechanism of gene regulation is normally reserved for exceptional situations such as the inactive X-chromosome in women and a few genes whereby only one of the two copies of the gene is expressed depending on the parent of origin, a process termed imprinting. Epigenetic silencing, though used for a limited number of genes, is essential for the normal development of mammalian cells.2
The mechanisms of chromatin remodeling for epigenetic purposes have been the subject of intense investigation. In mammals, two molecular mechanisms are key to the process—DNA methylation and histone modifications.1,3 Methylation is mediated by the biochemical addition of a CH3 group to various molecules. Methylation can affect DNA, and the cytosine base is a specific physiological target in mammalian cells. DNA methylation plays important roles in development and differentiation and, over evolution, is thought to have been essential in suppressing the harmful effects of the myriad of retrotransposons (“jumping genes”) that litter the human genome. Studies of the inactive X-chromosome established DNA methylation in promoter regions as key to maintaining epigenetic silencing. This is now thought to be achieved through tight interactions between DNA methylation and histone modifications.2 This silencing cascade4 involves binding of methylated-DNA binding proteins (e.g., MeCP2) to the modified promoters, followed by recruitment of histone deacetylases, histone methylases, and eventually, a silencing complex of proteins including heterochromatin protein 1 (HP1). By this mechanism, chromatin is remodeled such that it becomes “invisible” to transcription factors, achieving a stable silenced state.
DNA Methylation in MDS
Epigenetic processes, while required for development, are so drastic that they are not used for the dynamic regulation of gene expression. However, over the past 15 years, it has become apparent that cancer cells usurp the process of DNA methylation and use it to their advantage by silencing the expression and function of genes that counteract the malignant phenotype, such as tumor-suppressor genes.5 Data from a variety of tumor types has clearly established that gene silencing associated with promoter DNA methylation is as powerful as gene mutations in functionally inactivating genes. It is used in cancer cells to affect most pathways required for transformation such as proliferation, apoptosis, angiogenesis, invasion, and immune evasion. Recent experiments have shown that epigenetic reprogramming by nuclear transplantation erases the malignant phenotype in some cell lines despite the persistence of genetic changes,6 demonstrating that epigenetic abnormalities are full participants in malignant conversion.
Hematologic malignancies also demonstrate a link between methylation and the neoplastic phenotype. Leukemias and MDS are characterized by the hypermethylation and silencing of multiple genes.7 This process can occur early and has been detected in cases of low-risk MDS but, in general, it is associated with disease progression. In MDS, for example, the cyclin-dependent kinase inhibitor P15 is a frequent target of aberrant methylation, and its inactivation is associated with an increased risk of progression to AML.8 A number of other genes are similarly affected, included CDH1, CDH13, RIL, and others. There are data suggesting that aberrant methylation is associated with resistance to chemotherapy in AML9 and acute lymphocytic leukemia (ALL),10 and it is likely to play a similar role in MDS.
DNA Methylation Inhibitors
The discovery that hypermethylation contributes to the malignant process has rekindled interest in DNA methylation inhibition as a therapeutic strategy (“epigenetic therapy”) in cancer.11 Two cytosine analogs, 5-azacytidine (AZA) and 5-aza-2′-deoxycytidine (DAC) were found 25 years ago to specifically inhibit DNA methylation by trapping DNA-methyltransferases (MTases).12 AZA can incorporate into RNA and also is a pro-drug of DAC. DAC is phosphorylated by deoxycytidine kinase and incorporates efficiently into DNA. MTases, upon encountering DAC, form irreversible covalent bonds with the incorporated base and are then targeted for degradation in the proteosome. Cells then divide in the absence of MTases, which results in progressive DNA hypomethylation and reactivation of previously silenced genes. The covalent binding of MTases to DNA can also result in cytotoxicity at high doses of DAC and/or high levels of MTases.13 Both AZA and DAC were found to be active in vitro against a variety of transformed cell lines, with particular efficacy in hematologic malignancies.
AZA
Clinical trials with azacytidine were initiated two decades ago and revealed efficacy at high doses in acute myelogenous leukemia and at lower doses in MDS.11 A Phase III randomized study comparing AZA to supportive care in treatment-naïve MDS at various stages demonstrated response rates of 60% in the AZA arm (CR 7%, partial remission [PR] 16%, hematologic improvement [HI] 37%) compared to 5% in the supportive care arm (P < .001).14 These relatively durable responses (median 15 months) translated into an improved quality of life and a prolongation of median time to leukemic transformation or death from 13 months in the supportive care arm to 21 months in the AZA arm. Side effects were relatively modest, consisting primarily of myelosuppression. These results led to the recent FDA approval of AZA for the treatment of MDS.
DAC
DAC is more active than AZA in vitro at equimolar doses and may have a different spectrum of activity and side effects compared to AZA because it does not incorporate into RNA.11 Clinical trials with DAC were also initiated two decades ago and revealed promising efficacy in hematologic malignancies. Phase II studies of DAC in MDS revealed promising response rates of around 50% (CR rate around 20%), including cytogenetic responses, and minimal nonhematologic toxicity.15 These results led to a multi-institution Phase III study of DAC compared to supportive care in MDS, the results of which will be announced at the 2004 ASH meeting. Data recently made public (but not subjected to peer review) suggested an improved time to AML or death in the DAC arm compared to the supportive care arm, particularly in treatment-naïve patients (354 days vs 189 days, P = .03) and high-risk MDS (260 days vs 79 days, P = .001).
Other DNA Methylation Inhibitors
Favorable results with AZA and DAC led to intense recent interest in identifying additional MTase inhibitors, particularly orally available ones, or molecules that do not require DNA incorporation. A number of interesting approaches have been described, including antisense and RNA interference approaches,16 the identification of Procainamide as a weak MTase inhibitor,17 the suggestion that a green tea component might inhibit DNA methylation indirectly,18 and the recent discovery that Zebularine, an inhibitor of cytidine deaminase, also inhibits MTases.19 Clinical trials with these agents have either not shown promising results or not been initiated yet.
Moving Forward
The emerging field of hypomethylation therapy has raised many interesting and occasionally crucial issues in the treatment of malignancies.
Dose
Given that DAC has dual activity (hypomethylating at low doses, cytotoxic at high doses), the issue of optimal dosing of this agent (or its congener AZA) needs to be reevaluated. Here, the classical maximally tolerated dose (MTD) route to drug development is not indicated, and may have hindered the full evaluation of these drugs. Indeed, a recent study of low-dose DAC reported favorable responses at doses 10–30 times lower than the MTD, with a suggestion of loss of response at higher doses.20 Also, the best reported responses with AZA and DAC are at relatively low doses, developed specifically for the treatment of MDS, where most patients are older and tolerate cytotoxic therapy poorly. Correlative studies suggest that the in vitro observation of rapid saturation of the hypomethylation effect (and loss of the differentiation effect) with increasing doses21 is also true in vivo. Current studies are exploring optimal dosing schedules for both AZA and DAC.
Mechanism of response
It is not entirely clear whether responses to DAC and AZA are related to hypomethylation or cytotoxicity. The observation of decreasing responses with increasing dose favor hypomethylation,20 but this issue is far from completely settled. Moreover, even if hypomethylation is the mechanism mediating responses, events downstream of hypomethylation remain to be defined. Loss of methylation of the p15 tumor-suppressor gene was observed in patients with MDS treated with DAC,22 but p15 hypomethylation did not correlate with response in a separate study.20 Possibilities include direct cell death signaling by hypomethylation (perhaps through reactivation of retrotransposons), induction of differentiation, induction of senescence, induction of apoptosis through reactivation of proapoptotic molecules,23 or immune responses through modulation of tumor antigens24 or even the host’s immune system. Pharmacodynamic studies of DAC in chronic myeloid leukemia (CML) suggest little effect in the first 5 days of treatment and hypomethylation-related cell death in the second week of therapy, but the mediators of this effect remain to be clarified.
Combinations
Perhaps the most exciting prospects of this field are the opportunities to improve clinical response by using combinations of active drugs. These could be thought of in two broad categories. Combinations to improve epigenetic reactivation of silenced genes center on the mechanism of methylation-associated chromatin remodeling that has been uncovered over the past few years. Thus, combinations of DAC and histone deacetylase inhibitors are synergistic in reactivating gene expression,25 and combinations with inhibitors of methylated-DNA binding proteins or histone lysine 9 methyltransferases are also attractive possibilities. Combination therapy may also be directed at optimally exploiting gene reactivation. Indeed, DAC has been shown in vivo to sensitize cells to the effects of biologic therapy such as retinoic acid26 and to increase the expression of pro-apoptotic molecules,23 which may enhance the efficacy of classical chemotherapeutic agents. It has also been demonstrated to reverse drug resistance in selected cases.27 Clinical trials exploiting combination epigenetic therapy (e.g., DAC and a histone deacetylase inhibitor such as valproic acid) or making use of gene reactivation (e.g., DAC and all-trans retinoic acid [ATRA]) are currently ongoing.
IV. Hematopoietic Stem Cell Transplant Strategies in Patients with Myelodysplastic Syndrome and Secondary Acute Myeloid Leukemia: The Role of Reduced Intensity Conditioning Regimens
Theo de Witte, MD, PhD*, and Margriet Oosterveld, MD
University Medical Center, Geert Grooteplein 8, PO Box 9101, Nijmegen 6500 HB, The Netherlands Acknowledgments: The authors wish to thank the physicians and data managers of the transplant centers of the European Bone Marrow Transplant Group (EBMT) for contributing their data and their experience. In addition they would like to thank the EBMT data managers and statisticians for their analyses and support. Finally, they would like to thank the members of the MDS subcommittee of the Chronic Leukemia Working Party of the EBMT for their valuable contributions to the analyses and the interpretation of the data.
Allogeneic Stem Cell Transplantation
The primary curative treatment option for patients with MDS is allogeneic stem cell transplantation (SCT). Disease-free survival (DFS) ranges from 29% to 40%, with corresponding non-relapse mortality of 37% to 50% and rate of relapse ranging from 23% to 48% with an HLA-identical sibling donor.1– 5 Risk factors having an impact on the outcome of transplantation include age, disease duration, disease stage at time of transplantation, percentage of blasts in the bone marrow, the presence of cytogenetic abnormalities, the source of stem cells, the application of T cell depletion of the graft, the type of donor and the intensity of the pretransplant conditioning.
In general the results of allogeneic SCT have improved in the past decade. The European Bone Marrow Transplant Group (EBMT) analyzed the treatment outcome of patients transplanted in three periods. The 3-year survival and DFS were better in patients transplanted after 1989. This was due to a decrease in treatment-related mortality (TRM) over recent years.6 The Seattle team recently reported favorable results in patients with MDS treated with a busulphan-based regimen in which the busulphan dosage was adjusted to maintain blood levels at 800–900 ng/mL. The 3-year nonrelapse mortality was 31% (28% related donors, 30% unrelated donors) and relapse occurred in 16% of the patients with a related donor and 11% of the patients with an unrelated donor.7
An EBMT survey in 234 patients with MDS comparing marrow and G-CSF–mobilized peripheral blood stem cells (PBSC) showed a lower treatment failure when PBSC were used as stem cell source.8 Use of PBSC reduced the median duration of neutropenia and thrombocytopenia by 4 and 12 days, respectively, with a corresponding reduction in TRM (P = 0.007) except for patients with RA. Chronic GVHD was more common with PBSC (odds ratio: 1.62). The low treatment failure observed with PBSC in more advanced MDS stages suggests that a “graft-versus-MDS” effect exists and that it could be enhanced by the use of G-CSF-mobilized PBSC. The EBMT started a prospective study to compare the value of PBSC versus bone marrow stem cells in April 2004. This study also addresses the question of whether remission-induction chemotherapy should be administered to these patients prior to the transplant conditioning.
Disease stage and age
Patients with less advanced stages of MDS such as RA and RARS may profit optimally from allogeneic SCT with a myeloablative regimen, with long-term DFS in more than 50% of patients,9–,10 owing largely to the substantially lower relapse rate compared to patients with more advanced disease.2
Longer disease duration before transplantation and older age are associated with an increased risk of death after transplantation,10 thereby mandating consideration of transplant early in the course of the disease. Transplantation may be postponed in selected patients without life-threatening cytopenias and cytogenetic abnormalities. A recent analysis by the Seattle group confirmed that delayed transplantation may result in maximized overall survival for low and intermediate-1 IPSS groups.11 They hypothesized that the optimal timing of transplantation for this cohort is at the time of development of a new cytogenetic abnormality, the appearance of a clinically important cytopenia or an increase in the percentage of marrow blasts.
Data on allogeneic SCT in CMML are limited.1,12,13 Prognostic modeling shows that marrow infiltration with more than 5% monoblasts, a neutrophil count of more than 16 × 109/L and/or a monocyte count of more than 2.6 × 109/L are associated with an unfavorable prognosis and therefore patients with these features should be considered for for allogeneic SCT. In an analysis of 50 CMML patients reported to the EBMT registry, the estimated 2-year DFS was 18% with a relapse risk of 42%.13
Outcome of SCT in patients with RAEB and RAEBt is less favorable than the outcome in patients with RA(RS), due largely to a higher risk of relapse. The EBMT reported a 5-year actuarial relapse rate of 44% and 52% in 35 RAEB patients and 28 RAEBt patients.14 The Fred Hutchinson Cancer Research Center (FHCRC) reported a 49% relapse rate for patients with excess of blasts compared to 4% for patients without marrow blast elevations,12 with actuarial DFS of 31% versus 54%, respectively. Among 885 patients transplanted with an HLA-identical sibling in the EBMT registry, 3-year probability of DFS, overall survival and relapse were 36%, 41% and 36% respectively.6 Both age and disease stage had independent prognostic significance for all three end-points. A similar report by the International Bone Marrow Transplant Registry (IBMTR) in 452 recipients of HLA-identical sibling transplants performed between 1989 and 1997 confirmed the influence of young age and low percentage of marrow blasts at time of transplantation on high DFS and overall survival rates.15
In patients with secondary AML (sAML) after MDS, most European transplant centers have adopted the strategy of SCT after remission-induction chemotherapy based on the high failure rate of SCT in patients with active leukemia.2,3,16
Whether patients with advanced stages of MDS or sAML benefit from chemotherapy prior to transplantation is still unresolved. The superior outcome for patients with a lower blast percentage supports the use of chemotherapy to lower the disease burden before transplantation. Only prospective randomized studies with analyses based on the intention-to-treat principle will overcome the selection bias inherent in retrospective analyses. As noted above, the EBMT has launched such a study.
Cytogenetic abnormalities
Cytogenetic abnormalities have a major influence on the outcome after SCT. A French study3 reported a 7-year relapse rate of 83% in patients with complex anomalies. Using cytogenetic risk categories defined by the IPSS, event-free survival for the poor-risk, intermediate-risk and good-risk groups were 6%, 40% and 51%, respectively, with actuarial risk of relapse 82%, 12% and 19%, respectively.17
Therapy-related MDS/AML
A recent report from French investigators involving 70 patients with therapy-related MDS and AML18 included 34% of patients in complete remission at the time of transplantation. Two-year event-free survival, relapse and TRM rates were 28%, 42% and 49%, respectively. Only 5 of the 46 patients with active disease at the time of transplantation were long-term survivors. A large study from Seattle reported on 99 patients (47 tMDS, 52 tAML). Sixty-five patients received marrow from a family member and 34 received marrow from an unrelated donor. The probability of survival, relapse and non-relapse mortality was 13%, 47% and 78%, respectively.19
T cell depletion
T cell depletion did not influence outcome in a recent multivariate analysis performed by the IBMTR despite an increased relapse risk.15 However, an earlier, single center study, study showed a 73% DFS at 2 years after transplantation for RA with T cell–depleted grafts from HLA-identical siblings using elutriation.20
Reduced-intensity conditioning regimens
The principle of reduced-intensity conditioning (RIC) is to minimize toxicity associated with conventional myeloablative regimens and harness the graft-versus-MDS effect of the infused donor lymphocytes. RIC regimens depend largely upon intensive immune suppression either during conditioning and/or after stem cell infusion to facilitate donor engraftment and establish complete donor chimerism. Kröger et al21 reported 37 patients with MDS or secondary AML, half of whom had a related donor, who were ineligible for conventionally conditioned transplants. The reduced-intensity conditioning consisted of fludarabine, busulphan and antithymocyte globulin. Overall TRM was 27%, with significantly higher mortality in those with poor-risk cytogenetics (75% vs 29%) or with an HLA-matched unrelated donor (45% vs 12%). In total, 32% of patients relapsed, and actuarial DFS at 3 years was 38% with a median follow-up of 20 months. A Spanish study showed a TRM of only 5% after transplantation of 37 patients with MDS and AML (median age: 57 years) utilizing a regimen of fludarabine and busulphan 10 mg/kg.22 The 1-year progression-free survival was 66% with a corresponding frequency of disease-progression in patients with and without graft-versus-host disease (GVHD) of 13% (95% CI, 4%–34%) and 58% (95% CI, 36%–96%), respectively (P = 0.008). These results support the notion that a graft-versus-MDS/AML response is critical in reducing the risk of relapse after an RIC transplant.22 Stuart et al23 described the results of 91 patients with a diagnosis of MDS (n = 77) or MPD except CML (n = 14) who were conditioned with fludarabine and a single fraction of total body irradiation (2 Gy) followed by infusion of stem cells from an HLA-matched related (n = 49) or unrelated (n = 42) donor. Patients with low-risk MDS (RA, RARS, RAEB) at the time of transplant (n = 33) had an 18-month relapse rate of 32% ± 18%, resulting in overall survival rates at 18 months of 40% ± 18%. This relatively high relapse risk is in line with the observations of a recent EBMT study. The EBMT analysis showed a 54% relapse risk for the 24 patients transplanted with RIC protocols, which translated into an increased HR of 6.0 (P = 0.02) in the multivariate Cox model.24
The King’s College Hospital group from London reported more favorable results following conditioning with fludarabine, busulphan and alemtuzimab (Campath-1H) in 62 patients with MDS (24 matched sibling donors or 38 unrelated donors). One-year DFS was 61% and 59% in patients transplanted with sibling and unrelated donors, respectively. The favorable results may be explained by the low estimated 1-year TRM of 15% (5% sibling, 21% voluntary unrelated donors [VUD]), the relatively high number of patients transplanted with less than 5% marrow blasts (> 75% of the patients) at the time of transplant conditioning, the high number of patients who received donor lymphocyte infusions (67% of sibling recipients and 26% of VUD recipients) and the relatively short period of follow-up.25 No long-term surviving patients were observed in patients with progressive disease.
Martino analyzed 196 cases of MDS reported to the EBMT after transplantation with RIC regimens. In a multivariate analysis the survival and DFS were not influenced by the type of conditioning despite an increase in the risk of relapse after RIC.26 It is difficult to reconcile the contribution of RIC regimens to the improved outcome of allogeneic SCT for patients with MDS in view of the recently improved outcome of transplantation with marrow ablative regimens and the heterogeneity of the patient populations (age, co-morbidity, stage of disease). For this reason, the EBMT has launched a prospective randomized study comparing RIC regimens with standard conditioning regimens in patients with MDS older than 50 years for whom an HLA-identical sibling is available and in all age categories for patients with potential unrelated donors. Patient accrual started in April 2004.
Transplantation with alternative donors
Among patients with MDS transplanted at the FHCRC with an unrelated donor following myeloablative conditioning, 2-year DFS was 38% with a relapse rate of 28%, and non-relapse mortality of 48%.27 Both older age and longer disease duration were associated with a greater risk of death from non-relapse causes. Among 118 patients who received an SCT from an unrelated donor in the EBMT database, DFS at 2 years, relapse risk and TRM were 28%, 35% and 58%, respectively.28 The TRM was significantly influenced by age (younger than 18 years: 40%; 18–35 years: 61%; older than 35 years: 81%). Patients with more severe acute GVHD experienced a lower relapse risk, suggesting an increased graft-versus-MDS effect in these patients. The American National Marrow Donor Program (NMDP) reported an improved DFS in more recent transplantations in a cohort of 510 patients with MDS transplanted with unrelated donors. The relative risk for DFS was 1.43 (95% confidence interval: 1.01–2.01) for transplantations performed between 1988 and 1993 versus more recent transplantations.29
By comparison, among 91 patients transplanted with stem cells from genotypically non-identical related donors in the EBMT database,6 3-year DFS, survival and relapse rate were 28%, 31% and 18%, respectively. It is noteworthy that the TRM was 66%, higher than in any other type of transplantation. Table 4 shows a summary of studies published in the past years on allogeneic BMT for MDS and sAML.
Autologous Stem Cell Transplantation
For those patients lacking a suitable donor, intensive chemotherapy with AML-like schedules may be an alternative approach. Complete remission rates have improved in recent years, ranging between 15% and 65%.30–,34 Remission duration, however, is brief due to the high rate of relapse. Karyotype is the most important prognostic factor influencing DFS with a median of 16.5 months for patients with a normal karyotype compared to 4 months in those with an abnormal karyotype.32 In 1995 the Leukemia Cooperative Group of the European Organisation for the Research and Treatment in Cancer (EORTC) reported results of the first prospective multicenter study using cytarabine and idarubicin as remission-induction treatment in patients with high-risk MDS and sAML.33 There was difference in remission rates between patients with MDS (50%) and sAML patients (63%), with outcome adversely affected by an abnormal karyotype. In an analysis of 158 patients with high-risk RAEB and RAEBt and 372 AML patients with AML treated at the MD Anderson Cancer Center, remission rates were comparable for RAEB, RAEBt and AML, but EFS and overall survival were inferior in RAEB compared to AML or RAEBt.35 Multivariate analysis indicated that the poor outcome in this morphologic group was linked to disproportionate adverse prognostic features, in particular, complex cytogenetic abnormalities.
In view of the high relapse rate after chemotherapy alone, transplantation with autologous stem cells has been applied in an attempt to intensify the postremission therapy. In 1997 the EBMT reported the results of 79 patients autografted for MDS and sAML in first complete remission.36 Two-year survival, DFS and relapse rate were 39%, 34% and 64%, respectively. Patients younger than 40 years showed a significantly better DFS (39%) than patients older than 40 years (25%). In 1999 the first prospective study on autologous SCT in MDS was published.37 A complete remission was attained in 42/83 patients (51%). In 24 out of 39 patients (62%) transplantation with autologous bone marrow (ABMT: 16 patients) or peripheral blood stem cells (APSCT: 8 patients) was performed. Hematological reconstitution occurred in all autografted patients. However, this study, perhaps given its size limitations, did not confirm a faster hematopoietic recovery for peripheral blood stem cells compared to bone marrow. The median DFS of the autografted patients was 29 months from transplantation.
The results of several studies employing intensive chemotherapy with or without SCT are summarized in Table 5 . A multicenter study of the EORTC, EBMT, the Swiss Group for Clinical Cancer research (SAKK) and Gruppo Italiano Malattie Ematologiche dell’ Adulto (GIMEMA) compared the results on 159 patients who had received remission-induction chemotherapy and then were candidates for allogeneic and autologous stem cell transplantation depending on the availability of an HLA-identical sibling.38 Sixty-nine percent of the patients with a donor underwent allogeneic SCT and 49% received an autograft. The 4-year EFS was 23% for patients with a donor and 22% for patients without a donor (P = 0.66). This study suggests that patients with high-risk MDS and sAML may benefit from either allogeneic or autologous SCT. The results of this study were compared with the outcome of 215 MDS and MDS-AML patients treated at the MD Anderson Cancer Center.39 MDS patients had received varied high-dose cytarabine-containing induction regimens, and after remission continued to receive these regimens at reduced dosage for 6–12 months. Remission rates were 54% and 63% respectively (P = 0.09). Sixty-five of the EORTC patients who entered CR received a transplant in first CR. DFS in patients achieving CR was superior in the EORTC cohort, the 4-year DFS rates were 29% EORTC versus 17% MDA (P = 0.02), but the survival was not significantly different between the two study groups.
Since MDS is a clonal stem cell disorder, there remains concern regarding contamination of the graft by residual malignant cells, and residual normal stem cells are sufficient to support rapid reconstitution. However, several studies reported that patients with an abnormal karyotype can achieve a cytogenetic remission if a morphological remission is reached after chemotherapy. This is supported by murine models in which no clonal (cytogenetically aberrant) precursors were identified in NOD/SCID mice transplanted with marrow from patients with MDS more than 2 months after transplantation.40
Delforge et al reported that polyclonal primitive hematopoietic progenitors can be mobilized in patients with high-risk MDS after treatment with intensive chemotherapy.41 Clonality analysis was performed in females heterozygous for the X-linked human androgen-receptor (HUMARA) gene demonstrating a polyclonal pattern in the CD34+ cell population in 4 of 5 patients.
In a separate report involving 11 patients in CR after chemotherapy, stem cell mobilization was attempted either with G-CSF alone or with recovery from consolidation. In 7/11 patients CD34 cell yields exceeded 1 × 106/kg,42 and karyotypically normal progenitors were recovered exclusively in 6/9 patients who presented with an abnormal karyotype. In our own experience stem cell mobilization was feasible in about 50% of 24 patients in the recovery phase after chemotherapy with G-CSF.
Conclusions
Allogeneic SCT is the treatment of choice for the majority of young patients with MDS or sAML who have a histocompatible sibling. Long-term DFS can be attained if SCT is performed early in the disease course. Since transplant outcome is superior for patients with a low blast percentage, successful suppression of leukemia burden by chemotherapy may be justified prior to SCT in patients with advanced disease. A definitive answer to this long-standing question must await the results of the EBMT trial. Transplant outcome for patients with MDS remains inferior to that for de novo AML owing to the high TRM and rate of relapse. Subsequent studies must optimize selection schedule, conditioning regimens and posttransplant immunemodulation. Immunotherapy with donor lymphocyte infusions has been successful in selected cases of relapse after allogeneic SCT.43–45s RIC regimens allow allogeneic transplantation in recipients of older age or with co-morbidity, a frequent reality in the treatment of patients with MDS. Moreover, RIC allows optimal utilization of posttransplant immunomodulation with donor lymphocyte transfusions. However, the place of RIC remains to be determined since the results of conventional, bone marrow ablative regimens have improved in recent years. Prospective, randomized studies, such as that initiated by the EBMT, are necessary to elucidate the contribution of RIC regimens to the treatment of MDS patients.
For patients lacking an HLA-identical sibling, the outcome with autologous SCT appears comparable to allogeneic transplantation with donors other than HLA-identical siblings and phenotypically identical family members with lower TRM. Achievement of complete remission and harvest of a sufficient number of autologous stem cells are prerequisites for autologous SCT. For patients who fail to achieve remission, allogeneic SCT with unrelated donors remains an alternative for younger patients.
The World Health Organization (WHO) classification of myelodysplastic syndromes (MDS).
| Disease . | Blood Findings . | Bone Marrow Findings . |
|---|---|---|
| Refractory anemia (RA) | Anemia No or rare blasts < 1 × 109/L monocytes | Erythroid dysplasia only < 10% grans or megas dysplastic < 5% blasts < 15% ringed sideroblasts |
| Refractory anemia with ringed sideroblasts (RARS) | Anemia No blasts | Erythroid dysplasia only < 10% grans or megas dysplastic ≥ 15% ringed sideroblasts < 5% blasts |
| Refractory cytopenia with multilineage dysplasia (RCMD) | Cytopenias (bicytopenia or pancytopenia) No or rare blasts No Auer rods < 1 × 109/L monocytes | Dysplasia in ≥ 10% of cells in two or more myeloid cell lines < 5% blasts in marrow No Auer rods < 15% ringed sideroblasts |
| Refractory cytopenia with multilineage dysplasia and ringed sideroblasts (RCMD-RS) | Cytopenias (bicytopenia or pancytopenia) No or rare blasts No Auer rods < 1 × 109/L monocytes | Dysplasia in ≥ 10% of cells in two or more myeloid cell lines ≥ 15% ringed sideroblasts < 5% blasts No Auer rods |
| Refractory anemia with excess blasts – 1 (RAEB-1) | Cytopenias < 5% blasts No Auer rods < 1 × 109/L monocytes | Unilineage or multilineage dysplasia 5%–9% blasts No Auer rods |
| Refractory anemia with excess blasts – 2 (RAEB-2) | Cytopenias 5%–19% blasts Auer rods +/− < 1 × 109/L monocytes | Unilineage or multilineage dysplasia 10%–19% blasts Auer rods +/− |
| Myelodysplastic syndrome, unclassified (MDS-U) | Cytopenias No or rare blasts No Auer rods | Unilineage gran or mega dysplasia < 5% blasts No Auer rods |
| MDS associated with isolated del (5q) | Anemia < 5% blasts Platelets normal or increased | Normal to increased megakaryocytes with hypolobulated nuclei < 5% blasts No Auer rods Isolated del (5q) |
| Disease . | Blood Findings . | Bone Marrow Findings . |
|---|---|---|
| Refractory anemia (RA) | Anemia No or rare blasts < 1 × 109/L monocytes | Erythroid dysplasia only < 10% grans or megas dysplastic < 5% blasts < 15% ringed sideroblasts |
| Refractory anemia with ringed sideroblasts (RARS) | Anemia No blasts | Erythroid dysplasia only < 10% grans or megas dysplastic ≥ 15% ringed sideroblasts < 5% blasts |
| Refractory cytopenia with multilineage dysplasia (RCMD) | Cytopenias (bicytopenia or pancytopenia) No or rare blasts No Auer rods < 1 × 109/L monocytes | Dysplasia in ≥ 10% of cells in two or more myeloid cell lines < 5% blasts in marrow No Auer rods < 15% ringed sideroblasts |
| Refractory cytopenia with multilineage dysplasia and ringed sideroblasts (RCMD-RS) | Cytopenias (bicytopenia or pancytopenia) No or rare blasts No Auer rods < 1 × 109/L monocytes | Dysplasia in ≥ 10% of cells in two or more myeloid cell lines ≥ 15% ringed sideroblasts < 5% blasts No Auer rods |
| Refractory anemia with excess blasts – 1 (RAEB-1) | Cytopenias < 5% blasts No Auer rods < 1 × 109/L monocytes | Unilineage or multilineage dysplasia 5%–9% blasts No Auer rods |
| Refractory anemia with excess blasts – 2 (RAEB-2) | Cytopenias 5%–19% blasts Auer rods +/− < 1 × 109/L monocytes | Unilineage or multilineage dysplasia 10%–19% blasts Auer rods +/− |
| Myelodysplastic syndrome, unclassified (MDS-U) | Cytopenias No or rare blasts No Auer rods | Unilineage gran or mega dysplasia < 5% blasts No Auer rods |
| MDS associated with isolated del (5q) | Anemia < 5% blasts Platelets normal or increased | Normal to increased megakaryocytes with hypolobulated nuclei < 5% blasts No Auer rods Isolated del (5q) |
International Prognostic Scoring System (IPSS).
| *Good: normal, -Y, del(5q), del (20q); Poor: complex (≥ 3 abnormalities) or chromosome 7 anomalies; Intermediate: other abnormalities. | |||||
| Adapted from Greenberg et al, International Scoring System for Evaluating Prognosis in Myelodysplastic Syndromes.4 | |||||
| Prognostic variable | 0 | 0.5 | 1.0 | 1.5 | 2.0 |
| BM blast % | < 5 | 5–10 | — | 11–20 | 21–30 |
| Karyotype* | Good | Intermediate | — | Poor | — |
| Cytopenias | 0/1 | 2/3 | — | — | — |
| *Good: normal, -Y, del(5q), del (20q); Poor: complex (≥ 3 abnormalities) or chromosome 7 anomalies; Intermediate: other abnormalities. | |||||
| Adapted from Greenberg et al, International Scoring System for Evaluating Prognosis in Myelodysplastic Syndromes.4 | |||||
| Prognostic variable | 0 | 0.5 | 1.0 | 1.5 | 2.0 |
| BM blast % | < 5 | 5–10 | — | 11–20 | 21–30 |
| Karyotype* | Good | Intermediate | — | Poor | — |
| Cytopenias | 0/1 | 2/3 | — | — | — |
Survival and risk of AML evolution by International Prognostic Scoring System (IPSS) score.
| . | . | IPSS Risk Group . | ||
|---|---|---|---|---|
| . | Low . | Int-1 . | Int-2 . | High . |
| Score | 0 | 0.5–1.0 | 1.5–2.0 | ≥ 2.5 |
| Lifetime AML Evolution | 19% | 30% | 33% | 45% |
| Median Years to AML | 9.4 | 3.3 | 1.1 | 0.2 |
| Median Survival (years) | 5.7 | 3.5 | 1.2 | 0.4 |
| . | . | IPSS Risk Group . | ||
|---|---|---|---|---|
| . | Low . | Int-1 . | Int-2 . | High . |
| Score | 0 | 0.5–1.0 | 1.5–2.0 | ≥ 2.5 |
| Lifetime AML Evolution | 19% | 30% | 33% | 45% |
| Median Years to AML | 9.4 | 3.3 | 1.1 | 0.2 |
| Median Survival (years) | 5.7 | 3.5 | 1.2 | 0.4 |
Novel therapeutics categorized by pharmacologic class and target of interaction.
| Target and Pharmacologic Class . | Agent . |
|---|---|
| Survival signals | |
| Anti-Angiogenic | thalidomide (Thalomid™) CC5013 (lenalidomide, RevliMid™) bevacizumab (Avastin™) arsenic trioxide (Trisenox™) |
| Receptor Tyrosine Kinase Inhibitors | PTK787 imatinib mesylate (Gleevec™) |
| Protein Kinase C Inhibitor | PKC412 |
| p38αMAPK Inhibitor | SC10469 |
| Matrix Metalloprotease Inhibitor | AG3340 (Prinomastat™) |
| Farnesyl transferase Inhibitors | R115777 (tipifarnib, Zarnestra™) SCH66336 (lonafarnib, Sarasar™) |
| Pharmacologic Differentiators | TLK199 (Telintra™) |
| Target and Pharmacologic Class . | Agent . |
|---|---|
| Survival signals | |
| Anti-Angiogenic | thalidomide (Thalomid™) CC5013 (lenalidomide, RevliMid™) bevacizumab (Avastin™) arsenic trioxide (Trisenox™) |
| Receptor Tyrosine Kinase Inhibitors | PTK787 imatinib mesylate (Gleevec™) |
| Protein Kinase C Inhibitor | PKC412 |
| p38αMAPK Inhibitor | SC10469 |
| Matrix Metalloprotease Inhibitor | AG3340 (Prinomastat™) |
| Farnesyl transferase Inhibitors | R115777 (tipifarnib, Zarnestra™) SCH66336 (lonafarnib, Sarasar™) |
| Pharmacologic Differentiators | TLK199 (Telintra™) |
Allogeneic stem cell transplantation for myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (sAML).
| Source . | Number of Patients . | Median Age (years) . | Outcome Calculated at N yrs. . | DFS or EFS (%) . | Relapse (%) . | TRM (%) . |
|---|---|---|---|---|---|---|
| # Transplantation for therapy-related MDS and AML (tMDS/tAML) | ||||||
| Notes: Nevill 1998: including 22 unrelated donors; Deeg 2002: age 46 yr overall; Anderson 1997: including 17 tAML and 29 sAML patients; Yakoub: including 8 unrelated donors, 3 mismatched related donors; Martino 2002: including 17 AML patients | ||||||
| Abbreviations: DFS, disease-free survival; EFS, event-free survival; TRM, treatment-related mortality | ||||||
| HLA-Identical Sibling | ||||||
| Anderson 1995 12 | 93 | 30 | 5 | 40 | 29 | 44 |
| Sutton 1996 3 | 71 | 37 | 7 | 32 | 48 | 39 |
| Runde 1998 14 | 131 | 33 | 5 | 34 | 39 | 44 |
| Nevill 1998 13 | 60 | 40 | 7 | 29 | 42 | 50 |
| De Witte 2000 6 | 885 | 33 | 3 | 36 | 36 | 43 |
| Sierra 2002 15 | 452 | 38 | 3 | 40 | 23 | 37 |
| Deeg 2002 7 | 41 | 46 | 3 | 56 | 16 | 28 |
| Anderson 1997 16# | 46 | 42 | 5 | 24 | 31 | 44 |
| Yakoub-Agha 2000 18# | 70 | 37 | 2 | 28 | 42 | 49 |
| Voluntary Unrelated Donor | ||||||
| Anderson 1996 27 | 52 | 33 | 2 | 38 | 28 | 48 |
| Arnold 1998 28 | 118 | 24 | 2 | 28 | 35 | 58 |
| De Witte 2000 6 | 198 | — | 3 | 25 | 41 | 58 |
| Deeg 2002 7 | 64 | 46 | 3 | 59 | 11 | 30 |
| Castro-Malaspina 2002 29 | 510 | 38 | 2 | 29 | 14 | 54 |
| Reduced Intensity Conditioning | ||||||
| Martino 2002 26 | 37 | 57 | 1 | 66 | 28 | 5 |
| Ho 2004 25 | 24 | 56 | 1 | 61 | 5 | |
| Ho 2004 25(VUD) | 38 | 52 | 1 | 59 | 21 | |
| Source . | Number of Patients . | Median Age (years) . | Outcome Calculated at N yrs. . | DFS or EFS (%) . | Relapse (%) . | TRM (%) . |
|---|---|---|---|---|---|---|
| # Transplantation for therapy-related MDS and AML (tMDS/tAML) | ||||||
| Notes: Nevill 1998: including 22 unrelated donors; Deeg 2002: age 46 yr overall; Anderson 1997: including 17 tAML and 29 sAML patients; Yakoub: including 8 unrelated donors, 3 mismatched related donors; Martino 2002: including 17 AML patients | ||||||
| Abbreviations: DFS, disease-free survival; EFS, event-free survival; TRM, treatment-related mortality | ||||||
| HLA-Identical Sibling | ||||||
| Anderson 1995 12 | 93 | 30 | 5 | 40 | 29 | 44 |
| Sutton 1996 3 | 71 | 37 | 7 | 32 | 48 | 39 |
| Runde 1998 14 | 131 | 33 | 5 | 34 | 39 | 44 |
| Nevill 1998 13 | 60 | 40 | 7 | 29 | 42 | 50 |
| De Witte 2000 6 | 885 | 33 | 3 | 36 | 36 | 43 |
| Sierra 2002 15 | 452 | 38 | 3 | 40 | 23 | 37 |
| Deeg 2002 7 | 41 | 46 | 3 | 56 | 16 | 28 |
| Anderson 1997 16# | 46 | 42 | 5 | 24 | 31 | 44 |
| Yakoub-Agha 2000 18# | 70 | 37 | 2 | 28 | 42 | 49 |
| Voluntary Unrelated Donor | ||||||
| Anderson 1996 27 | 52 | 33 | 2 | 38 | 28 | 48 |
| Arnold 1998 28 | 118 | 24 | 2 | 28 | 35 | 58 |
| De Witte 2000 6 | 198 | — | 3 | 25 | 41 | 58 |
| Deeg 2002 7 | 64 | 46 | 3 | 59 | 11 | 30 |
| Castro-Malaspina 2002 29 | 510 | 38 | 2 | 29 | 14 | 54 |
| Reduced Intensity Conditioning | ||||||
| Martino 2002 26 | 37 | 57 | 1 | 66 | 28 | 5 |
| Ho 2004 25 | 24 | 56 | 1 | 61 | 5 | |
| Ho 2004 25(VUD) | 38 | 52 | 1 | 59 | 21 | |
Intensive chemotherapy with or without autologous stem cell transplantation in myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (sAML).
| Source . | Number of Patients . | Median Age (years) . | Induction Chemotherapy . | CR (%) . | Number of Patients Transplanted . | Outcome # . |
|---|---|---|---|---|---|---|
| #: Outcome: median duration in months or percentage at 2 years or 4 years | ||||||
| ##: 3 allogeneic BMT, 3 autologous stem cell transplantation | ||||||
| ###: Report on 79 patients transplanted in first CR | ||||||
| *: DFS and OS with or without fludarabine respectively | ||||||
| **: 65 patients without HLA-identical sibling donor | ||||||
| Abbreviations: CR, complete remission; A, cytarabine; Z, zorubicine; I, idarubicin; M, mitoxantrone; Q, quinine; E, etoposide; G-CSF, granulocyte colony-stimulating factor; DFS, disease-free survival; OS, overall survival; EFS, event-free survival; RAEB, refractory anemia with excess blasts; RAEBt, RAEB in transformation | ||||||
| Ossenkoppele 2004 30 | MDS 91 AML 43 | 65/69 | A + G-CSF + F | 68 | — | DFS 23/16% OS 39/24%* |
| Fenaux 1991 32 | MDS 31 sAML 16 | 54 | Z + A | 47 | — | DFS: 11 mo OS: 14 mo |
| De Witte 1995 33 | MDS 34 sAML 16 | 46 | I + A | 54 | — | DFS: 11 mo OS: 15 mo |
| Parker 1997 34 | MDS 13 sAML 3 | 44 | I + A + F + G-CSF | 63 | 6 ## | Too short follow-up |
| Estey 1997 35 | MDS 158 | 60 | I + A or F + A ± G-CSF | 65 | — (RAEB/RAEBt) | DFS : 5–12 mo |
| De Witte 1997 36### | MDS 19 sAML 60 | 39 | relapse: 64% | — 79 DFS: 34% OS: 39% | ||
| Wattel 1999 37 | MDS 37 sAML 46 | 45 | A + M ± Q | 51 | 24 | DFS: 29 mo OS: 33 mo |
| Oosterveld 2003 38 | MDS 91 sAML 28 | 47 | A + I + E | 54 | 32/65*** | EFS: 23* |
| Source . | Number of Patients . | Median Age (years) . | Induction Chemotherapy . | CR (%) . | Number of Patients Transplanted . | Outcome # . |
|---|---|---|---|---|---|---|
| #: Outcome: median duration in months or percentage at 2 years or 4 years | ||||||
| ##: 3 allogeneic BMT, 3 autologous stem cell transplantation | ||||||
| ###: Report on 79 patients transplanted in first CR | ||||||
| *: DFS and OS with or without fludarabine respectively | ||||||
| **: 65 patients without HLA-identical sibling donor | ||||||
| Abbreviations: CR, complete remission; A, cytarabine; Z, zorubicine; I, idarubicin; M, mitoxantrone; Q, quinine; E, etoposide; G-CSF, granulocyte colony-stimulating factor; DFS, disease-free survival; OS, overall survival; EFS, event-free survival; RAEB, refractory anemia with excess blasts; RAEBt, RAEB in transformation | ||||||
| Ossenkoppele 2004 30 | MDS 91 AML 43 | 65/69 | A + G-CSF + F | 68 | — | DFS 23/16% OS 39/24%* |
| Fenaux 1991 32 | MDS 31 sAML 16 | 54 | Z + A | 47 | — | DFS: 11 mo OS: 14 mo |
| De Witte 1995 33 | MDS 34 sAML 16 | 46 | I + A | 54 | — | DFS: 11 mo OS: 15 mo |
| Parker 1997 34 | MDS 13 sAML 3 | 44 | I + A + F + G-CSF | 63 | 6 ## | Too short follow-up |
| Estey 1997 35 | MDS 158 | 60 | I + A or F + A ± G-CSF | 65 | — (RAEB/RAEBt) | DFS : 5–12 mo |
| De Witte 1997 36### | MDS 19 sAML 60 | 39 | relapse: 64% | — 79 DFS: 34% OS: 39% | ||
| Wattel 1999 37 | MDS 37 sAML 46 | 45 | A + M ± Q | 51 | 24 | DFS: 29 mo OS: 33 mo |
| Oosterveld 2003 38 | MDS 91 sAML 28 | 47 | A + I + E | 54 | 32/65*** | EFS: 23* |
Relative percentage of various cytogenetic abnormalities in de novo myelodysplastic syndrome (MDS).
Cytogenetic abnormalities are found in 40%–70% of patients with de novo MDS and nearly 95% of patients with therapy-related MDS. This chart illustrates the relative distribution of the most common cytogenetic abnormalities in de novo MDS.
Percentage of WHO subtypes showing cytogenetic abnormalities: refractory anemia 24%, refractory anemia with ringed sideroblasts (RARS) 29%, refractory anemia with excess blasts–1 (RAEB-1) 35%, refractory cytopenia with multilineage dysplasia and ringed sideroblasts (RCMD-RS) 37%, refractory anemia with excess blasts–2 (RAEB-2) 38%.
Relative percentage of various cytogenetic abnormalities in de novo myelodysplastic syndrome (MDS).
Cytogenetic abnormalities are found in 40%–70% of patients with de novo MDS and nearly 95% of patients with therapy-related MDS. This chart illustrates the relative distribution of the most common cytogenetic abnormalities in de novo MDS.
Percentage of WHO subtypes showing cytogenetic abnormalities: refractory anemia 24%, refractory anemia with ringed sideroblasts (RARS) 29%, refractory anemia with excess blasts–1 (RAEB-1) 35%, refractory cytopenia with multilineage dysplasia and ringed sideroblasts (RCMD-RS) 37%, refractory anemia with excess blasts–2 (RAEB-2) 38%.