Abstract
Some immunologic diseases are characterized by profound loss or primary dysfunction of a given population of cells. The atypical cellular disorders discussed here all bear some similarities in that abnormal proliferations of lymphocytes and macrophages or dendritic cells result in lymphadenopathy, skin rashes, bone lesions and infiltrations of nearly any other organ system. What are the similarities and the differences between Langerhans cell histiocytosis (LCH), sinus histiocytosis with massive lymphadenopathy (SHML) or Rosai-Dorfman disease, and Castleman’s disease (CD)? Studies on LCH have some advantages since it was described before the others, and organized clinical trials have been done since the 1980s. The understanding of SHML benefited from a registry maintained by Drs. Rosai and Dorfman. CD was described fifty years ago and for one subtype has the most clearly defined etiology (HHV-8 infection) of the three atypical cellular disorders discussed here.
In Section I, Dr. Kenneth McClain examines the unanswered question of whether LCH is a malignant clonal disorder or an inflammatory response triggered by aberrant cytokine expression or a virus. Advocates of the malignant proliferation theory rest their case primarily on the following two points: Clonality of the CD1a+ Langerhans cells was demonstrated by analysis of the human androgen receptor in patients with single bone lesions (Low Risk) or multisystem disease including spleen, liver, bone marrow, or lung (High Risk). Although no consistent chromosomal abnormalities have been reported, loss of heterozygosity (LOH) has been defined by comparative genomic hybridization. Those in the “inflammatory response” camp note that non-clonal proliferation of Langerhans cells in adult pulmonary LCH also have LOH by the same method. The pathologic cells have not been successfully grown in culture or immune-deficient mice and don’t have a “malignant” morphology. While the basic scientific arguments continue, important advances in the treatment of LCH have been made by international collaborations of the Histiocyte Society. Risk groups have been clearly defined and the response to therapy after the initial 6 weeks is known to be the strongest prognostic variable for outcome.
In Section II, Dr. Yasodha Natkunam reviews the features of SHML, which most often presents as painless cervical lymphadenopathy, although many patients can have extranodal involvement as well. These sites include the skin, respiratory tract, bone, lung, gastrointestinal tract, and brain. The diagnosis rests on finding intact lymphocytes in the cytoplasm of activated macrophages as well as accumulation of mature plasma cells. Hemolytic or non-hemolytic anemias, hypergammaglobulinemia, and elevated erythrocyte sedimentatin rate (ESR) are often found with SHML. An intriguing finding of human herpesvirus (HHV)-6 viral proteins in SHML has been reported in several patients, but needs further study. SHML associated with lymphoproliferations triggered by defects in apoptosis are discussed since this mechanism may provide a clue to the etiology. Therapy for SHML varies greatly in reported case series. Many patients have spontaneous regression or resolution after surgical removal of isolated node groups. Others with systemic involvement may benefit from chemotherapy, but no clinical trials have been done.
In Section III, Dr. Steven Swerdlow clarifies key features of the four types of CD. Localized cases are divided into the hyaline vascular type and plasma cell type. Both are usually cured by surgical excision and have symptoms mainly of a mass lesion, although the latter often also has constitutional symptoms. The two types are distinguished largely by the nature of the follicles and the number of interfollicular plasma cells. Interleukin (IL)-6 expression is increased in the plasma cell type. Multicentric CD of the plasmablastic type is most often found in HIV-positive patients with coincident HHV-8 infection. Many have lymphomas or Kaposi sarcomas. Other cases of multicentric CD are also most like the plama cell type, however, with disseminated disease and constitutional symptoms. A wide variety of anti-neoplastic drugs, radiation therapy, anti-IL-6 and rituximab or atlizumab have been used with varying success in patients with multicentric CD. Clinical trials are needed for SHML and CD and registration of adult and pediatric patients on current LCH trials are encouraged.
I. Langerhans Cell Histiocytosis: What Is the Orphan Telling Us?
Kenneth L. McClain, MD, PhD*
Texas Children’s Cancer Center/Hematology Service, Baylor College of Medicine, 6621 Fannin St. (MC 1510.00), Houston TX 77030
Orphan diseases such as Langerhans cell histiocytosis (LCH) can be frustrating to physicians and patients alike when there is apparently little direction for treatments or data about biology. This review will illustrate some major advances in our understanding of LCH, but also reveal the many gaps in our knowledge. Historical eponyms for LCH have suggested a variety of diseases, but the unifying designation LCH is preferred. Modern treatment results from collaborative studies have shown increased therapeutic options and solid reasons for systemic treatment in many children and probably adults also. However, therapeutic trials for adults are just being initiated, so hard data are not available. More importantly, the role of extensive surgery or radiation therapy has diminished and some new “biologic” therapies are being used or are on the horizon. Late effects of LCH are now better documented and these provide additional reasons to not assume that this is a benign disorder that “will just go away by itself.” The biology of LCH remains complicated with the controversy about it being a clonal, e.g., malignant, disorder versus a reactive immunologic response still unsettled.
LCH Biology
Langerhans cells (LC) were identified over 140 years ago at the dermal/epidermal border of the skin and subsequently as the most efficient antigen-presenting cell of the dendritic cell family. Nezelof et al proved in 1973 that the LC were the principal cells of LCH lesions, although lymphocytes, eosinophils and macrophages are present also. For decades most investigators have assumed the LC was the “pathologic” cell, but there is increasing evidence that the other cells may be playing extremely important roles in controlling the behavior of LC. Geissmann et al found that the LC in bone, lymph node, and some skin lesions were immature dendritic cells lacking expression of CD83, CD86, or DC-Lamp and had intracellular major histocompatibility complex (MHC) class II antigen.1 When cells were isolated from the lesions and incubated with CD40L they gained the maturation markers listed above and were better able to function in an allogeneic lymphocyte proliferation assay. Many macrophages in bone or lymph node lesions expressed interleukin (IL)-10, but this was not found in skin lesions. The authors hypothesized that IL-10 as well as tumor growth factor (TGF)β could be key factors inhibiting the maturation of LC. IL-10 is noteworthy since it also downregulates B7 and class II antigen expression, thus inhibiting effective antigen presentation. This is consistent with the known defect of this function of LC from LCH patients. The authors concluded that LC in LCH are not intrinsically malfunctioning but are heavily influenced by the microenvironment. Egeler and colleagues had earlier identified the types of cells producing many of the cytokines in the microenvironment.2 They found LC, macrophages, and T cells produced granulocyte-macrophage colony-stimulating factor (GM-CSF) and interferon-γ; IL-1α came exclusively from LC, T cells produced IL-2, IL-4, IL-5 and tumor necrosis factor (TNF)-α; IL-10 came from LC and macrophages, and eosinophils produced IL-5, interferon-γ, GM-CSF, IL-10, and IL-7. In another study this group has reported extensive expression of CD40 on LCH and CD40L on T cells. Since Geissman et al did not stain their specimens for CD40L we do not know if there could have been a deficiency in those lesions. One would expect not and thus hypothesize that pharmacologic doses of exogenous CD40L caused the LC in Geissmann’s in vitro studies to overcome the effects of other cytokines that inhibit LC maturation. The close proximity of T cells and LC suggested that the “cytokine storm” in the lesions resulted from autocrine and paracrine amplification of signals between cell types. Ultimately this would be a favorable environment for proliferation of LC (and other cell types). Not surprisingly many of the cytokines could be responsible for the features of LCH: bone osteolysis, lung necrosis, liver and lung fibrosis. Others have found similar cytokine profiles (without IL-10 expression) in adult pulmonary LCH along with increased expression of the co-stimulatory molecules CD80/86 (B7-1/B7-2).3
Additional evidence of the “immature” state of the LC and a possible mechanism for their accumulation in lesions comes from analysis of chemokines on the cell surface. CD1a+ cells express the chemokine of immature dendritic cells, CCR6, and its ligand CCL20/MIP-3α.4 Among the other cytokines expressed by LC are CCL5/RANTES and CXCL11/I-TAC, which may help recruit eosinophils and CD4+ T cells. Thus, the abnormal CD1a+ cells would remain at the site of origin and recruit T cells that are also retained by the expression of CXCR3 and CCR6. Conversely, others have found that CCR7 was also expressed on the LC, suggesting they could migrate to skin and bone or to lymph nodes.5
LCH = Malignancy?
A central controversy about LCH is whether this is a malignant or inflammatory disorder. Clonal expansion of LC, but not lesional T cells, was defined by the human androgen receptor (HUMARA) DNA assay as well as T-cell receptor analysis.6 These findings have led many aficionados of LCH to strongly state that it is a malignant proliferation. Clinical data that are considered supportive of this concept include the finding of familial cases and the higher than expected incidence of malignancies in LCH patients. Until recently there had been scant data on chromosome analysis. Now there are two papers in the literature showing molecular cytogenetic abnormalities in LCH by comparative genomic hybridization (CGH) and loss of heterozygosity (LOH) analysis.7,8 The first study evaluated 7 bone lesions and found losses of DNA sequences on chromosomes 1p, 5, 6, 9, 16, and 22q.7 There was gain of DNA copy number on 2q, 4q, and 12. The highest frequencies of LOH (3/7 cases) were found on chromosome 1p and chromosome 7 (4/7 cases). Allele loss occurred on chromosomes 9 (2 cases) and 22q (1 case). The authors noted that 1p35-p36.3 contains several putative tumor suppressor genes and 22q12 contains the neurofibromatosis type 2 gene. They hypothesized that gains of DNA at sites similar to those found in malignancies (4q and 12) could relate to presence of a proliferation sequence. The above CGH data seem supportive of the malignancy theory given that cells from bone lesions are clonal by the HUMARA assay. However, analysis of adult pulmonary LCH by HUMARA has determined that it is not a clonal proliferation. Dacic et al reported that by CGH/LOH analysis of pulmonary LCH there was allelic loss at 1 or more tumor suppressor gene loci in 80% of the nodules dissected from 14 patients using essentially the same polymorphic microsatellite markers as noted in the first CGH study.8 Given similar CGH/LOH results from clonal versus nonclonal LCH, the controversy on the malignant nature of LCH seems far from settled.
LCH = Inflammation?
For those who like the “inflammatory” disease label, the collection of morphologically normal LC along with other players in common inflammatory lesions (macrophages, lymphocytes, eosinophils) augur for a nonmalignant biology. Until recently there had been few data linking a specific viral infection to LCH. Glotzbecker et al found immunohistochemical evidence for human herpesvirus-6 in 71% of LCH biopsies.9 However, there was no serologic evidence to show that a recent infection had occurred. Given the latent nature of herpes viruses and their propensity for reactivation, the link of HHV-6 and LCH is controversial. Another argument against a transformed LC phenotype is the fact that no one has been able to propagate these cells in vitro for more than a few days or successfully maintain LCH cells in immune-deficient mice. Furthermore, the generation of “clonal proliferations” does not always signal a malignancy. Clonal proliferation of T cells in the peripheral blood of normal individuals or those with cutaneous lymphomas have been termed “clonality of uncertain significance.” The familial LCH cases are extraordinarily rare, and HLA data show that siblings of LCH patients who don’t have the disease share same HLA type.10 A study of Scandinavian LCH patients revealed immunogenetic heterogeneity (as did the US study) with the HLA-DRB1*03 phenotype present in 38% of single-system LCH and only 3% of multisystem LCH.11 It is well known that individuals with immune deficiencies have high rates of malignancies so it is difficult to use this data to argue that LCH is an a priori (pre)malignant condition. Strong TGFβ receptor expression and heterogeneous staining for MDM2, p53, and p21 in LCH lesions are thought to be evidence of a normal cellular response, not of mutational damage.12 Given the tumor suppressor actions of TGFβ, p53, and p16 it seems unlikely that all of these would be functional in a truly malignant cell. High expression of Bcl-2 in LCH lesions has been reported by more than one group and is thought to enhance cell survival. A Danish group has evaluated the expression of p53, Fas and Fas-ligand in LCH and found strong expression of p53 and some cells co-expressing Fas/Fas-ligand creating an “autocrine apoptotic shortcut.” This mechanism could possibly explain the spontaneous regression of some LCH lesions.
Treatment of LCH
The focus on clinical features and response to therapy has sharpened because of international cooperative studies of childhood LCH. Hopefully an adult trial will commence soon. The clinical classification of pediatric LCH patients has been divided between “Low Risk” sites: skin, bone, lymph nodes, and pituitary, and “High Risk” sites: lung, liver, bone marrow, and spleen. Since adult pulmonary disease is closely linked to smoking and has different biologic characteristics it should not be automatically put in the latter group. Analysis of data from the second international study for pediatric LCH (LCH-II) has shown some “Special Site” lesions of the skull (mastoid, orbit, temporal bones) that are associated with much higher frequency of diabetes insipidus and parenchymal brain lesions (see later).
Should all LCH patients receive therapy? The simple answer is no when including single skull lesions in the frontal, parietal, or occipital areas and other skeletal lesions. However, it would be a mistake to say LCH is a slowly progressing disease in which a “wait and see” approach should be taken. This is especially true of pulmonary, jaw, and skin disease of adults. Smoking cessation may be effective in some patients with lung disease, but they need to be monitored carefully since the insidious progression of cystic changes and fibrosis can rob the patient of vital lung function. Heroic surgery for jaw disease results in disfigurement and loss of teeth, whereas a 6-month course of vinblastine and prednisone can cure the disease and allow reformation of the bone with no loss of dentition. Painful and disturbing perineal ulcers of LCH in women are best treated with chemotherapy or thalidomide, not radiation.
LCH-II results helped settle some questions, but left several others for the current (LCH-III) and future studies to answer.13 LCH-II compared 24 weeks of treatment with vinblastine/prednisone to etoposide/prednisone for treatment of multisystem LCH. There was no difference in survival, number of reactivations, or toxicity between the two regimens. Thus, etoposide is no longer considered a reasonable therapeutic agent for LCH because of the known complication of causing secondary myeloid leukemia. It was concerning that 50% of patients in either treatment arm had at least one recurrence. Patients with bone, skin, and/or lymph node involvement were all eventually cured with one or two salvage regimens. Earlier trials (DAL-HX 83/90) that extended therapy to 1 year and were more intense (additional maintenance therapy with mercaptopurine plus oral methotrexate in addition to vinblastine/prednisone pulses) had only a 32% recurrence rate.14 The current LCH-III study is randomizing low-risk patients to 6 or 12 months of therapy with vinblastine/prednisone alone and treating all high-risk patients for 1 year. The randomization for high-risk patients is between vinblastine/prednisone (Arm A) vs vinblastine/prednisone/IV methotrexate (Arm B) in the first 6 weeks followed by maintenance with vinblastine/prednisone pulses every 3 weeks plus daily oral mercaptopurine for 1 year with oral methotrexate weekly for patients in Arm B (Figure 1; see Color Figures, page 503).
Two other important results of the LCH-II trial were that age was not a prognostic factor, but response at week 6 was. High-risk patients who had not improved by the sixth week had only a 17% chance of survival in contrast to the 88% survival of those who had a good initial response.
Further analysis of the patients with only bone LCH who had 1 year of therapy (DAL-HX 83/90 Trial) revealed that 18% had recurrences, with essentially all occurring in the first 2 years off therapy.14 There was no difference in the frequency of recurrence whether the patients had unifocal or multifocal bone lesions. All patients with multifocal bone disease had the multi-agent chemotherapy mentioned above with the modest recurrence rate that is strikingly better than the 50%–80% recurrence rate for patients treated with only single agent chemotherapy, radiotherapy or only observed.
Another group of patients for whom multi-agent chemotherapy treatment is definitely indicated are those with lesions in the orbit, mastoid, or temporal bones. Forty percent of these individuals will develop diabetes insipidus if they receive single agent therapy or surgery only as opposed to 20% who are treated with multi-agent chemotherapy. Patients with these bone lesions also may have parenchymal brain disease, which could be masses or infiltrative, that causes a neurodegenerative process.
Long-Term Problems and Adult LCH
Late effects of the disease and treatment occur in 50% of LCH patients, making long-term follow-up a necessity.15 These include diabetes insipidus (24%), orthopedic abnormalities (20%), hearing loss (13%) and neurologic problems (11%). The latter is characterized by cerebellar symptoms that may not be manifest until 10 or more years after diagnosis. Over 70% of multisystem patients had one or more of the late effects compared to 24% of single system patients. Another study of 59 patients with diabetes insipidus and serial magnetic resonance imaging (MRI) studies suggested that those with a thickened pituitary stalk were at higher risk of developing anterior pituitary deficiencies that ultimately occurred in 58% of all those with diabetes insipidus.16
Adults with LCH continue to have difficulty in finding physicians familiar with the disease or comfortable with choosing treatments. A survey of adult LCH patients treated at 18 different centers throughout the world revealed a median latency time to diagnosis of 4 months, but was 11 months for those initially presenting with diabetes insipidus.17 Unfortunately there are patients with pulmonary LCH who may not be diagnosed for years and then die of lung or secondary cardiac failure. Seventy-seven percent of pulmonary LCH patients were smokers compared to 44% of those with nonpulmonary LCH. Although only 16% of patients had isolated lung disease, it was present in 62% of those with multisystem disease. The optimal therapy for pulmonary LCH is not known.18 Hence, in the adult LCH trial that should open soon, we will be assessing the natural history of patients who quit smoking and then offer a short course of steroids for progressive disease. An organized trial of chemotherapy for these patients must await a better understanding of the time course of the disease. Patients with lung plus LCH in other sites who receive chemotherapy may show us if there is some benefit for treatment of nodular or progressive cystic lung changes.
Results of salvage therapy studies for LCH have been frustratingly slow coming to the literature because of insufficient accrual. Cladribine (2-CdA) has been used effectively for patients with bone disease,19 but is probably not adequate for patients with “risk organ” involvement. The addition of cytosine arabinoside with 2-CdA is being considered for the next salvage protocol. Stem cell transplants have been successful in a number of patients, but the true rate of efficacy remains unknown since the total number of attempted stem cell transplants is not available.20 Obviously, it is preferable to use this modality after only one or two salvage attempts since the patients are better able to withstand the rigors of the procedure. Antibodies to the CD1a epitope have been developed and may be available for diagnostic and therapeutic use in the future. Recently we found that the pathologic, but not normal LC express CD52 so a trial of alemtuzumab in patients refractory to standard therapy is needed.21
Protocols and information for patients and health care professional can be obtained from the Histiocytosis Association of America. Phone 856-589-6606, FAX 856-589-6614. Email: HistioSociety@aol.com; Web: www.histio.org/society.
II. Sinus Histiocytosis with Massive Lymphadenopathy (Rosai-Dorfman Disease): An Update
Yasodha Natkunam, MD, PhD*
Stanford University Medical Center, Dept. of Pathology, L235, 300 Pasteur Drive, Stanford CA 94305 Acknowledgments: I thank Drs. Ronald Dorfman, Roger Warnke, and Daniel Arber for their critical review of this manuscript.
Disorders arising from cells of histiocytic and dendritic derivation are the epitome of confusing medical terminology, incomplete disease definitions and partial biologic and immunologic understanding. They are among the most rare of disorders of the hematopoietic system and thus, among the most difficult to study. They comprise a spectrum from benign proliferations of unknown etiology to frankly malignant tumors. The scarcity of markers specific to histiocytes, the lack of consistent means for detection of monoclonality, and the clinocopathologic overlap with reactive and infectious proliferations further complicate understanding their pathogenesis.
Formulating a classification that incorporates the heterogeneity of histiocytic disorders has been challenging. The classification adopted by the Histiocyte Society separates dendritic cell- and macrophage-related entities of varied biological behavior from those that are malignant.1,2 A recent proposal expounded by the International Lymphoma Study Group classifies tumors of histiocyte and accessory dendritic cells into four groups based on their immunophenotype: histiocytic sarcoma, Langerhans cell tumor/sarcoma (LCH), follicular dendritic cell tumor/sarcoma and interdigitating dendritic cell tumor/sarcoma.3 This classification only incorporates histiocytic disorders currently recognized as neoplastic. Thus, nonmalignant proliferations of histiocytic derivation, where the histiocyte may be central to the pathogenesis or a mere bystander, are still poorly understood.
Sinus histiocytosis with massive lymphadenopathy (SHML) is a rare disorder characterized by a nonmalignant proliferation of distinctive histiocytic/phagocytic cells within lymph node sinuses and lymphatics in extranodal sites. Much of what is known of SHML today was pioneered by two pathologists, Juan Rosai and Ronald Dorfman. They recognized SHML as a distinct clinicopathologic entity and established a registry to collect and catalogue cases of SHML worldwide such that this unusual disorder may be better studied.4 This scholarly contribution is well recognized today by the popularity of the eponym Rosai-Dorfman disease (RDD), particularly to refer to the extranodal form of this disorder. Since its initial description in 1969, SHML remains a disorder defined primarily by its histopathologic features. Due to its rarity, neither community-based surveys nor systematic treatment studies have been undertaken. Although a few hundred publications related to SHML can be found in the literature, many are single case reports or descriptions of small series of patients, limiting comprehensive appraisal of the subject.
Clinical Features
SHML occurs worldwide and is primarily a disease of childhood and early adulthood. No specific gender, ethnic or socioeconomic predilection is encountered, and the 423 cases in the SHML registry reflect the fact that people of Caucasian and African descent are equally affected while the disease is less common in those of Asian descent.4 Occasional familial cases have been reported. Painless lymphadenopathy is the most frequent presenting symptom and involves the cervical region in up to 90% of patients. Axillary, para-aortic, inguinal and mediastinal lymph nodes are also commonly affected. Antecedent non-specific fevers and pharyngitis may herald the onset of SHML, occasionally accompanied by pain, tenderness, malaise, night sweats or weight loss. Extranodal disease is documented in 43% of patients, in some without associated lymphadenopathy which may or may not develop later in the disease course.4 The most often affected extranodal sites include skin and soft tissues, upper respiratory tract and bone followed by genitourinary tract, lower respiratory tract and the oral cavity. Simultaneous involvement of multiple extranodal sites is not unusual. Involvement of the kidney, lower respiratory tract and liver are associated with a worse clinical outcome as is the number of extranodal sites involved. Hepatosplenomegaly is uncommon.
SHML exhibits a broad range of clinical presentations and thus, elicits a wide differential diagnosis. Although not comprehensive, the salient clinical features of SHML affecting various anatomic sites are provided to illustrate its heterogeneous presentations (Table 1 ). SHML in lymph nodes may clinically mimic metastatic carcinoma or melanoma, Hodgkin and non-Hodgkin lymphoma, infectious processes, granulomatous lymphadenitis, reactive lymphadenopathy or other histiocytoses including Langerhans cell histiocytosis. Histiocytic disorders, particularly Langerhans cell histiocytosis must be distinguished from RDD affecting skin, soft tissue and bone. In the nasopharynx, nasal polyps, nasopharyngeal carcinoma, lymphoma and rhinoscleroma enter the spectrum of diagnostic considerations. In the lung, the differential diagnosis includes granulomatous inflammation such as sarcoid, infectious processes, Erdheim-Chester disease, foreign-body giant cell reaction and aspiration pneumonia. In addition to these entities, RDD affecting the heart may resemble giant cell myocarditis, granulomatous myocarditis or a foreign-body giant cell response. Tonsilar involvement may simulate Epstein-Barr virus (EBV)-associated lymphoproliferative disorders and infectious mononucleosis. Since its recognition 35 years ago, the constellation of clinical findings has formed an ever-expanding almanac as more and more cases of SHML are diagnosed.
Pathologic Features
Clinical laboratory findings include hematologic abnormalities such as normocytic or microcytic anemia. Immunologic abnormalities are found in a significant number of patients and often lead to a clinically unfavorable disease course. Up to 90% of patients demonstrate an elevated erythrocyte sedimentation rate. The most frequent immune dysfunction is autoimmune hemolytic anemia, which leads to severe hemolysis or fatality in a subset of patients.4,5 Polyarthralgia, rheumatoid arthritis, glomerulonephritis, asthma and diabetes mellitus are also known to complicate SHML. Polyclonal hypergammaglobulinemia is found in 90% of patients. Rarely, patients exhibit rheumatoid factor, antinuclear antibodies, and a reversal of CD4/CD8 ratio among peripheral lymphocytes. A small subset of patients also has concurrent neoplasia including non-Hodgkin lymphoma, other histiocytic proliferations, myeloma, melanoma and carcinoma. Consistent serologic findings are lacking, although antibodies to pathogenic organisms including EBV and human herpesvirus (HHV)-6 have been reported.6– 8
Surgically excised lymph nodes are yellow-white with frequent capsular and pericapsular fibrosis. Generally, normal lymph node architecture is preserved and effacement is only seen in patients with longstanding lymphadenopathy. Typically, the lymph node sinuses are expanded by a proliferation of distinctive histiocytes exhibiting enlarged round or oval vesicular nuclei with well-defined, delicate nuclear membranes and a single prominent nucleolus. Multilobulated nuclei, nuclei with multiple nucleoli and nuclear atypia are rare. Mitoses are infrequent, although increased mitotic activity can be apparent occasionally. The histiocytes possess abundant pale eosinophilic cytoplasm; however, on occasion numerous histiocytes with foamy cytoplasm may predominate the cellular milieu. The hallmark of the SHML histiocyte is the presence within the cytoplasm of variable numbers of intact lymphocytes, a phenomenon referred to as lymphophagocytosis or emperipolesis, defined as lymphocytic penetration of and movement within another cell, in this case, the histiocyte.4 These lymphocytes are often housed within vacuoles and escape degradation during their transit through the histiocyte (Figure 2; see Color Figures, page 504). Plasma cells, neutrophils and red blood cells may also occupy this unique intracytoplasmic niche. Associated with the histiocytic proliferation are numerous, morphologically typical plasma cells often aggregated around post-capillary venules. Eosinophils are not a salient feature of SHML as compared with LCH, classical Hodgkin or T-cell lymphoma. Collections of neutrophils, eosinophilic microabscesses and reactive germinal centers are occasionally seen but are not prominent findings in SHML. Rosai-Dorfman disease involving extranodal sites shows similar morphologic features to its nodal counterpart although more fibrosis and fewer histiocytes with emperipolesis are encountered.
The histologic differential diagnosis includes LCH, histiocytic sarcoma, storage disorders such as Gaucher’s disease, classical Hodgkin lymphoma, metastatic melanoma and carcinoma, and infections caused by Histoplasma and mycobacterial organisms involving the lymph node. In the nasal cavity, rhinoscleroma may morphologically resemble RDD but is distinguished by the identification of Klebsiella rhinoscleroma. Perhaps the most frequently faced histologic challenge is that of distinguishing SHML from reactive sinus histiocytic proliferations which occur as nonspecific responses to a variety of instigating agents. Emperipolesis is rare or absent in reactive histiocytic proliferations. Although erythrophagocytosis is seen in reactive and neoplastic histiocytic proliferations, including LCH, emperipolesis in a setting outside of SHML is extremely rare. The appearance of lymph node sinuses expanded by a histiocytic proliferation is a feature common to LCH and SHML. However, unlike SHML, Langerhans cells have smaller, frequently folded or grooved nuclei and are associated with eosinophilic microabscesses.
Immunohistologic Studies
The most useful immunohistologic marker for SHML histiocytes is the expression of the S100 protein.9,10 In addition, SHML histiocytes express pan-macrophage antigens (CD68, HAM 56, CD14, CD64, CD15), antigens associated with phagocytosis (CD64 or Fc receptor for IgG), lysosomal activity (lysozyme, alpha-1-antitrypsin) and immune activation (transferring receptor, interleukin-2 receptor). We have recently found that SHML histiocytes express CD163, a hemoglobin scavenger receptor and acute phase-regulated transmembrane protein found on tissue macrophages and monocytes.11 These studies support the hypothesis that the effector cell in SHML is a functionally activated macrophage and is distinct from Langerhans cells, follicular dendritic cells and interdigitating dendritic cells. While Langerhans cells stain for the S100 protein and CD1a, only rare CD1a-positive cells are found in SHML. In addition, SHML histiocytes do not express CD21, CD23, or CD35 (markers of dendritic differentiation). The overlap in morphologic and immunophenotypic features among reactive and neoplastic histiocytic proliferations, especially in unusual nodal presentations or extranodal sites, can make the diagnosis of SHML particularly problematic.
Clinical Course and Treatment
The clinical course of SHML is characterized by spontaneous resolution in most cases, especially in those patients presenting with localized lymph node involvement. Some patients have episodes of exacerbation alternating with periods of remission that continue for many years, where the timing and duration of each phase is entirely unpredictable. Persistence of lymphadenopathy or progression to widespread dissemination may occur, particularly in SHML involving the kidney, lower respiratory tract or liver with associated immunologic dysfunction. Of 423 cases in the SHML registry 17 patients died of the disease or with persistent disease.12
Treatment is not necessary in the majority of patients as the disease is self-limiting in most cases. Only in a minority of patients where massive nodal or extranodal enlargement infringes on organ function or threatens life is treatment warranted to halt the natural progression of SHML. As randomized clinical studies of treatment for SHML have not been undertaken, precise and objective measures of therapeutic response are unavailable. In a recent review of treatment strategies for SHML,13 50% of patients (40/80) did not require any treatment. Patients given antibiotics or anti-tuberculosis drugs showed no response. Steroid therapy resulted in reduction of lymphadenopathy and associated fevers. Of 9 patients treated with radiation therapy for lymphadenopathy 3 achieved complete remission, 3 had persistent SHML and 3 died of disease. Of 12 patients treated with currently used chemotherapy regimens involving a combination of Vinca alkaloids, anthracyclines and alkylating agents, 10 showed no response while 2 others achieved complete and durable remission with methotrexate and 6-mercaptopurine. Surgery and radiation therapy employed in 8 patients led to complete remission in 1 case and partial responses in 6 cases. High-dose interferon-α was given to 1 patient with long-term remission. In a recent report, 2 children with intracranial RDD were treated with the purine analog 2-chlorodeoxyadenosine found to be of clinical utility in the treatment of refractory LCH; although short-term symptomatic relief was obtained there was no objective response.14
The ideal treatment for SHML is as yet undefined. For SHML that does not involve or compress vital organs the patient may be followed with observation. For patients with high fever without other symptomatology steroid therapy may be instituted, although a concurrent infection should be carefully investigated before the administration of steroid therapy. Surgical debulking, radiation therapy or a combination of both may be used in cases where vital organ function is compromised. The lack of response to high-dose chemotherapy for severe SHML suggests that chemotherapy should not be a mainstay of therapy in the management of this disease. More data are needed on newer agents such as interferon-α to permit evaluation of their efficacy in the treatment of SHML.
Pathogenetic Mechanisms
Since its original description in 1969, SHML has remained a disorder of unknown etiology. Infectious agents have been suspected, but no clinical response to antibacterial or anti-tubercular drugs has been documented. Although serologic evidence of EBV was found in 3 of 6 cases, no evidence of latent or lytic EBV infection was identified within histiocytes of SHML.8 Thus, it is unlikely that EBV plays a causative role in SHML. In situ DNA hybridization has demonstrated HHV-6-specific DNA within histiocytes of SHML in 7 of 9 cases.6 In addition, immunohistochemistry for the ORF-1 protein of HHV-6 demonstrates viral protein in the SHML histiocytes in both cases investigated.7 In the latter study, the expression of HHV-6–specific p101K antigen was also found in follicular dendritic cells (FDC) in SHML, the relevance of which is unclear.
The unfavorable clinical outcome in patients with associated immune dysfunction has led to the consideration that SHML results from an exuberant response of the hematopoietic system to an as yet undetermined immunologic trigger. Recently, cases of SHML associated with autoimmune lymphoproliferative syndrome (ALPS) have been described.15 ALPS occurs primarily in early childhood and is an inherited disorder of lymphocyte programmed cell death orchestrated by mutations in death receptor genes that specifically abrogate apoptosis in lymphocyte subsets. First described by Canale and Smith in 1967 with the constellation of findings that include lymphadenopathy, splenomegaly and autoimmune cytopenias, ALPS shares similarities with mouse models of defective apoptosis (MRL/lpr phenotype) and shows an increase in mature T cell receptor alpha/beta-positive, CD4-negative, CD8-negative (double-negative, DN) T cells. The classical form of ALPS harbors specific mutations of Fas and Fas ligand, although additional ALPS-like syndromes have since been identified and involve mutations of genes downstream of the Fas receptor.16– 18 Recent data suggest that mutations of the Fas gene are found in a small subset of patients with SHML unassociated with ALPS (George TI, Arber DA, unpublished observation). Thus, SHML may represent an acquired disorder of deregulation of apoptotic signaling pathways.
Additionally, familial hemophagocytic lymphohistiocytosis (FHL), a rare and often acutely fatal autosomal recessive disorder, results from gene defects in perforin, a central mediator in lymphocyte cytotoxicity.19 FHL is characterized by deregulated proliferation and accumulation of cytotoxic T cells and histiocytes accompanied by overproduction of inflammatory cytokines leading to fever, pancytopenia, coagulopathy and neurologic deficits. Similar to the Fas/FasL pathway, the perforin pathway is another mediator of cytotoxic activation and a potential target for study in unraveling the pathogenesis of SHML.
Conclusion
Sinus histiocytosis with massive lymphadenopathy is characterized by a rare, acquired, nonmalignant proliferation of distinctive histiocytes that presents with lympadenopathy or extranodal disease primarily in children and young adults. The causative agent is unknown. However, its diverse clinical manifestations and frequent association with subtle or severe immunologic abnormalities suggest an immune-mediated etiology. Recent studies of other immune-mediated histiocytic and cytotoxic T-cell proliferations have raised interesting parallels worthy of further investigation in SHML. Defective Fas/FasL signaling leading to defective apoptosis, as demonstrated in patients with ALPS, may be an important mechanism whereby the uncontrolled histiocytic proliferation of SHML is triggered. However, a single gene defect is unlikely to explain the various clinical manifestations of SHML, which are most likely modulated by other immunologic factors or infectious agents. Additional studies are needed to characterize the interplay between death receptors and cytotoxic mediators and to further elucidate the loss of immune hemostasis that may underlie idiopathic histiocytic proliferations such as SHML.
III. Castleman Disease: One Disease or Several?
Steven H. Swerdlow, MD*
University of Pittsburgh School of Medicine, Division of Hematopathology, UPMC Presbyterian, Room C606, 200 Lothrop Street, Pittsburgh PA 15213-2582
Castleman disease (CD, angiofollicular lymph node hyperplasia) was described almost a half a century ago by Benjamin Castleman and colleagues as a localized mediastinal mass in otherwise usually asymptomatic patients.1 These cases are now known as the hyaline vascular type of CD (HV-CD). More than three decades ago the much less common plasma cell type of CD (PC-CD) was described that was also localized but had very different clinical and histopathologic features.2 More than two decades ago multicentric CD (M-CD) was described and, most recently, an aggressive “plasmablastic” variant of M-CD.3 Understanding CD and the literature about CD requires not only recognizing these very different types of CD but also recognizing that, because CD in many circumstances is a diagnosis made by exclusion, there can easily be overusage of this diagnosis by pathologists, and, at the same time the diagnosis can also be easily overlooked in certain circumstances.
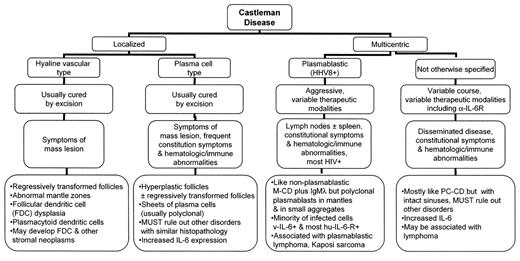
This update will review the major clinical features of each of these types of CD, the nature of the basic pathologic lesions, their pathophysiology, the secondary or associated neoplasms that have been reported and the varied therapeutic implications (Figure 3 ). This is not intended to be a diagnostic pathology primer but will use the pathologic findings to help convey the nature of the disease. Neither is it intended to be a therapeutic recipe book, which would be presumptuous and dangerous for a pathologist to write. It will look at how current therapeutic options often follow from a good understanding of these disorders. Finally, this update cannot deal with all the complexities of this topic, particularly given some variation between diagnostic criteria utilized in different studies, the inherent difficulties in reproducibly identifying and subclassifying cases, and the varied methods used to assess some of the reported biologic characteristics of the disease.
Localized Hyaline Vascular Type Castleman Disease
HV-CD occurs in children and adults (median age in the 30s) who present with large solitary masses of the mediastinum, or markedly enlarged single peripheral or abdominal lymph nodes but in > 90% of cases without constitutional symptoms.2,4,5 Although most cases are believed to arise in lymph nodes, HV-CD can also form soft tissue masses.6 HV-CD is a lymphoid proliferation that demonstrates characteristic abnormalities both in the lymphoid follicles and in the interfollicular regions (Figure 4A; see Color Figures, page 505). The follicles have widened mantle zones composed of concentric rings of small lymphocytes that surround one or more abnormal small regressively transformed germinal centers. The regressively transformed germinal centers have lymphoid depletion, vascular proliferation often with penetrating small hyalinized vessels and prominent follicular dendritic cells (FDC) that sometimes appear dysplastic or show an expanded/disrupted pattern.6,7 The mantle zone cells have been reported to have an abnormal immunophenotype.8 The variably expanded interfollicular region is composed of small lymphocytes, mostly of T cell type, a variably hyalinized vascular proliferation including many high endothelial venules, and frequently foci of plasmacytoid dendritic cells (also known as plasmacytoid monocytes) (Figure 4B; see Color Figures, page 505). The lymphoid cells in the vast majority of cases are polyclonal. Plasmacytoid dendritic cells, identified morphologically and based on their CD68+, CD45RA+, CD123+ phenotype, produce large amounts of type 1 interferon (IFN-α) after stimulation and may play a role in the response to viral infections or prolong survival of antigen-activated T cells.9 There is a variably prominent interfollicular stromal component including fibroblastic reticulum cells, vessels, myoid cells and a variety of dendritic cells. Some cases have a predominance of the interfollicular component, and the stromal components can even form nodular masses. Other inflammatory cells, such as plasma cells, eosinophils or histiocytes, may be present but are not numerous. Significant fibrosis and calcification can also be seen. The explanation for these varied abnormalities remains uncertain. Viral infection including EBV or human herpesvirus 8 (HHV-8 or Kaposi’s sarcoma-associated herpes virus) and interleukin-6 (IL-6) overexpression are not considered to play a role in at least most cases of HV-CD.10,11 The vascular proliferation may, in some cases, be due to abnormal, usually intrafollicular, vascular endothelial growth factor (VEGF) expression.12 A clonal cytogenetic abnormality has been reported in at least two cases of HV-CD including one where the abnormality that involved the HMGIC gene was documented in the CD21+ FDC.13HMGIC (high mobility group protein I-C) abnormalities are reported in a variety of benign mesenchymal neoplasms. Although this suggests that the FDC in HV-CD are neoplastic, it could be a secondary event unrelated to the basic pathogenesis of the CD. FDC dysplasia, however, is a relatively frequent finding in HV-CD.6,7 In addition, like many FDC sarcomas and unlike normal germinal centers, there is often moderate or strong expression of the epidermal growth factor receptor (EGFR) in the FDC of CD, a feature that seemed to correlate with the degree of FDC dysplasia present.14 Consistent with the importance of FDC and other stromal elements in HV-CD is the development of FDC sarcomas and vascular neoplasms in some patients with HV-CD. Therapy for HV-CD is generally surgical with only rare recurrences.4,5,15 Radiation therapy has also been utilized.15
Localized Plasma Cell Type Castleman Disease
Localized PC-CD also occurs in children and adults (median age in the 20s) and presents with a mass lesion that, in contrast to HV-CD, is typically an aggregate of lymph nodes, most frequently in the mediastinum or abdomen.2,4,5 Unlike those with HV-CD, these patients often present with constitutional symptoms and 50%–90% demonstrate hematologic disorders, including anemia and a leukocytosis, with many having hypergammaglobulinemia and hypoalbuminemia. Other disorders have occasionally been associated with PC-CD, such as growth retardation.5
In contrast to HV-CD, the follicles are mostly hyperplastic rather than regressively transformed, FDC patterns are usually unremarkable, and there are sheets of usually polyclonal but sometimes monoclonal plasma cells (Figure 4C; see Color Figures, page 505). The distinction of the latter cases from a small B cell lymphoma with plasmacytic differentiation is problematic. In addition to the sometime similar follicular abnormalities, the mantle zones in PC-CD have been reported by one group of investigators to have the same phenotypic abnormality as in HV-CD8 and others have reported similar upregulation of the EGFR in the follicles (including at least some cases that are probably not localized).14
In complete contrast to HV-CD and normal lymph nodes, there is abnormal IL-6 expression by cells in the germinal centers and elsewhere in the lymph nodes that may be very important in the pathogenesis of this disorder.10,16 IL-6 levels are also increased in the serum. IL-6 leads to the differentiation of B-cells into plasma cells, may play a role in vascular proliferation and also induces acute phase reactant proteins. It thus can account for many of the pathologic findings, systemic symptoms and laboratory abnormalities in PC-CD. Abnormal expression of VEGF by interfollicular plasma cells may also be of importance.17 VEGF expression in fibroblast-like cells in germinal centers of CD of uncertain type has also been reported.12 Rare cases of lymphoma or plasmacytoma have been described following localized CD.18 As with HV-CD, most cases are cured by surgical excision including resolution of the systemic signs and symptoms.4,5 Some patients have also received steroids and/or radiation therapy.
Multicentric Castleman Disease
M-CD is a systemic disorder that is reported to occur in children19 but which most typically occurs in older individuals (median age 50s).4,5,15 A specific plasmablastic type (PB-M-CD) has been recognized more recently (see below).3,20 These latter cases are undoubtedly present and not distinguished in many series of M-CD, thus complicating interpretation of this literature. Patients with M-CD present with more than one site of disease including nodal involvement plus frequent hepatosplenomegaly together with the types of constitutional symptoms and laboratory abnormalities as seen in PC-CD.4,5,21,22 M-CD occurs in patients with human immunodeficiency virus (HIV) infection, in older individuals with Kaposi’s sarcoma (KS) and in others as well. It has been suggested that, independent of HIV infection, M-CD “probably arises in a setting of immunoregulatory deficit” perhaps related to immune senescence.5 Although often considered to be an aggressive disorder, the reported course is variable, with some cases that may wax and wane over long periods of time. Pediatric cases are described as having a better prognosis.19
Perhaps with the exception of PB-M-CD, M-CD is a diagnosis made by exclusion as the pathologic findings are non-specific. Most cases closely resemble PC-CD except for the presence of intact and often dilated sinuses in lymph nodes (Figure 4D; see Color Figures, page 505). There may be significant fibrosis. Spleens demonstrate numerous plasma cells in the white pulp surrounded by a zone of fibrosis.3 Most but not all cases are polyclonal. As in PC-CD, IL-6 is of pathogenetic importance and VEGF may be as well.16,17 MC-CD is associated with HHV-8 infection in the HIV-positive patients and in about 40% of HIV-negative cases. M-CD is not generally considered to be associated with EBV; however, EBV+ cases have been reported.19 The development of lymphoma is an important complication of M-CD (see below).
As noted recently “...no standard of care for the treatment of MCD has been established....”23 Numerous modalities including steroids, radiation therapy and chemotherapeutic agents15,24 as well as more targeted therapies have been utilized. Use of a humanized anti-IL6-R antibody has led to symptomatic relief and partial or complete regression of the adenopathy.25 The status of this investigational agent, Atlizumab, was recently reviewed.26 Anti-IL6 antibodies and rituximab have also been variably successful, although the latter led to exacerbation of KS.27 Both decreases in viral load and clinical responses to antiviral agents such as ganciclovir have been reported in HHV-8/HIV–associated disease, although antiviral therapy, like many other therapies, is not always successful.23
Plasmablastic variant of multicentric Castleman disease
PB-M-CD is distinguished by the presence of plasmablasts that express the HHV-8 latent nuclear antigen and are present mostly in follicular mantles (Figure 4E–G; see Color Figures, page 505).3,20 A subset of the HHV-8 infected cells in M-CD express other HHV-8 encoded proteins including v-IL-6 and lytic antigens.28 HHV-8 infected cells in KS or primary effusion lymphomas (PEL) have more restricted protein expression.28 The v-IL-6 not only contributes to the plasmacytosis but can induce VEGF and enhance angiogenesis.29 Most of the infected cells also express the human IL-6 receptor, suggesting the importance of this pathway.20 HIV+ patients with HHV-8+ M-CD also have high plasma IL-6 (and IL-10) levels.30 Other HHV-8 encoded proteins may play an important role in the pathogenesis of these lesions (a subject beyond the scope of this discussion).31 HHV-8+ CD has also been reported to show EGFR overexpression in the FDC.14
The infected cells are naïve CD20+ plasmablastic/immunoblastic B-cells that usually express IgMλ but are not monoclonal.20 CD20 negative infected cells are also described and, in some studies of HHV-8+ CD, many infected cells are small. Some cases are associated with small aggregates of polyclonal or, less often, monoclonal plasmablasts that have been termed “microlymphomas.” A significant number of cases have simultaneous or subsequent overt plasmablastic lymphomas and some have an associated PEL. Many of these patients also have or develop KS. PB-M-CD have behaved in a very aggressive fashion; in one study most patients died within one year, suggesting that it is more aggressive than HHV-8− M-CD.3 This conclusion would be consistent with the reportedly better prognosis of “idiopathic plasmacytic lymphadenopathy with polyclonal hypergammaglobulinemia,” an entity described in Japan that has many features of M-CD but is HHV-8−.32
Whether, as in the classic description of PB-M-CD, HHV-8 positivity in M-CD is synonymous with the plasmablastic variant remains to be determined. It is important to distinguish PB-M-CD from other HHV-8–associated disorders including PEL that is also often found in HIV+ individuals and “KSHV-associated germinotropic lymphoproliferative disorder,” both of which are most typically EBV+.33 The latter is a monotypic but polyclonal/oligoclonal plasmablastic proliferation that occurs largely in germinal centers. It presents with localized adenopathy in immunocompetent patients who respond well to therapy.
Other Disorders That May Be Associated with CD-Like Histopathologic Findings
There are several disorders that have unique clinical features but in which lymph node biopsies may resemble or possibly truly represent CD. CD is considered a minor criteria for the diagnosis of the POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein and skin changes) syndrome, with the reported estimate of 11%–30% of cases having CD considered a conservative estimate.34 As in PB-M-CD, the POEMS syndrome is a λ-restricted “plasmaproliferative” disorder34 and most POEMS-associated CD is HHV-8+.35 These cases are included in some CD series but probably should be separated as this is a well-defined, distinct clinicopathologic entity. “Cutaneous plasmacytosis,” a chronic benign disorder usually but not exclusively reported in Japanese patients who present with multiple cutaneous plaques with numerous polyclonal plasma cells, is another entity that can be associated with lymphadenopathy and other signs and symptoms seen in PC or M-CD.36 Hodgkin lymphoma is sometimes described with adjacent CD-like changes or in association with CD or CD-like changes, perhaps reemphasizing the nonspecific nature of many of the pathologic findings of CD and the need to exercise caution before making the diagnosis.37
Conclusion
CD is a set of complex disorders that range from localized benign tumor masses to extremely aggressive disseminated proliferations. Although the major subtypes of CD do have some features in common, suggesting a possible relationship to one another, their differences are much greater and must be recognized both for clinical purposes and to best study their underlying biologic features.
Clinical manifestations of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease).
| Anatomic site . | Frequency* . | Clinical Manifestation . |
|---|---|---|
| * RDD patients from the SHML registry4 with involvement at multiple extranodal sites are included under each anatomic site. The rarity of SHML precludes accurate statistics on the incidence of SHML or the frequency of specific clinical findings in the general community. | ||
| Lymph nodes | 87% | Massive painless adenopathy: cervical most frequent, may be bilateral, occasionally accompanied by pain or tenderness |
| Skin and Soft tissue | 16% | Broad range of non-specific presentations: typically painless, maculopapular rash, yellow xanthomatous solitary or multiple, usually raised reddish or bluish lesions, scaly Subcutaneous nodules or soft tissue masses |
| Nasal cavity/Paranasal sinuses | 16% | Mucosal thickening, nasal polyps (may be recurrent) or masses causing mild or complete nasal obstruction, epistaxis, displacement of septum, infiltrative mass extending into maxillary, ethmoid, or sphenoid sinuses |
| Eye/Orbit/Ocular adnexa | 11% | Eyelid or orbital mass with proptosis; may require enucleation |
| Bone | 11% | Bone pain with osteolytic lesions in any bone of the axial or appendicular skeleton; on radiography, may be purely lucent or mixed with sclerosis or exhibit irregular borders or a broad sclerotic rim |
| Salivary gland | 7% | Bilateral parotid enlargement or submandibular mass; may be associated with dry mouth |
| Central nervous system | 7% | Intracranial epidural or dural, single or multiple masses, spinal cord masses, associated lytic bone lesions in skull or spine, cranial or spinal nerve palsies, weakness, syncope, headache |
| Oral cavity | 4% | Infiltrative mass of soft or hard palate, diffuse mucosal thickening, papillomatosis, gingival swelling |
| Kidney/Genitourinary tract | 3% | Unilateral or bilateral renal masses accompanied by obstruction: hydronephrosis, hydroureter, ureteral obstruction, glomerulonephritis, chronic pyelonephritis Testicular, scrotal or epididymal masses with intermittent penile or pubic edema |
| Respiratory tract/Larynx/Lungs | 3% | Laryngeal obstruction, thickening and fibrosis associated with hoarseness |
| Liver | 1% | Enlargement with congestion, milliary infiltrates, masses |
| Tonsil | 1% | Enlargement, may be accompanied by tonsillitis |
| Breast | < 1% | Predominantly subcutaneous involvement, rarely involves breast parenchyma, associated axillary adenopathy |
| Gastrointestinal tract | < 1% | Hyperplastic mucosal folds, single or multiple exophytic masses |
| Heart | < 1% | Subendocardial or valvular infiltrates causing arrythmias |
| Anatomic site . | Frequency* . | Clinical Manifestation . |
|---|---|---|
| * RDD patients from the SHML registry4 with involvement at multiple extranodal sites are included under each anatomic site. The rarity of SHML precludes accurate statistics on the incidence of SHML or the frequency of specific clinical findings in the general community. | ||
| Lymph nodes | 87% | Massive painless adenopathy: cervical most frequent, may be bilateral, occasionally accompanied by pain or tenderness |
| Skin and Soft tissue | 16% | Broad range of non-specific presentations: typically painless, maculopapular rash, yellow xanthomatous solitary or multiple, usually raised reddish or bluish lesions, scaly Subcutaneous nodules or soft tissue masses |
| Nasal cavity/Paranasal sinuses | 16% | Mucosal thickening, nasal polyps (may be recurrent) or masses causing mild or complete nasal obstruction, epistaxis, displacement of septum, infiltrative mass extending into maxillary, ethmoid, or sphenoid sinuses |
| Eye/Orbit/Ocular adnexa | 11% | Eyelid or orbital mass with proptosis; may require enucleation |
| Bone | 11% | Bone pain with osteolytic lesions in any bone of the axial or appendicular skeleton; on radiography, may be purely lucent or mixed with sclerosis or exhibit irregular borders or a broad sclerotic rim |
| Salivary gland | 7% | Bilateral parotid enlargement or submandibular mass; may be associated with dry mouth |
| Central nervous system | 7% | Intracranial epidural or dural, single or multiple masses, spinal cord masses, associated lytic bone lesions in skull or spine, cranial or spinal nerve palsies, weakness, syncope, headache |
| Oral cavity | 4% | Infiltrative mass of soft or hard palate, diffuse mucosal thickening, papillomatosis, gingival swelling |
| Kidney/Genitourinary tract | 3% | Unilateral or bilateral renal masses accompanied by obstruction: hydronephrosis, hydroureter, ureteral obstruction, glomerulonephritis, chronic pyelonephritis Testicular, scrotal or epididymal masses with intermittent penile or pubic edema |
| Respiratory tract/Larynx/Lungs | 3% | Laryngeal obstruction, thickening and fibrosis associated with hoarseness |
| Liver | 1% | Enlargement with congestion, milliary infiltrates, masses |
| Tonsil | 1% | Enlargement, may be accompanied by tonsillitis |
| Breast | < 1% | Predominantly subcutaneous involvement, rarely involves breast parenchyma, associated axillary adenopathy |
| Gastrointestinal tract | < 1% | Hyperplastic mucosal folds, single or multiple exophytic masses |
| Heart | < 1% | Subendocardial or valvular infiltrates causing arrythmias |
Summary of the clinicopathologic and biologic features of the major types of Castleman disease.
Summary of the clinicopathologic and biologic features of the major types of Castleman disease.