Abstract
One of the most common complications involved in treating patients with hematologic cancer is infection. In many cases there are multiple factors that predispose these patients to infections such as neutropenia induced by therapy or bone marrow involvement, hypogammaglobulinemia, T-cell dysfunction, and mucosal damage. In addition, newer therapies have changed the spectrum of infection that is seen in these patients.
In Section I, Dr. Blijlevens discusses mucosal damage as a major risk factor for complications of cytotoxic chemotherapy. She focuses on mucosal barrier injury (MBI) as manifest in the GI tract and will describe a pathological model to explain MBI, evaluate risk factors for development of this syndrome, explain the relationship between MBI and infection, and discuss treatment and prevention of this injury.
Invasive fungal infections continue to represent a significant problem in patients with hematologic cancer. In Section II, Drs. Anaissie and Mahfouz review the latest developments in the diagnosis, prevention, and management of invasive fungal infections with a focus on a risk-adjusted approach to this problem.
Finally, in Section III, Dr. O’Brien reviews infections associated with newer therapeutic regimens in hematologic cancers. The spectrum of infections has changed with the use of purine analogs and the advent of monoclonal antibodies. The profound T-cell suppression associated with these therapies has led to the emergence of previously rare infections such as cytomegalovirus. An approach to both prophylaxis and management of these infections is discussed.
I. Mucosal Damage: A Major Risk Factor for Severe Complications after Cytotoxic Therapy?
Nicole M.A. Blijlevens, MD*
University Medical Center St. Radboud, Department of Hematology, Radboud Centraal, Geert Grooteplein 8, Postbox 9010, Nijmegen 6500 HB, The Netherlands
Today there are more than 10 million patients worldwide with cancer and this number is expected to increase with an increasingly elderly population. Intensive chemotherapy alone or in combination with radiation therapy is available for a variety of different cancers. Unfortunately, this treatment is often complicated by damage to the integument of the mouth and, to a larger extent, of the gastrointestinal (GI) tract, which results in significant morbidity and a lower quality of life.1 While the severe pain of oral mucositis is often the most obvious and troublesome symptom of patients undergoing intensive cytotoxic therapy, gastrointestinal injury is more life threatening.2 There is some evidence that intensive cytotoxic chemotherapy also damages the mucosa of the upper and lower airways, the bladder, and the vagina. However, the focus of this review is on what has been termed “mucosal barrier injury” (MBI) as manifest in the mouth and GI tract, and will present a pathological model to explain MBI, explore the risk factors involved, highlight the relationship between MBI, infection, and other complications and discuss treatment and prevention.
Defining Mucosal Barrier Injury
Oral mucositis
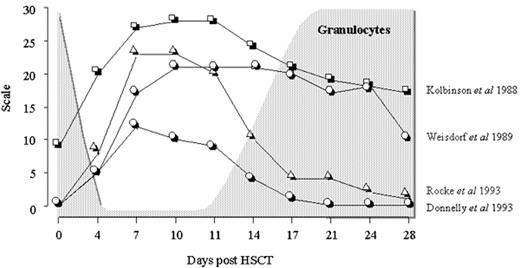
Each regimen used in anticancer treatment is associated with varying degrees of mucosal damage. Oral mucositis is reported to affect 60% to 100% of hematopoietic stem cell transplant recipients when myeloablative preparative regimens are used. In general, oral mucositis is characterized by functional complaints such as pain and difficulty in swallowing (dysphagia) and anatomical changes such as edema, erythema, ulceration, pseudomembrane formation, alterations in mucus consistency, and changes in saliva production (xerostomia). When severe or protracted, oral mucositis prevents patients from eating and drinking normally. This problem is frequently managed by giving parenteral nutrition. A rational basis for managing oral mucositis is still a distant goal because of the lack of a single, uniform, practical, validated tool to measure oral mucositis. Hence, grading systems like that of the World Health Organization or the National Cancer Institute3 and center-specific systems such as the Daily Mucositis Score4 are widely used although never formally validated. The modified oral mucositis assessment scale (OMAS) was developed and validated to provide a scoring system that would be generally accepted and used for observational studies and in future multicenter clinical trials of prevention and treatment.5 Only time will tell whether the OMAS scheme will become generally accepted. Besides recording the presence of mucositis, the OMAS scheme lends itself to repeated, regular monitoring of oral mucositis, a feature shared with other scoring systems.4,6– 8 This, in turn, allows the extent of oral mucositis to be estimated by the area under the oral mucositis curve. Irrespective of the grading system used, oral mucositis is known to begin around a week after starting conditioning and to worsen until a peak is reached after which resolution takes place (Figure 1 ).
Risk factors for oral mucositis
Risk factors for oral mucositis have not been systematically explored thereby preventing the use as stratification for evaluating remedies. Intuitively, the risk factors can be divided into patient-related and therapy-related factors.
Patient-related risk factors: Patient-related risk factors include age, gender, nutritional status, composition of the oral microflora, salivary function, and the status of oral health and hygiene. The data are sometimes conflicting. For instance, age and gender can either increase or decrease the severity of oral mucositis. General malnutrition, specific deficiencies in vitamins, trace elements and/or antioxidants are thought to exacerbate mucositis.9 Xerostomia that is already present before starting chemotherapy also makes mucositis worse. Contradictory data also exist regarding factors like body mass index, body surface area, or total body nitrogen since these can also adversely influence mucositis. Whether these factors truly indicate the risk of mucositis or simply reflect an altered pharmacodynamic profile of the drugs used for chemotherapy is unclear. Dental care and oral hygiene are also held to be important although randomized studies evaluating the effect of mouth rinses, especially chlorhexidine and oral decontamination regimens, could not show any benefit in reducing mucositis, pain, or oral infection beyond the use of a systematic oral hygiene program.10
Therapy-related risk factors: A large number of cytotoxic agents produce oral mucositis, although specific agents with comparable cytotoxic potential injure the oral mucosa in a wide variety of ways. Antimetabolites and alkylating agents produce a high incidence of oral mucositis. Hematopoietic stem cell transplant (HSCT) recipients treated with regimens containing total body irradiation (TBI) experience more severe mucositis than do patients treated with busulfan containing regimens, although other reports disagree. Addition of idarubicin to the preparative regimen prolongs bone marrow aplasia but also worsens oral mucositis. Wardley and colleagues used multivariate analysis to identify risk factors including age, HSC source, use of myeloid growth factors, and drug regimen and found that the conditioning regimen was the only independent risk factor for oral mucositis.11 Furthermore, high-dose melphalan-based regimens resulted in the highest mean peak mucositis although the onset of mucositis was significantly delayed in comparison with the other regimens (busulfan, busulfan-cyclophosphamide, cyclophosphamide-TBI, cyclophosphamide-carmustine with or without etoposide). In patients undergoing an autologous transplant, prior radiation therapy, a diagnosis of non-Hodgkin’s lymphoma (NHL), and a regimen containing etoposide for the mobilization of progenitor cells was associated with more severe mucositis and a prolonged length of hospital stay. Certain chemotherapeutic agents such as etoposide and methotrexate may also be secreted in the saliva thereby intensifying oral mucositis.
Gut Injury
Signs and symptoms
Much more is known about oral mucositis than its gastrointestinal counterpart. Indeed, there are no reliable data on the incidence of gut injury although bowel symptoms like nausea, vomiting, diarrhea, and abdominal complaints affect almost every HSCT recipient. Moreover, the exact course and severity of gut injury are difficult to ascertain because many patients receive narcotic analgesics to relieve pain associated with oral mucositis thereby masking or dampening gastrointestinal complaints. Measurements like fecal volume according to the National Cancer Institute (NCI)–Common Toxicity Criteria and parameters such as the days of diarrhea and duration in days of parenteral nutrition are commonly used to evaluate gut injury. Use of the latter, although convenient, is heavily influenced by several other factors like oral pain, dysphagia, and the patency of central venous catheters but also by physician preference, department protocol and hospital policy. Whether patient-related risk factors play any role in gut injury remains to be seen. By contrast, treatment-specific risk factors have been reported. Diarrhea is related to specific drugs like 5-fluorouracil, docetaxel, and cytosine arabinoside. The active metabolite of irinotecan (CPT-11), SN-38, can be deconjugated in the gut by bacterial β-glucuronidase resulting in mucosal damage. When TBI is included in the conditioning regimen, significantly more HSCT recipients experience severe diarrhea12; the duration of parenteral nutrition is prolonged in younger patients, those who receive a bone marrow transplant, and when TBI is included in the regimen.2 Diarrhea that develops following allogeneic HSCT is most likely attributed to acute graft-versus-host disease (GVHD) but this was only true in 72 of the 150 (48%) of the episodes in a prospective study in which biopsies were obtained endoscopically.13 Nausea and vomiting are universal in the first 24 hours after high-dose chemotherapy without antiemetic therapy and a later onset is particularly common with cyclophosphamide. Nausea and vomiting can also persist for 2–8 weeks after autologous HSCT without evident signs of mucositis or obstruction. Gastroparesis appears to play an important role because symptoms of bloating, nausea, and vomiting are consistent with delayed gastric emptying,14 which, in turn, is influenced by the use of opiates but could also be due to autonomic neurologic damage induced by the preparative regimen.
Noninvasive tests
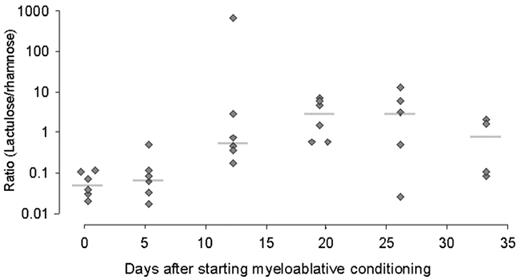
Hence, it is difficult to determine gut toxicity based solely on signs and symptoms. Alternative tests are therefore necessary to describe gut injury. For instance, radionuclide imaging and ultrasound can be used to document the severity or extent of bowel mucositis by measuring bowel wall thickness. Significant bowel thickening > 4 mm was observed almost exclusively in patients having the clinical picture of neutropenic enterocolitis,15 which is considered an extreme manifestation of gut MBI. Permeability tests are noninvasive and generally accepted to be surrogate markers of intestinal disease or damage in patients treated for cancer.16 Alterations in permeability and loss of epithelial surface are the principle features of gut injury. Permeability can be measured in vivo by means of urinary excretion of test substances that have been ingested or by detecting their presence in blood. Various sugars (lactulose, rhamnose, xylose, mannitol), polymers of polyethylene glycol (PEG) or 51Cr-labelled ethylenediaminetetraacetic acid (51Cr-EDTA) have been used to determine gut permeability in different populations of patients given different chemotherapy regimens. Permeability increases within 2 days after initial therapy and continues to increase for about another 10 days when it reaches a plateau, which persists for another 1 to 3 weeks.17
The lactulose/rhamnose ratio
The lactulose/rhamnose ratio is commonly used as an index to reflect changes in the permeability and gut surface area following chemotherapy, which assumes that premucosal and postmucosal factors equally affect both sugars. The lactulose/rhamnose ratio will be higher after high-dose chemotherapy due to an increase of lactulose absorption through open tight junctions together with a decrease of rhamnose absorption due to loss of surface area of the gut epithelium18 (Figure 2 ). However, the permeability test is nonspecific and is also altered when acute GVHD develops after allogeneic HSCT.19 When using a nonmyeloablative regimen for HSCT, intestinal permeability as measured by 51Cr-EDTA is preserved, just as diminished gut complaints would indicate.20 After myeloablative therapy, clinically scored gastrointestinal but not oral toxicity was consistent with a significant increase in permeability, which, nonetheless, could not predict the clinical severity.21 The lactulose/rhamnose ratio is correlated poorly with clinically scored gut toxicity indicating that permeability tests measure another feature of gut injury than do gut complaints.22 The altered permeability reflects damage to the small intestinal mucosa that is associated with an increase of apoptosis in crypts that precedes hypoplastic villous atrophy and loss of enterocyte height as has been documented by biopsies of duodenal tissue after intensive chemotherapy.23
Pathophysiology of Mucosal Damage
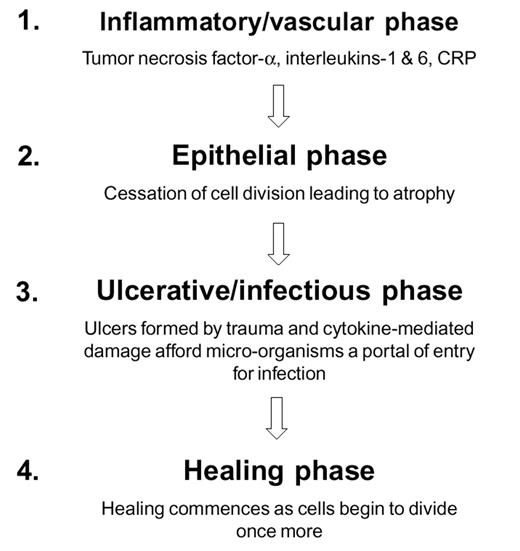
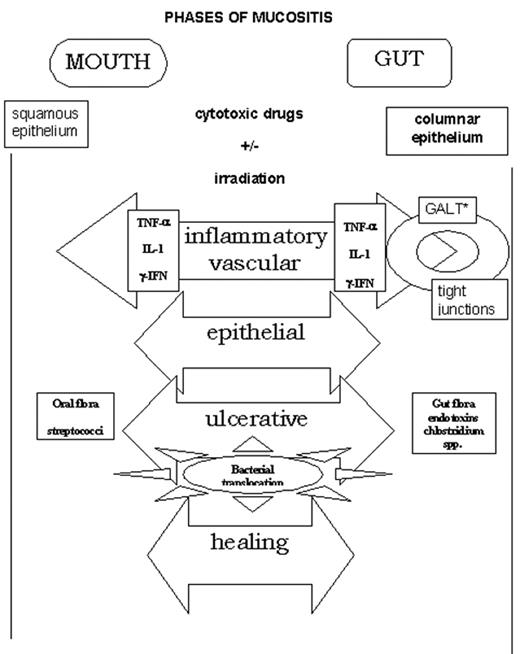
In the past, mucositis was assumed to be the result of both the direct effect of cytotoxic therapy on cells with a high mitotic index, including normal epithelial cells of the mouth and gastrointestinal tract, and the indirect effect of local infections associated with evolving neutropenia. However, this does not explain why similar patients receiving comparable chemotherapy regimens develop different degrees of MBI. Sonis described a new model to explain the development of oral mucositis. It comprises 4 different phases—inflammatory, epithelial, ulcerative, and healing. For the first time the role of the proinflammatory cytokines, interleukin (IL)-1, and tumor necrosis factor (TNF)-α released in response to cytotoxic therapy is integrated with the effects upon local immunity and the oral microflora24 (Figure 3 ). This model has been adapted to the gastrointestinal tract because of major differences in architecture of mucosal tissue between mouth and the other parts of the digestive tract17 (Figure 4 ).
In contrast to the mouth, resident lymphocytes, intraepithelial lymphocytes, and macrophages located in the extensive gastrointestinal–associated lymphoid tissue (GALT), rather than circulating cells, are the main targets that respond to irradiation and chemotherapy by releasing proinflammatory cytokines (inflammatory phase). Moreover, gut epithelial cells are capable of producing and secreting proinflammatory cytokines themselves and upregulate the production of MHC class II molecules and other adhesion molecules. The next phase (epithelial phase) is different because of the hierarchical distribution of mitotic activity of clonal stem cells, located in the crypts. This results in different sensitivity to cytotoxic drugs and irradiation and leads to apoptosis of crypt cells and decreased cell renewal.25 The microflora play a key role in the next phase (ulcerative phase) in the formation of ulcers as atrophy of the epithelial lining occurs. The intestinal epithelial cells are active participants in innate and adaptive immune response and produce cytokines in response to specific antigens of microorganisms like endotoxin, peptidoglycan, and other bacterial antigens. Bacterial translocation via several different pathways occurs when the barrier is disrupted. The last phase (healing phase) depends upon the rate of recovery of stem cells that are able to repopulate the epithelium to a decreasing extent toward the rectum and also upon the production of site-specific factors like trefoils that stimulate local restoration and migration of crypt cells.
Variability in the extent of mucositis
The variability in the extent and degree of mucositis might be explained by genetic differences in the expression of proinflammatory cytokines or proteins that control stem cell apoptosis, for example p53 and bcl-2 along the GI-tract.25 The role of activated transcription factors like nuclear factor-kappa B (NF-κB) by cytototoxic drugs is now the subject of study with respect to inflammation, cell growth, differentiation and apoptosis of epithelial cells (Bea van’t Land, personal communication).
Until recently, pharmacokinetics received little attention. Indeed, the simple question of whether dose determinations of cytostatic drugs should be based upon surface area or actual or ideal weight is still unanswered for many cytotoxic drugs. Variability in the timing of administration of chemotherapeutic agents that act on topoisomerase II has implications for the rate of mucositis because of the circadian rhythm of their expression. Genetic variability in drug response as a result of molecular alterations of drug-metabolizing enzymes, receptors, and transport proteins may be of greater importance and perhaps might predict drug concentrations to optimize the therapeutic effect while minimizing toxicity.26 Genetic polymorphism of the cytochrome P450 enzyme family located in the liver and gut is becoming of interest as reports are being published about interactions between alkylating chemotherapeutic agents and drugs given for other purposes particularly in the transplant setting. For instance, the antifungal, itraconazole, decreases clearance of busulfan. The resultant increase in the area under the curve of busulfan is related with more gastrointestinal toxicity. This might explain the greater incidence of some gut toxicity reported after treatment with itraconazole.27 The interval between busulfan and cyclophosphamide administration can negatively affect the pharmacokinetics of cyclophosphamide.28 The antibacterial fluoroquinolone, ciprofloxacin, also suppresses certain cytochromes P450 at the transcriptional level decreasing clearance of cyclophosphamide.29 A very interesting observation was made 20 years ago but never followed up on; it was observed that a priming injection of cyclophosphamide was shown to reduce gut toxicity in patients receiving high-dose melphalan. This suggests that clearance of cytotoxic drugs is important in the development of MBI. The variability in the pharmacokinetics and pharmacodynamics of anticancer chemotherapy is also influenced by the presence of an inflammatory response to conditions such as infections, cancer, and, of course, mucosal injury.30
Clinical Consequences of Mucosal Damage
Damaged mucosa leads to significant morbidity and impaired quality of life, especially in the setting of HSCT. The extent and severity of oral mucositis is significantly correlated with days of fever, parenteral narcotic use, total parenteral nutrition, antibiotic therapy, and the total number of days in hospital. The risk of significant infections and mortality was higher in those transplant recipients who had ulcerative mucositis with total hospital charges that were almost $43,000 higher.1 An increased risk of bacterial infection and mortality has been associated with more severe mucositis.2 Oral viridans streptococcal (OVS) infections are related to MBI of the upper part of the digestive tract, particularly the oral cavity, whereas enteric Gram-negative bacillary infections and neutropenic enterocolitis are related to the lower part of the digestive tract.
Gram-positive infections—oral viridans streptococci
Although infection can originate from exogenous sources, the majority of organisms responsible for infection arise from the patient’s own endogenous microflora, particularly from those residing on the mucosal surfaces of the mouth and gastrointestinal tract. There has been a shift from Gram-negative bacilli to Gram-positive cocci which now account for 3 of every 4 bacteremic isolates in patients with hematological malignancies (SCOPE project).31 Recipients of autologous HSCT with oral ulcerative mucositis were found to be 3 times more likely to develop OVS bacteremia than those without mucositis and OVS bacteria were isolated from the blood at a median of 6 days (range 2–8 days) after transplant.32 Drug-induced achlorhydria and the use of antimicrobial prophylaxis with fluoroquinolones contribute toward the development of OVS bacteremia.33 One particular group of OVS, the mitis group which comprises the species S mitis and S oralis, is reported to be associated with more serious complications like sepsis and adult respiratory distress syndrome particularly following chemotherapy with high-dose cytarabine. These complications are associated with a high mortality (80%) and occur within a few days of the onset of bacteremia. Recently, a simple scoring system for predicting streptococcal infection was proposed.34
Gram-positive infections—coagulase-negative staphylococci
Coagulase-negative staphylococci (CoNS) were the most frequently isolated microorganisms of the SCOPE project.31 CoNS bacteremia is frequently related to the use of central venous catheters, as CoNS are predominant members of the skin microflora. However, CoNS are also present in the endogenous flora of the mucosa of mouth and gastrointestinal tract of neutropenic patients. Plasmid pattern analysis on CoNS bloodstream isolates revealed that the mucosa was the origin in 70% of hematology patients. CoNS bacteremia mostly occurred within the first 2 weeks after transplant when gut integrity was markedly disturbed.35
Gram-negative bacillary infections
Escherichia coli and Klebsiella species remain the most common causes of Gram-negative bacillary bacteremia, although Pseudomonas aeruginosa accounted for 14% of the isolates and was associated with the highest mortality (40%) amongst HSCT recipients despite adequate empiric antibiotic therapy. Unusual but more resistant pathogens, such as Acinetobacter species, Stenotrophomonas maltophilia and Capnocytophaga species are occasionally isolated and are related to either gastrointestinal or oral mucositis.36
Anaerobic infections
The severity of mucosal damage was an independent predictor of anaerobic bloodstream infections, which occurred in 23 of 611 transplants with a rate of 4 infections per 100 HSCT procedures. Fusobacterium nucleatum was the most frequently isolated pathogen a week after transplant.
Neutropenic enterocolitis
Neutropenic enterocolitis (NE) or typhlitis is the most severe example of cytotoxic therapy-induced mucosal damage of the gut and cannot be diagnosed by clinical signs of fever, abdominal pain, and diarrhea alone. Mortality rates vary between 40% and 90% depending on the selection criteria.37 Patients fitting the clinical picture of NE undergoing ultrasonography can be graded by their bowel wall thickness as a bowel wall of more than 10 mm indicates poorer outcome. This serious complication illustrates how the delicate balance between host and microflora can be totally disturbed by MBI in the setting of prolonged exposure to antibiotics prophylaxis. Necrosis of the mucosal surfaces provides a portal of entry for several toxin-producing bacteria like Staphylococcus aureus, Pseudomonas aeruginosa, and Clostridium species. Not surprisingly, fungal pathogens were cultured as new microorganisms at autopsy in more than 50% of patients who died with NE. Interestingly, xylose malabsorption occurred earlier after remisson-induction chemotherapy among patients who developed NE.
Candida infections
Candida species are commensal yeast that normally reside on the mucosal surfaces of the digestive tract and vagina. Adherence to these surfaces appears to be a prerequisite for local mucosal infection and subsequent invasive disease since regular surveillance cultures of hematological patients have shown that colonization invariably precedes infection.38 The local oral infection with Candida species on top of mucositis increases local discomfort and pain and reduces oral intake. Mucosal damage is a risk factor for invasive candidiasis (candidemia and/or hepatosplenic candidiasis) among the patients receiving antineoplastic therapy. Patients treated for AML who developed invasive candidiasis had significantly lower serum D-xylose levels with the maximum difference noted at week 2 after start than patients without invasive disease. Candidemia is also correlated with NE.39 HSCT recipients prepared with regimens composed of TBI and patients treated with remission-induction regimens containing either high-dose cytarabine or an anthracycline, have an increased risk of developing invasive disease.
These data support the contention that mucosal damage provides a portal of entry to the systemic circulation for commensal oral and gastrointestinal microorganisms (Figure 3 ). Thus, the ability to treat or prevent severe MBI is an important step toward decreasing the infectious complications experienced by patients given intensive chemotherapy.
Acute graft-versus-host disease
MBI induced by cytotoxic chemotherapy and GVHD of the gut present with similar signs and symptoms and even permeability tests are unable to distinguish these entities. Hence only histology can confirm the diagnosis. Tissue damage of the gut caused by conditioning regimens plays a role in triggering acute GVHD. The epithelial cells and immune cells of the GALT release proinflammatory cytokines, which upregulate expression of adhesion molecules and histocompatibility proteins. Donor T-lymphocytes recognize unregulated host mucosal antigens and macrophages of acceptor and donor are further activated by translocated endotoxin leading to mucosal damage of the gut by acute GVHD (aGVHD). The gut is not only a major target organ of aGVHD but also a critical amplifier of systemic GVHD.40 Intensification of conditioning may also result in more mucositis and correlate with more severe GVHD directly as a result of toxicity or indirectly as a result of inadequate GVHD prophylaxis.41–,43 Prior animal and clinical studies of monoclonal antibodies to TNF-α, soluble IL-1 receptor, and IL-1 receptor antagonist (IL-1RA) suggested that specific inhibition might be effective in preventing GVHD. However, a double-blind randomized trial of recombinant human IL-1 receptor antagonist given to 186 HSCT recipients failed to show any reduction in GVHD or toxicity, including mucositis.44 Polymorphisms of the IL-1 genes might have influenced severity or TNF-α is the dominant cytokine in mediating GVHD and its production is stimulated by translocated endotoxin (which is a constituent of Gram-negative bacilli of the normal commensal bowel flora). Mice given an allogeneic HSCT with LPS-resistant bone marrow had significantly less TNF-α production and less severe GVHD.45 Decontamination of the gut reduces clinical GVHD although there are several other bacterial products, besides endotoxin, that might play a role in triggering GVHD.46
Systemic inflammatory response syndrome
Patients who develop severe complications after HSCT tend to have significantly higher levels of cytokines around the time of transplant with an excess of proinflammatory cytokines. This results in vascular endothelial damage and is known as the systemic inflammatory response syndrome (SIRS). Major complications such as GVHD, acute respiratory distress syndrome (ARDS), thrombotic microangiopathy, veno-occlusive disease, and central nervous system disorders can be considered manifestations of SIRS.47 The MBI that occurs following conditioning therapy is accompanied by the release of proinflammatory cytokines—the first manifestation of SIRS. Mucosal damage could therefore be used as a marker of systemic toxicity and damage to other organs. However, unlike C-reactive protein, which is easy to measure, the detection of cytokines is not straightforward. Regular monitoring of CRP after transplant can be used to identify patients at risk of severe transplant-related complications and even mortality.48 As mentioned previously, inflammation influences the pharmacokinetics and pharmacodynamics of drugs. Exposure of intestinal epithelial cells to proinflammatory cytokines decreases the mRNA expression of CYP3A4 and increases the multidrug resistance by increasing Pgp expression. The functional linkage in the enterocytes of CYP3A4 and Pgp will therefore limit bioavailability, even when oral intake of medication is successful.
Malnutrition
Metabolism in patients undergoing intensive cytotoxic treatment changes over time with the highest energy expenditure occurring about 14 days after transplant. At that time, oral intake is negligible and malnutrition together with severe weight loss would occur without total parenteral nutrition (TPN). However, even with TPN, there will still be a profound loss of fluid and electrolytes together with malabsorption and the hypoproteinemia associated with protein-losing enteropathy.
Summary and Treatment Options
MBI is a risk factor for severe infectious complications but is more than just a toxicological side effect of anticancer treatment (Table 1 ). The intact mucosa not only form a line of first defense against systemic infections but also play an active role in the crucial balance between host and environment, particularly in the setting of an allogeneic HSC transplant. The therapeutic potential of allogeneic transplant is related to the graft-versus-malignancy effect and protection of the mucosa could lessen any immunostimulatory effects of MBI and reduce GVHD.
All attempts thus far to prevent or ameliorate oral mucositis including mouth rinses with chlorhexidine and numerous other products have failed (Table 2 ). Trials of oral or parenteral glutamine supplementation yield conflicting results showing either no effect on mucositis or a decrease of infections. Allogeneic transplant recipients seemed to benefit the most, especially when TBI is used. Topical or systemically administrated granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) met a similar fate. The first clinical randomized Phase 1 trial of recombinant human keratinocyte growth factor indicated that patients treated with this agent had a lower rate of WHO grade 2–4 mucositis.49 IL-11 ameliorates thrombocytopenia following myelosuppresive chemotherapy, increases proliferation of mucosal basal cell layer, and partially suppresses apoptosis. In experimental studies, IL-11 almost completely prevented GvHD of the gut and reduced serum endotoxin levels after HSCT. However, a randomized placebo-controlled dose-finding study of IL-11 among patients given high-dose chemotherapy found no difference in the rate of WHO grade 3 or 4 mucositis between the active treatment group and the placebo group although incidence of bacteremia was decreased.50 Another double-blind randomized trial in which 40 patients underwent intensive chemotherapy demonstrated a reduction in bacteremia together with more normal lactulose/rhamnose ratios in the group given IL-11.51 This agent might therefore offer a new approach toward managing infectious complications occurring in patients with MBI.
However, better means of measuring oral and gut MBI are necessary and clearer definitions are required if progress is to be made in identifying those at greatest risk, and in designing and evaluating agents for treatment or, better still, prevention.
II. Invasive Fungal Infections: New Developments in Prevention, Diagnosis and Management
University of Arkansas for Medical Sciences, 4301 W. Markham Street, Mail Slot 776, Little Rock AR 72205-7101
Invasive fungal infections (IFIs) continue to represent a significant problem for patients with hematologic cancer. In this manuscript, we present an update on the newest developments in the diagnosis, prevention and management of IFIs in this patient population with a focus on a risk-adjusted approach and the practical implications for clinicians.
Pathogens Associated with Invasive Fungal Infections in Patients with Hematologic Cancer
With the introduction of novel antifungal, antiviral and antineoplastic therapies, significant changes in the epidemiology of fungal infections have been noted. These changes include a decrease in candidiasis with C. albicans, C. tropicalis and C. parapsilosis with an emergence of occasional infections by azole-resistant species such as C. glabrata, and C. krusei.1 In addition, an increase in the incidence of mould infections, particularly aspergillosis, has been observed together with an emergence of infections by non-fumigatus Aspergillus spp.2 and other moulds (Fusarium spp., Zygomycetes and others).3
The incidence of IFIs varies widely among patients with hematologic cancers and is highest among those undergoing allogeneic bone marrow transplantation (alloBMT) (Table 3 ).
Pathogenesis and Risk Factors for Fungal Infections in Patients with Hematologic Cancer
The pathogenesis of IFIs is closely related to the presence and severity of risk factors. It is, therefore, critical to identify these risk factors in patients at risk for these infections.3
The incidence and severity of IFIs result from the interaction of three factors (Table 4 ):
Previous fungal infection and colonization
Net state of immunosuppression (e.g., older age, active cancer, and prolonged and profound neutropenia and lymphopenia)
Organ dysfunction (e.g., renal failure, pulmonary dysfunction, mucositis, and severe GVHD)
Strategy for a Risk-Adjusted Approach to Fungal Infections in Patients with Hematologic Cancer
Determine the risk for fungal infection in specific patient populations
This is best accomplished by evaluating various risk factors (exposure, net state of immunosuppression, and organ damage) (Table 4 ). For practical purposes, one can distinguish 3 risk groups: high, moderate and low.
High-risk group: 15–30% incidence of IFI. This group includes alloBMT [and peripheral blood stem cell transplant (PBSCT)] recipients who have several of the following risk factors: age > 40 years, malignancy other than chronic myeloid leukemia, mismatched or matched unrelated transplant, graft failure, GVHD or corticosteroid therapy; Patients with acute myeloid leukemia who are > 55 years, receiving gut-damaging cytotoxic therapy (such as high dose cytarabine), and with poor performance status also belong in this high-risk category.
Low-risk group: < 5% incidence of IFI: younger (< 19 years) patients who have none of the risk factors mentioned above, but are undergoing alloBMT/PBSCT or therapy for acute leukemia. Autologous PBSCT patients also belong in this category.
Moderate-risk group: 5–15% incidence of IFI. These are patients who exhibit a small number of the risk factors mentioned under the high-risk group.
Perform radiologic and laboratory evaluation according to risk category
Laboratory and radiological evaluation depend on the risk group.
Cultures: Sputum, nose and bronchoalveolar lavage (BAL) cultures are the most common cultures obtained for invasive aspergillosis. For serious candidal infections, blood cultures remain the standard for diagnosis although serial mucosal cultures have an excellent negative predictive value (NPV) and an acceptable positive predictive value (PPV) for these infections.4
Radiological evaluation: High resolution computed tomography (CT) chest and sinus-CT are both useful at baseline (prior to commencing antineoplastic therapy) and for monitoring high-risk patients. The lung findings in invasive aspergillosis include nodules with an early halo sign during severe neutropenia that may be followed by air-crescent sign with recovery from myelosuppression.5 Of note, an increase in size of pulmonary lesions does not necessarily mean progression of infection as it could be related to the immune recovery.
Serology: Galactomannan antigen test is now FDA-approved for the detection of infection with Aspergillus spp. Surveillance testing of blood specimens is associated with good sensitivity, specificity, PPV and NPV.
False positive results may be observed in patients receiving piperacillin/tazobactam or amoxicillin/clavulanate.6,7 Decreased sensitivity was shown in children, and may also occur in patients with circulating aspergillus antibodies and those receiving antifungal agents active against Aspergillus spp. The cut-off point for true positive Galactomannan antigen test ranges from 0.5 to 1.5 though a consensus may be emerging for a 0.5 cut-off point for positivity.8 The Galactomannan antigen test has several advantages: it is non-invasive, easy to use, quantitative and specific for Aspergillus spp. A negative test may give a false sense of security, as patients may be infected with fungi that are not detected by this test. This test is best reserved for intermediate and high-risk patients.
1-3-beta-d-glucan antigen is a new promising diagnostic test that appears to be useful in detecting all fungal infections (yeasts and moulds) in the bloodstream and is currently undergoing evaluation (not yet approved by the FDA).
Polymerase chain reaction (PCR) is a very sensitive test for detecting aspergillus nucleic acids in various tissues.9 Problems with PCR are related to lack of a commercial product, cost, and risk of false positivity. Real-time PCR has been suggested as a promising diagnostic method for invasive aspergillosis.
Prevent and manage fungal infections according to risk category
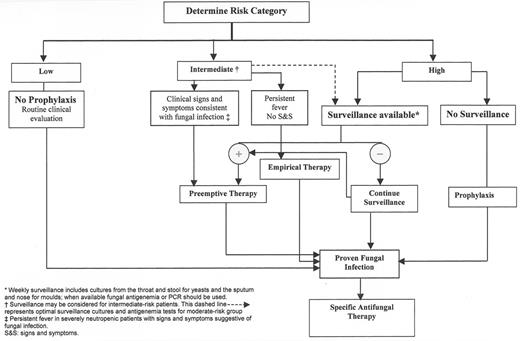
In view of the above-mentioned risk stratification, it is critical to develop a practical approach for managing IFIs according to risk in specific patients or patient populations. This approach may utilize antifungal agents for prophylaxis, pre-emptive, or empirical therapy or reserve their use for patients with established fungal infections. A description of these approaches follows:
Therapy of established infection: This approach relies on providing antifungal therapy to patients in whom an IFI has been documented.
Pre-emptive antifungal therapy: Pre-emptive therapy consists of initiating antifungal agents in persistently febrile neutropenic patients who have a probable IFI on the basis of significant colonization, positive serology, clinical, or radiological findings.
Empirical antifungal therapy: Empirical therapy provides antifungal agents to neutropenic patients who have persistent fever despite broad-spectrum antibiotics but do not exhibit the clinical or laboratory findings suggestive of IFI (mentioned above).
Antifungal prophylaxis: Primary prophylaxis provides antifungal agents before any evidence of fungal colonization or infection; it is usually given at initiation of immunosuppression. Secondary prophylaxis provides antifungal agents after recovery from a documented fungal infection and prior to additional immunosuppressive therapy.
Infection control: Sources of Aspergillus spores in hospitals include air, dust, construction area, ventilation system, potted plants, flowers, cereals, nuts, spices and carpets. A newly investigated source is water with secondary aerosolization.10
Measures to reduce exposure of high-risk patients to airborne moulds include:
Preventing dust accumulation by cleaning of all surfaces, isolating patient care wards from outside air, maintaining negative pressure in construction areas and providing patients with masks when moving into unprotected areas.
Installing HEPA filtration in high-risk areas. HEPA filtration is inexpensive and reduces mould airborne spores. By contrast, laminar airflow is costly and is built-in only.
Investigating potential outbreaks.
Avoiding patient exposure to tap water during severe immunosuppression, using sponge baths instead of showers and cleaning the showering facility prior to use.
Measures to reduce hospital-acquired candidal infections in these patients rely on handwashing, an important, simple and inexpensive infection control strategy.
Antifungal Armamentarium
Antifungal agents11
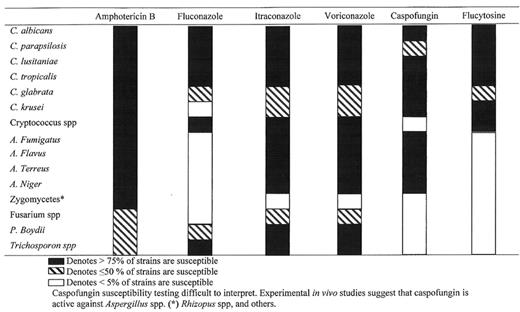
Several antifungal agents are currently available. The properties, in vitro activities and dosing schedules of the available antifungal agents are summarized in Tables 5–Table 6,Table 7,Table 8,9 , and Figure 5 .
Polyenes: Amphotericin B deoxycholate and lipid formulations of Amphotericin B (Table 5 and66,Figures 5 and66): Antifungal polyenes have a broad spectrum of activity including most Candida and Aspergillus spp., C. neoformans, and the zygomycetes. Their activity is limited against Fusarium spp., Trichosporon spp. and Scedosporium spp.11
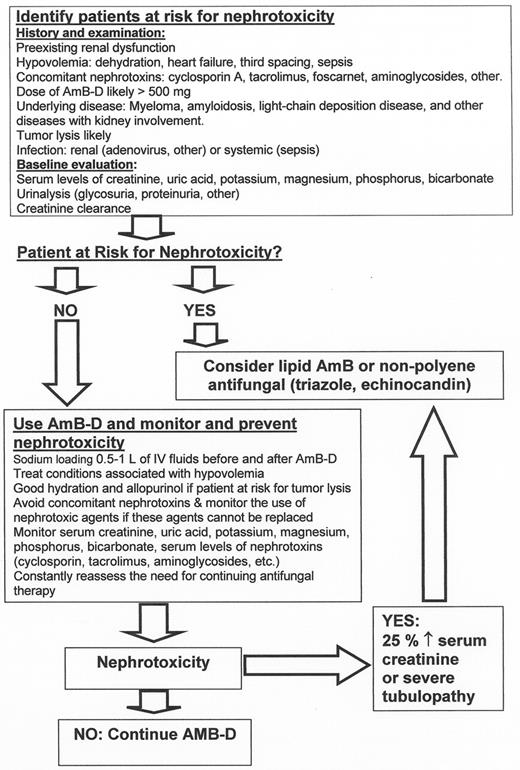
Amphotericin B deoxycholate (AmB-D) is associated with several adverse events:
Nephrotoxicity: azotemia (up to 50%), potassium and magnesium wasting (25–50%), renal tubular acidosis (50–100%), polyuria (50–100%), and anemia (up to 50%).
Infusion-related toxicities, including anaphylaxis, hypertension, hypotension, fever, rigors, hypoxemia and cardiac arrhythmias.
Nephrotoxicity is the most important dose-limiting toxicity of AmB-D. It is in general dose-dependent11 and is associated with prolonged hospitalization and increased mortality.
The newer lipid formulations of AmB-D are less nephrotoxic. These agents are, however, significantly more expensive and not necessarily problem-free. The risk of infusion reactions and nephrotoxicity is compound-specific and is in the following order: AmB-D > ABCD > ABLC > LAMB.11
Triazoles: Fluconazole, itraconazole, voriconazole (Tables 5–9T9, Figure 5 ): Fluconazole is characterized by water solubility, linear renal excretion, modest drug interactions, and relatively narrow spectrum (mostly yeasts).11 Absorption is generally excellent, and drug interactions are mild to modest. Fluconazole is also as efficacious as amphotericin B in treatment of non-neutropenic patients with candidemia, and is considered the antifungal of choice for prophylaxis aimed at Candida spp. infection.12
Itraconazole is characterized by poor water solubility, variable oral absorption, complex degradation in the liver, and extensive drug interactions. The drug is active against yeasts, and various moulds including Aspergillus species and appears to be effective in preventing invasive aspergillosis in high-risk patients.11,13– 15
Itraconazole is available both orally (capsule and solution) and via IV. Itraconazole capsule has an erratic bioavailability; especially in patients with hypochlorhydria (bioavailability improves if the drug is taken with fat-rich food and a cola beverage). Increased and more predictable absorption of itraconazole can be achieved by using the cyclodextrin-based solution, which does not require gastric acidity or fat-rich meals, and is better absorbed away from meals. The IV formulation is most useful for patients with gut dysfunction. Itraconazole suffers from significant drug-drug interaction requiring close monitoring.11 Patients should avoid taking concurrent drugs (antacids, H2 or proton pump blockers) that impair the absorption of the oral capsule or accelerate the metabolism of itraconazole (rifampin, rifabutin, phenytoin, and barbiturates among others) (Table 9 ). Dosage-adjustment and close monitoring for toxicity is required when agents whose metabolism is affected by itraconazole (cyclosporine A, tacrolimus, benzodiazepines, digoxin, HMG-CoA reductase inhibitors, others) are used concomitantly with this agent (Table 8 ). Itraconazole is well tolerated. Side effects include nausea, vomiting, diarrhea, and skin rashes. Transient elevations in serum transaminases may occur while fulminant hepatotoxicity and liver failure is extremely rare. Hypertension, edema, and hypokalemia have been reported in patients receiving higher doses of itraconazole. Itraconazole has also been associated with negative inotropic effect, a factor that should be considered in patients receiving cardiotoxic agents such as the anthracyclines or cyclophosphamide.
Voriconazole is available as IV and oral formulation. The drug has good oral bioavailability, and is active against moulds and yeasts. Voriconazole has been shown to be more effective and better tolerated than conventional amphotericin B in patients with invasive pulmonary aspergillosis16 and as effective as Liposomal amphotericin B as empirical treatment of febrile neutropenia.17
All azoles inhibit CYP3A4, the primary oxidative drug-metabolizing enzyme in humans. Voriconazole also inhibits CYP2C9/19. Some agents (Rifampin, long-acting barbiturates and carbamazepine) are con traindicated in patients receiving voriconazole because they greatly reduce voriconazole exposure (Table 9 ). Patients treated with certain agents (cyclosporine A, tacrolimus, sirolimus, HMG-CoA reductase inhibitors, others) should be closely monitored because the drug concentrations of these drugs are substantially increased by voriconazole (Table 8 ).18 Voriconazole is generally well tolerated and exhibits the same gut, skin and liver toxicity observed with itraconazole. In addition, voriconazole causes generally mild and transient visual disturbances (brightness, blurring, light sensitivity, or changes in color vision). Typically, these abnormalities are observed in around 30% of patients, starting 30 minutes after the voriconazole dose, and lasting about 30 minutes. Patients should avoid driving at night. Skin photosensitivity has also been observed (~6% of patients), particularly among those receiving longer (> 12 weeks) courses of therapy. Direct exposure to sunlight should be avoided.
Posaconazole is a triazole that is structurally related to itraconazole and possesses a broad spectrum of activity including yeasts and moulds. Among Candida spp., the agent appears to be least active against fluconazole- and itraconazole-resistant strains (C. glabrata, C. krusei, and others).19 Limitations of posaconazole are the lack of IV formulation and the somewhat erratic bioavailability of the drug in the setting of severe mucositis. Posaconazole is currently undergoing Phase III clinical trials.
Ravuconazole is structurally related to fluconazole and voriconazole. The agent possesses broad-spectrum antifungal activity and is formulated as a prodrug thus obviating the need for a solubilizing agent such as a cyclodextrin.19 Ravuconazole is available PO and IV and may be associated with less drug interaction than the other mould-active triazoles. Ravuconazole is currently in Phase II clinical trials.
Flucytosine (Table 5 andFigure 5 )11: Flucytosine is the only member of the group of antifungal antimetabolites. The drug is active against most Candida spp., C. neoformans and some of the dematiaceous fungi but should not be used as monotherapy because of the risk of emergence of resistance and should be given in combination. The major toxicities of flucytosine are myelosuppression, gastrointestinal intolerance, and hepatic toxicity and are related to drug accumulation
Echinocandins: Caspofungin (Tables 5 and6, Figure 5 )11: Echinocandins represent a new class of antifungals that inhibit the synthesis of β 1,3 d-glucan, an essential component of the fungal cell wall. The drug is available only IV. Caspofungin is fungicidal for Candida spp.20 and is most active against Candida albicans, C. tropicalis and C. glabrata. Caspofungin is active against Aspergillus spp. but lacks activity against Cryptococcus neoformans and various moulds.20
Caspofungin decreases the area under the curve (AUC) of tacrolimus requiring monitoring of tacrolimus concentrations. Some drugs may decrease the concentration of caspofungin such as phenytoin, rifampin, and dexamethasone. In patients receiving these agents, the daily maintenance dose of caspofungin should be raised to 70 mg IV.21 Cyclosporine increases the concentration of caspofungin by 30%. Caspofungin is generally well tolerated and may be occasionally associated with fever, skin reactions, rash, infusion site phlebitis and transient increase in liver function tests. Mild-moderate histamine-mediated infusion reactions may occur. In a randomized, double blind study comparing caspofungin and amphotericin B for invasive candidiasis, caspofungin therapy resulted in a higher response rate than amphotericin B and was better tolerated.22 A study of caspofungin as therapy for presumed or probable aspergillosis was conducted in 83 patients with various conditions (including hematologic cancer) who had failed to respond to or tolerate standard antifungal therapy. Response (complete and partial) was observed in 45% of patients.23
Micafungin is an echinocandin with potent in vitro and in vivo activity against a variety of pathogens including Candida spp. and Aspergillus spp. The drug is well tolerated and appears effective. Micafungin has been recently compared to fluconazole in a randomized, double blind trial for prophylaxis of IFIs in patients undergoing allogeneic and autologous BMT/PBSCT. Compared to fluconazole, micafungin significantly reduced the incidence of probable and proven invasive fungal infections (P < .05), was as effective as fluconazole for the prevention of candidiasis with a strong trend toward reduction in invasive aspergillosis (P = .08).24
Anidulafungin is another echinocandin that possesses similar antifungal spectrum and activity and is undergoing Phase II clinical trials.
Cytokines and granulocyte transfusions
Enhancing the patient’s immune status is another approach to managing IFIs in patients with hematologic cancer. Potentially useful approaches include:
Granulocyte colony-stimulating factor (G-CSF): Polymorphonuclear cells (PMNs) from patients treated with G-CSF possess enhanced in vitro activity against A. fumigatus, Rhizopus arrhizus, and C. albicans.25 Data to support the use of G-CSF in preventing IFIs are lacking and G-CSF elicits a Th-2 response, usually associated with poor outcome in patients with serious mycoses.
Granulocyte-macrophage colony-stimulating factor (GM-CSF): GM-CSF stimulates macrophages to release pro-inflammatory cytokines25 and can overcome steroid-mediated suppression of monocytic activity against Aspergillus.26
Gamma interferon (IFN-γ): IFN-γ enhances human neutrophil oxidative burst, and neutrophil and monocyte-mediated damage to A. fumigatus, F. solani hyphae, and C. albicans pseudohyphae.27 and reverses corticosteroid-induced immunosuppression of human neutrophil-induced damage to A. fumigatus hyphae.27 IFN-γ has been also shown to enhance the antifungal activity of elutriated human monocytes against A. fumigatus hyphae.28 In humans, IFN-γ enhances the ability of neutrophils to damage Aspergillus hyphae ex vivo,29 accelerates the sterilization of the cerebrospinal fluid in cryptococcal meningitis,30 and has been associated with improved clinical response in patients with acute leukemia and refractory IFIs.31
Granulocyte transfusions: Recent reports have provided encouraging results on the role of granulocyte transfusions, elicited with G-CSF with or without steroids in patients with IFIs.32 Related and or community donors can be utilized.
Granulocyte transfusions should be considered in patients with refractory IFI, who continue to be profoundly neutropenic but whose prospects for recovery from myelosuppression and disease control are good. Mild reactions to the granulocyte transfusion products are common, including fever and chills, and can be reduced if the infusion rate is slowed. Severe side effects, namely hypotension or respiratory distress, are estimated to occur in ~1% of recipients; GVHD is prevented by irradiation of the product pre-infusion with 15–30 Gy.
Risk-Adjusted Recommendations
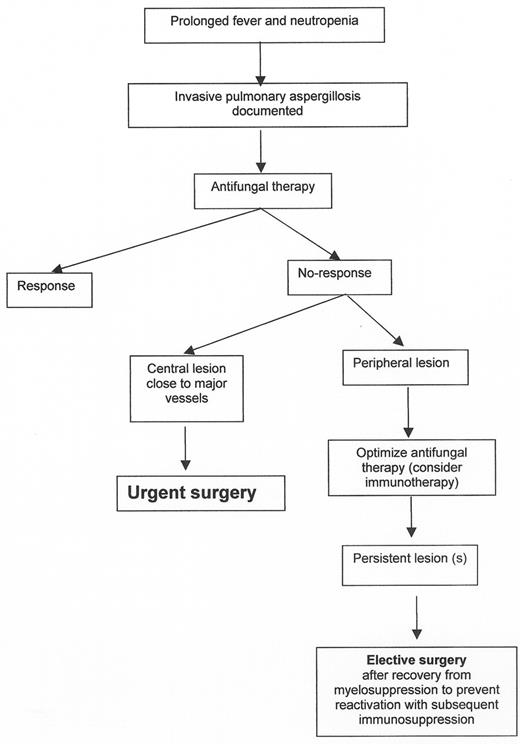
Primary antifungal prophylaxis (Figure 7)
Antifungal prophylaxis should be provided to patients at high-risk for IFI and should be tailored to the patient population. Those likely to develop hematogenous candidiasis only are best offered oral fluconazole while those at risk for aspergillosis should probably receive itraconazole (voriconazole is also likely to be effective but has not been tested in this setting).14,15,33,34 Itraconazole should be given at maximal tolerated dose (600–800 mg/d), preferably as solution (not capsule) or IV in the presence of significant gut dysfunction. Other options include micafungin (when it becomes available)24 and lipid amphotericin B.
Appropriate infection control measures should be optimized in these patients at high-risk for IFIs.
Secondary antifungal prophylaxis
Patients with a prior episode of mould infection and who are at risk for relapse when recieving additional immunosuppression should be given secondary antifungal prophylaxis. Following recovery from myelosuppression, these patients can be treated with itraconazole, voriconazole or an IV polyene that is safe for prolonged use (not AmB-D); all residual sites of infections should be resected if possible, and the patient closely monitored with serologic (PCR, Galactomannan antigen) and radiologic tests. Therapy for the primary infection should be continued despite achievement of complete response. Immediately prior to commencing additional immunosuppression, antifungal therapy should be optimized (agent, dose, schedule, and route), and consideration given to utilizing less immunosuppressive antineoplastic agents if possible. Prophylactic GM-CSF and in select patients, pre-emptive granulocyte transfusions to avoid profound neutropenia may be used.
Empirical and pre-emptive therapy (Figure 7)
Empirical/pre-emptive therapy should be utilized in patients at moderate risk for infection when an IFI is suspected (colonization, positive serology, clinical, or radiological findings). Agents effective in this setting include AmB-D, itraconazole, voriconazole, LAMB, and ABCD. Recent metaanalyses support the use of LAMB and itraconazole in pre-emptive/empirical antifungal therapy (Table 10 ).
Therapy of documented infection (Figure 8)
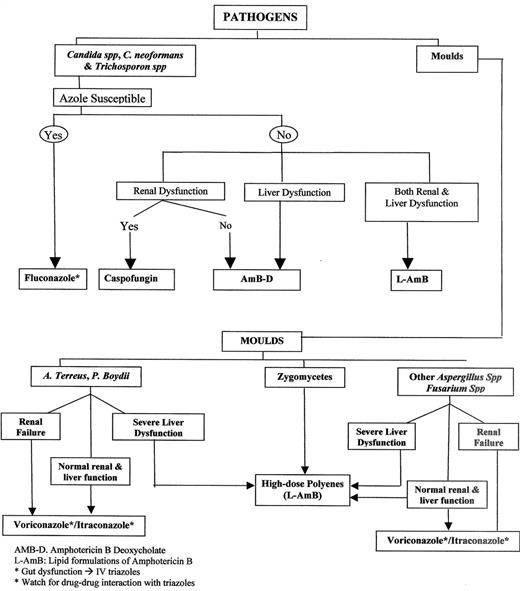
Candidiasis is effectively treated with caspofungin, an IV polyene or fluconazole. Selecting the optimal agent to use depends on the pathogen, the site (s) involved, and the presence of co-morbidities. Triazoles are in general safe on the kidneys, available PO for long-term outpatient therapy, and particularly effective against pathogens like A. terreus. Triazoles (other than fluconazole), however, suffer from extensive interaction with drugs frequently used in patients with hematologic cancers (Table 8 ) and may cause liver dysfunction.
Combination antifungal therapy (2 or 3 drugs: a polyene, a triazole and caspofungin) is currently being utilized for mould infections but without supporting evidence. Combinations may be considered, prior to identification of the mould pathogen, given the differential susceptibilities of fungi to the available antifungal drugs (Figure 5 ).
Therapy should be continued until complete response, resolution of immunosuppression (absolute neutrophil count > 2000, CD 4 count > 400), and cessation of immunosuppressive therapies.
Additional therapeutic measures include reduction or discontinuation of immunosuppressants and resection of infected foci (Figure 9 ). Consideration should also be given to granulocyte transfusions in the neutropenic patient and to cytokines (IFN-γ or GM-CSF) in patients with adequate neutrophil counts. Secondary antifungal prophylaxis should always be offered to patients undergoing subsequent immunosuppression.
Conclusion
The increase in the incidence and severity of IFI in patients with hematologic cancer has led to the development of various preventive strategies, including prophylaxis with antifungal agents. While effective, these strategies are associated with toxicity, high cost, and potential emergence of resistance. The risk-adjusted strategy we recommend provides a balance between the problems associated with antifungal prophylaxis and the high morbidity and mortality of IFI in this patient population.
III. Managing Infections Associated with Purine Analogues and Monoclonal Antibodies
Susan O’Brien, MD*
University of Texas, MD Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston TX 77030
Infections are a major cause of morbidity and mortality in patients with chronic lymphocytic leukemia (CLL).1–,7 Up to 50% of patients suffer from recurrent infections. The spectrum of infections in patients with CLL treated with conventional therapy is mainly bacterial.2,6 Infections with Streptococcus pneumoniae, Staphylococcus aureus, and enteric Gram-negative organisms are commonly seen.7–,9 Infections predominantly affect the respiratory tract, skin, or urinary tract, with a significant incidence of sepsis.1,7,10– 16
Herpes simplex and herpes zoster are also frequently diagnosed in patients with CLL, they are particularly common in advanced disease and after prior therapy.9,16–,18 In most cases these infections are localized but disseminated disease may be seen, particularly when there has been a delay in diagnosis. Fungal infections also are more common in patients with advanced disease and therapy or disease-related neutropenia.16,19,20
Opportunistic infections, such as Pneumocystis carinii, are uncommon in patients treated with conventional chemotherapy.21 Other infections, such as tuberculosis, are uncommon due to relative preservation of cellular immunity in early stage CLL. With the use of nucleoside analogs (e.g., fludarabine) or alemtuzumab, particularly in combination with corticosteroids, more unusual infections have been described, including listeriosis and P. carinii pneumonia (PCP).22–,24 There is also an increased frequency of invasive mycoses and mycobacterial infections in patients treated with nucleoside analog–containing regimens.22,25,26
Factors Predisposing to Infections in CLL
Hypogammaglobulinemia
Several studies have demonstrated a high incidence of hypogammaglobulinemia in patients with CLL.17,27–,33 There is a clear decline in immunoglobulin levels with increasing disease duration.17,28–,37 However, the effect of treatment on serum immunoglobulin levels is less clear. The etiology of hypogammaglobulinemia in CLL is poorly understood. Defects in cellular immunity, such as functional abnormalities of T cells resulting in dysregulation of nonclonal CD5− B cells, may be partly responsible.38– 39
Various studies have evaluated the frequency of hypogammaglobulinemia, the type of immunoglobulin (Ig) deficiency, and the impact on survival. Deficiencies of IgG, IgA, or IgM were not found to influence survival in a series reported by Ben-Bassat et al.31 In contrast, Rozman et al29 found that the survival of patients with CLL and an immunoglobulin level < 700 mg/dL was significantly shorter than that of patients with levels ≥ 700 mg/dL. Reduced levels of IgG and IgA were associated with reduced probability of survival (P = .027 and P = .014, respectively), whereas low IgM levels had no influence on survival.9,29
The association between low immunoglobulin levels and recurrent infections, usually with encapsulated organisms, has been well described in patients with CLL.2,15,17,18,27–,29,39–,43 However, patients with a normal serum immunoglobulin level may have recurrent infections, while some patients with severe hypogammaglobulinemia may remain infection-free for prolonged periods of time. Some authors have suggested that the relationship between a deficiency in a specific immunoglobulin class or subclass and the risk of infection may be more important. A recent report emphasized an association between infection and a low serum IgA level.38 Morrison et al44 found that 69% of patients with a low serum IgA concentration had at least four infections, compared with 27% of patients with a normal serum IgA level. Aittoniemi et al. also examined deficiencies of immunoglobulin classes and subclasses in patients with CLL.45 In multivariate analysis, the only independent risk factor for infection was a decreased IgA level. A specific IgG4 deficiency has been associated with severe, recurrent pulmonary infections.46
Copson et al47 reported lower levels of serum IgG3 and IgG4 in patients with CLL compared to those in age- and sex-matched controls. Deficiency of IgG3 may partly explain the increased risk for herpesvirus infections in patients with CLL because IgG3 is a known defense mechanism against these viruses.
Deficiencies in secondary antibody response to common antigens may further explain the increased risk for infection in patients with CLL.2,40 Low levels of pneumococcal antibodies were reported in 23 (39%) of 59 patients with CLL compared to 6 (11%) of 56 controls (P = .005). The association between low levels of antipneumococcal antibodies and hypogammaglobulinemia was not consistent: 12 patients with a low serum IgG level had a normal pneumococcal antibody level, and three patients with a low pneumococcal antibody level had a normal serum IgG level. The majority of patients with severe or multiple infections (13 of 18) had low levels of both total IgG and pneumococcal antibodies.40
Complement system
Several investigators have described abnormalities of the complement system in patients with CLL.43,48,49 Heath and Cheson50 described a defect in binding of the opsonin C3b to bacteria in 15 patients with CLL. Fust et al51 reported a reduction in mean C1 and C4 levels in more than 50% of the samples tested from 46 patients with CLL. No abnormalities of the alternative complement pathway were seen. Others have demonstrated depressed classic complement pathway activity in CLL.52,53 Schlesinger et al examined complement levels in 26 patients with CLL and reported that they were decreased in all of the patients with advanced stage disease and 40% of patients with early stage disease48 Interestingly, the authors also found deficiencies of at least one complement component in 11 first-degree relatives of these patients.
Cellular immunity
Although qualitative and quantitative abnormalities of T lymphocytes have been reported in CLL, the exact cause of these defects is not clear. However, it is likely that these T-cell abnormalities contribute to hypogammaglobulinemia and an increased risk of infection. Increased T-cell numbers with a relative increase of suppressor T cells (CD8) and a decrease of helper T cells (CD4) resulting in a reduced CD4/CD8 ratio is common in CLL.4,30,54,55 This is frequently seen with more advanced stages of disease. Both increased and decreased numbers of the suppressor-inducer phenotype (CD45Ra) have been reported.56,57
Studies of the qualitative function of cellular immunity have been inconclusive; both normal and decreased CD4 cell function have been reported58,59 while excessive, normal, or reduced CD8 cell function has also been described.55,58,60–,62 Burton et al63 suggested that T cells obtained from patients with CLL were not inherently dysfunctional but that this was caused by soluble factors elaborated by CLL B cells. Subsequently, Decker et al64 demonstrated no quantitative or qualitative differences in cytokine production or proliferative capacity when purified T cells from patients with CLL were compared with those from normal donors. However, addition of CLL cells as autologous accessory cells resulted in a marked increase in IL-2 production but not induction of IFN-γ or IL-4. Accessory cells, predominantly monocytes, from normal individuals resulted in a completely different pattern of cytokine stimulation. The authors concluded that T-helper cells from patients with CLL were intrinsically normal but that the predominance of CLL-B cells as accessory cells modulated the immune function of the T cells.
The most obvious clinical indication of impaired cellular immunity is lack of response to recall antigens on skin testing. A diminished response to skin testing for tuberculin, mumps, and Candida in patients with recurrent infections has been shown.43 Chapel and Bunch2 reported anergy to recall skin testing in 48% of 23 patients; the presence of anergy correlated with an increased frequency of infections. Whether the alternations in the T-cell repertoire of patients with CLL and the frequent oligoclonality of CD4 and CD8 T-cell populations play a role in the pathogenesis of infection in CLL patients remains to be demonstrated.65,66
Changes in cellular immune function are further complicated by newer treatment modalities. Nucleoside analogs have become established as the most effective single agents in patients with CLL.67 These agents are potent lymphotoxins and are not specific for B cells; they also produce T-cell depletion.68 O’Brien et al35 examined the CD4 count in 217 patients with CLL treated with fludarabine and prednisone. The median CD4 count prior to therapy was 1015 cells/mL (range, 47–17,791 cells/mL); after 3 months of therapy, the median decreased to 169 cells/mL (range, 0–4669 cells/mL), and after 6 months to 148 cells/mL (range, 6–1908 cells/mL). Wijermans et al68 demonstrated a significant decrease in CD3 cells occurring after only two courses of fludarabine. Although the number of CD4 cells had significantly increased after 1 year, the median count was still below normal.68 Similarly, Bergmann et al69 observed a decrease in CD4 cells from a median of 2935 cells/mL to 1316 cells/mL after fludarabine treatment in 10 patients with refractory CLL. The median CD8 cell count decreased from 5281 cells/mL to 1131 cells/mL and the CD4/CD8 ratio remained unchanged. Seymour et al reported prolonged suppression of CD4 counts in patients with hairy cell leukemia (HCL) after only one 7-day course of 2-chlorodeoxyadenosine (2-CdA). More than one year after treatment the median CD4 level was 309 cells/mL (range, 57–514 cells/mL), compared with a median pretreatment value of 749 cells/mL (range, 58–2201 cells/mL) (P < .002). Similarly, Juliusson et al70 reported that in 43 patients receiving a 7-day subcutaneous administration of 2-CdA, the median baseline CD4 level was 660 cells/mL (range, 409–1169 cells/mL) compared with 399 cells/mL (range, 325–486 cells/mL) at 12 months. Corresponding levels for CD8 lymphocytes were 546 cells/mL (range, 300–875 cells/mL) and 429 cells/mL (range, 301–661 cells/ml). Similar reductions in CD4 counts and severe lymphopenia have also been observed in CLL patients receiving the monoclonal antibody alemtuzumab. These changes in cellular immunity are of a magnitude seen in patients with human immunodeficiency virus (HIV)-related acquired immunodeficiency syndrome and are likely responsible for the increased incidence of opportunistic infections seen when nucleoside analogs are combined with corticosteroids.35,77– 78
Neutropenia
Patients with CLL frequently develop neutropenia with advancing disease and other myelotoxic chemotherapy.16 Mechanisms contributing to neutropenia include marrow infiltration by CLL, chemotherapy-related myelosuppression, and suppression of neutrophil progenitors by cellular and humoral factors.
Neutrophil function in patients with CLL has been reported to be deficient by some authors71 but not others.72 This dysfunction has been associated with enzyme deficiencies (lysozyme and myeloperoxidase)71 and thought to predict infection risk.71,73 Monocytopenia has also been suggested as a risk factor for infection in CLL.74
NK cells
NK cell activity, the capacity of cytotoxic effecter cells to bind to their target, and lymphocyte-activated killer (LAK) cell functions are reduced in patients with CLL.4,39,75,76 The clinical influence of these immune defects is not clear. However, the defect in NK cell activity could predispose to hypogammaglobulinemia since CD16+ CLL NK cells inhibit mitogen induced immunoglobulin secretion by normal B-cell.77,78
Mucosal immunity
Levels of serum and mucosal (salivary) IgA, IgG, and IgM were measured in a series of patients with CLL and controls.30,37,38 Despite serum immunoglobulin deficiencies, variable changes were seen in mucosal immunoglobulin levels. As in serum, salivary IgM levels were markedly decreased. There were no differences in levels of salivary IgG or IgA between patients and controls. These data suggest that mucosal B cells, in addition to systemic B cells, may also have defects in immune function. Additional studies are needed to clarify the role of mucosal immunity in patients with CLL.
Disease duration
Longer disease duration is associated with an increased predisposition to infections and is likely a result of progressive immune dysfunction, including hypogammaglobulinemia.4,9,16,18,27,38 Molica et al4 examined the frequency of infections in 125 patients over a 10-year period. The 5-year risk for severe infection was 26% and 21 (29.5%) of 71 deaths were related to infections. However, hazard function analysis revealed a constant risk pattern suggesting a lack of correlation between risk of infection and time. The increased risk of infection with time may be related to the greater likelihood of therapeutic intervention and the associated immunosuppression.
Treatment
Chemotherapy
Until recently, chlorambucil, alone or in combination with corticosteroids, was the primary therapy for patients with CLL. These agents, as well as combinations of cytotoxic agents, result in an increased risk of infection through their immunosuppressive properties79–,82 and the risk of infection increases with more extensive therapy.4 However, the incidence of infections is generally low when these combinations are used for first-line therapy of CLL.83
Nucleoside analogs have become established as the most effective agents in the treatment of patients with CLL.83–,87 Keating et al84 used fludarabine in 68 previously treated patients with CLL and reported 25 episodes of pneumonia and four episodes of septicemia. Major infections were more common in patients with resistant disease. In a study in 33 previously untreated patients with CLL, the same group reported three early infectious deaths in elderly patients with advanced disease.67 Robak et al88 administered 2-CdA to 113 patients with CLL ≤ 55 years of age. Infections or fever of unknown origin occurred in 26% of patients. There were no infections in previously untreated patients compared to 45% of previously treated patients. Van Den Neste et al89 compared the incidence of infections in the 6 months preceding treatment with 2-CdA with the 6 months following therapy in 95 patients, and showed a doubling of the infection rate after treatment with 2-CdA. A similarly increased incidence of infections was noted with the use of pentostatin.22,90 With more widespread use of nucleoside analogs, reports of opportunistic infections led to an increased concern for such an association.23,24,69,91–,93 The incidence of such infections increases in patients recently treated with steroids or receiving them concurrently.92,94
Anaissie et al94 reviewed the MD Anderson experience with 402 patients with CLL who received fludarabine (30 mg/m2/day for 5 days) with or without prednisone. Infections occurred more frequently in previously treated (58%) than untreated (34%) patients (P < .001). Infections with Listeria monocytogenes or PCP occurred in 12 (7%) of 170 previously treated patients who received fludarabine plus prednisone, in none of 78 previously treated patients who received fludarabine alone, and in 2 (1%) of 154 previously untreated patients who received the combination (P = .003). Multivariate analysis identified Rai stages III and IV, previous treatment, and elevated serum creatinine concentration as significant independent risk factors for major infection. Cutaneous zoster developed in five (26%) of 19 patients with CD4 counts < 50 cells/mL, compared with 9 (6%) of 139 patients with a CD4 count >50 cells/mL (P = .01). Recent data suggest that older age and low serum IgG levels may also be risk factors for herpes virus infections.95
Morrison et al. recently described the incidence and spectrum of infections seen in previously untreated patients with CLL treated with chlorambucil, fludarabine, or the combination of the two drugs.96 Five hundred forty-four patients were randomized into the three treatment arms. Concomitant therapy with corticosteroids was prohibited but prior use of steroids for autoimmune phenomena was allowed. A total of 1107 infections including 241 major infections occurred in 518 patients over a period beginning at study entry and lasting until subsequent therapy or death. Fewer patients received the combination because of early closure of that arm of the study after an interim analysis found increased rates of life-threatening toxicity. The follow-up interval was shorter for patients treated with chlorambucil as more of these patients crossed over to the fludarabine alone arm due to a lower response rate. Patients receiving the combination of chlorambucil and fludarabine had more infections than those receiving either drug alone (P < .0001). The mean number of infections per month of follow-up period was also significantly higher for patients treated with the combination (P = .008). Comparison of both single agents showed that the incidence of infection was higher in the fludarabine-treated patients (P = .055). Major infections, totaling 241 episodes, were also more common among patients who received fludarabine, either alone or in combination with chlorambucil. Although the patients who received the combination therapy were at greatest risk for major infections, the increased risk of major infections with fludarabine continued to be significant when compared to chlorambucil alone. Herpesvirus infections were most likely to occur in the combination arm (P < .0001) and occurred more frequently with fludarabine compared to chlorambucil (P = .0006).
Although this study shows an increased risk for infections associated with fludarabine-based regimens, most infections were not serious and involved skin, soft tissues and the respiratory tract, sites commonly associated with infections in CLL. No significant differences in infection-related mortality occurred between the three treatment arms and no treatment-related lethal toxicities attributable to infections were seen. The study design excluded the use of prophylactic antibiotics. Although the universal use of prophylactic antibiotics cannot be recommended (vide infra), a risk-based strategy for prophylactic therapy in patients receiving fludarabine-based regimens has been proposed.96
Other associations examined in this report included the impact of age, Rai stage, renal function, IgG status, and best response to initial therapy on the incidence of infections.96 Age was associated with occurrence of major infections in the combination arm but not the single-agent arms (P = .004). There were no association between infections and Rai stage, except for a marginal association in the combination arm (P = .05). Patients in the combination arm (and not the other arms) were at greater risk of developing at least one major infection and of having more major infections as their creatinine clearance declined (P = .03 and .04, respectively). When IgG levels were evaluated as a dichotomous variable (< or ≥ 500 mg/dL), a low value was associated with a greater risk of infections (P = .02).106
The development of refractoriness to fludarabine is associated with a significant likelihood of infection. Keating et al. reviewed the response to salvage therapy of 147 patients who were refractory to fludarabine or had a remission less than six months.97 Only a minority of patients (22%) responded to further therapy and the median survival was 10 months. The major cause of morbidity and death was infections. Gram-positive organisms were most commonly encountered. However, Gram-negative bacilli or opportunistic infections such as fungi, Pneumocystis carinii, acid-fast bacilli and legionella were also prominent.97 In a recent report by Perkins et al, 24 of 27 patients (89%) with fludarabine-refractory CLL developed serious infections.98 Patients had a median of 2 admissions (range, 0–11) for serious infection occurring at a median of 4 months (range, 0–21) from onset of fludarabine-refractoriness. Bacteria caused 69 of 88 infections (78.4%); viruses (varicella-zoster and herpes simplex) caused 11 of 88 (12.5%); fungi caused 4 of 88 (4.5%); and opportunistic infections caused 4 of 88 (4.5%). Median survival was 13.0 months (range, 1–44+).98 Thus, the frequency of serious infections in patients with fludarabine-refractory CLL is high irrespective of their subsequent therapy. This is likely to complicate the administration of potentially effective regimens and the interpretation of data pertaining to clinical trials of investigational agents in this setting.
Monoclonal antibodies
Rituximab: Rituximab, a chimeric monoclonal antibody, targets the B-cell antigen CD20. The standard schedule of rituximab, 375 mg/m2 weekly × 4, results in the reduction of peripheral B-lymphocyte counts by approximately 90% within 3 days; B-cell recovery occurs slowly over 9–12 months.99– 101 Despite this B-cell depletion, there is only a minimal decrease in serum immunoglobulin levels in some patients, and no effect on serum complement levels. Transient reductions in the absolute granulocyte count to 1500 cells/mL may be seen. Occasionally, isolated but reversible neutropenia of uncertain etiology develops after therapy with rituximab.
Infections may occur in patients receiving rituximab, but their incidence and severity may actually be decreased compared to that seen in similar cancer patients receiving chemotherapy. Most infections in this setting are limited and responsive to antimicrobial therapy.102– 105
Emerging data suggest that the use of rituximab with fludarabine may result in a higher incidence of neutropenia. In a recent trial conducted by the CALGB patients with CLL were randomized to fludarabine treatment monthly for 6 cycles or the same dose of fludarabine combined with single monthly doses of rituximab.106 Grade III-IV neutropenia occurred in 41% of patients receiving fludarabine alone versus 74% of patients treated with the combination. Despite the higher incidence of neutropenia in the combination arm, there was no increase in the incidence of infections. This is likely related to the fact that fludarabine does not produce mucosal damage. The etiology of the increased neutropenia seen with the combination of fludarabine and rituximab is uncertain; whether this effect is specific to the use of fludarabine or will also be seen when rituximab is combined with other agents remains to be seen.
Alemtuzumab: Alemtuzumab is a chimeric monoclonal antibody that binds to the CD52 antigen.107 Since this antigen is present on the surface of all lymphocytes, alemtuzumab significantly depletes both B and T cells and is associated with a pattern of infections similar to that seen with purine analogs.108 In an early study in patients with CLL by Rai et al opportunistic infections (OI) were common, occurring in 10 of 24 patients. Infections included 4 episodes of PCP, disseminated herpes zoster, invasive aspergillosis, cytomegalovirus (CMV) legionella pneumonitis, and 2 orbital candidal infections.108 In another trial of alemtuzumab in 29 patients with relapsed or refractory CLL, infections were the main toxicity.109 Localized HSV reactivation was noted in 11 patients (38%) and oral candidiasis in 5 (17%). Pneumonia was seen in 6 patients (21%) (2 with PCP) and bacteremia in 3 patients (10%). This significant incidence of infection led to the recommendation that all patients receiving alemtuzumab receive prophylactic trimethoprim sulfamethoxazole and anti-HSV therapy. In a recent study of 93 fludarabine–refractory patients with CLL treated with alemtuzumab, grade III-IV infections occurred in 26.9% of patients.110 Eleven patients developed OIs during treatment. A further 7 OIs occurred in the follow-up period. OIs included PCP (n = 1), in a patient not receiving trimethoprim sulfamethoxazole prophylaxis; Aspergillus pneumonia (n = 1); a fatal episode of rhinocerebral mucormycosis; systemic candidiasis (n = 1); cryptococcal pneumonia (n = 1, fatal); herpes zoster (n = 4 after study); pulmonary aspergillosis (n = 1 after study, fatal); invasive aspergillosis; and Listeria meningitis (n = 1, after study). The most commonly reported OI was CMV reactivation (n = 7), with 3 cases of grade 2 and 4 cases of grade 3 infection reported but no cases of CMV documented in the post-study period. Patients who developed OIs had received between 4 and 7 prior regimens.110
The incidence of CMV reactivation was likely underreported in the pivotal trial since recognition of this virus as a cause of persistent fever was just emerging. Nguyen et al reviewed their experience with alemtuzumab in 34 patients with CLL and reported a CMV reactivation rate of 15%.111 The typical presentation was persistent fever in the setting of broad-spectrum antibiotic therapy. Organ involvement was not seen and patients responded promptly to discontinuation of alemtuzumab therapy and initiation of ganciclovir.
At MD Anderson, alemtuzumab has been used in over 125 patients with CLL entered onto various protocols. The CMV reactivation rate has been consistent across protocols at 20–25%. As described persistent fever was the most common manifestation of reactivation and organ involvement, such as pneumonia or colitis, was rare (unpublished data).
The incidence of infections with alemtuzumab is lower when used in less refractory patients. Osterborg et al112 used alemtuzumab in nine previously untreated patients with CLL and reported one episode of CMV pneumonitis and oral thrush. Recently, Lundin et al. used prolonged (18-weeks) subcutaneous administration of alemtuzumab to treat 41 patients with symptomatic CLL who had no prior therapy.113 Grade IV neutropenia occurred in 21% of patients but bacterial infections were rare; 10% patients experienced CMV reactivation but responded promptly to intravenous ganciclovir. One patient, allergic to cotrimoxazole prophylaxis, developed PCP.113
Prophylaxis
Strategies for preventing infections in patients with CLL should take into consideration age, stage of disease, extent of prior therapy, and the immunosuppressive properties of the antineoplastic agent used.
Primary Prophylaxis
There are no randomized trials that have examined the role of prophylactic antibiotic therapy in patients with CLL. Thus the use of antibacterial prophylaxis is difficult to recommend without clear data indicating a beneficial effect. Nevertheless, patients receiving a purine analog and having risk factors for infection (advanced-stage disease, extensive prior therapy, or neutropenia) may benefit from antimicrobial prophylaxis. Anaissie et al94 suggested a risk-based strategy for prophylaxis in patients receiving fludarabine-based regimens. They recommended the use of antifungal prophylaxis in the form of triazoles in patients with persistent (>10–14 days) or severe (< 100 neutrophils/ml) neutropenia, particularly in the presence of fungal colonization or mucositis,114 Sudhoff et al25 recommended using ciprofloxacin during neutropenic periods, together with trimethoprim-sulfamethoxazole prophylaxis until 2 months after the discontinuation of fludarabine.
Evaluation of a large series of patients with CLL enrolled in the Intergroup trial showed that infections caused by VZV were more common in patients receiving fludarabine than chlorambucil.96 Herpes zoster is a frequent problem in patients with CLL; the incidence may be increased with the use of nucleoside analogs. Most infections are dermatomal; disseminated infections are rare, unless there is a delay in diagnosis. Thus, although these infections are rarely life-threatening, they are a major cause of morbidity because of the development of post-herpetic neuralgia.95 This complication is more frequent and severe in older adults and has been reported to last longer than 1 year in almost half of patients ≥ 70 years of age.115 The use of oral prophylaxis to prevent such infections has been effective in patients undergoing stem cell transplantation. However, there are no clear-cut prognostic factors to identify a high-risk CLL population. Anaissie et al94 found a correlation between very low CD4 counts post-fludarabine and reactivation of herpes. Whether a high-risk group can be targeted to receive prophylaxis for zoster before the first episode is still unclear. On the basis of the limited data available, prophylaxis may be provided to older patients who are seropositive and have severely depressed CD4 counts (< 50 cells/mL). However, the duration of prophylaxis remains uncertain.
Concomitant use of nucleoside analogs and corticosteroids should be avoided. However, such a combination may be necessary if autoimmune phenomena develop. All patients receiving nucleoside analogs and concomitant corticosteroids should receive P. carinii pneumonia prophylaxis with trimethoprim-sulfamethoxazole or pentamidine.
Given the pan-lymphocyte depletion seen in patients treated with alemtuzumab, as well as the high likelihood of HSV reactivation, all patients should receive HSV and PCP prophylaxis during treatment and for several months after completion of therapy. The fact that CMV reactivation occurs in 15%–25% of patients treated with alemtuzumab raises the issue of monitoring for this infection as well as the question of whether prophylaxis is indicated. Faderl et al described 13 patients (27%) who developed CMV antigenemia while receiving treatment with a combination of alemtuzumab and rituximab. However, only seven patients (15%) were symptomatic. In the remaining six patients, various interventions occurred. Two patients were treated, although they were asymptomatic, two patients were removed from therapy, and two patients continued therapy without developing reactivation of CMV. Thus, the significance of a few antigen positive cells in an asymptomatic patient being treated with alemtuzumab is uncertain. Given that most patients will not develop CMV reactivation and the cost associated with monitoring, this is not recommended outside a clinical trial setting in which low levels of positivity can be further evaluated in terms of prognostic significance. Rather, it is more important for the physician to be aware that CMV reactivation may occur and be the cause of fever in a patient receiving alemtuzumab; a persistent febrile episode should always raise the question of reactivation of CMV in these patients.
Prophylaxis of CMV has also not been examined in a clinical trial. Valganciclovir is the oral prodrug of ganciclovir with very good oral absorption. This agent would also provide protection against HSV reactivation. However, the main side effect of this drug is myelosuppression. Thus, a theoretical concern in using this agent for phophylaxis would be increased myelosuppression in patients being treated with alemtuzumab; this could result in a tradeoff in terms of development of other more common infections. MD Anderson Cancer Center is beginning a randomized trial in patients with CLL receiving alemtuzumab. Patients will be randomized to receive the standard prophylactic regimen with PCP prophylaxis and valacyclovir or PCP prophylaxis and valganciclovir. This trial will address the issue of whether oral valganciclovir can prevent CMV reactivation and also examine whether there is any difference in the overall incidence of infections between the two arms.
Intravenous immunoglobulin replacement
Initial studies of intravenous immunoglobulin (IVIG) suggested a decreased risk of infection in patients with CLL receiving this treatment prophylactically.116,117 In a randomized, double-blind study comparing IVIG (Gammagard, Baxter Healthcare Corp., Glendale, CA) with placebo, 81 patients with CLL and either hypogammaglobulinemia or a history of infections received IVIG (400 mg/kg body weight) every 3 weeks for 1 year. Patients treated with IVIG had a significantly lower incidence of bacterial infections (P = .01). In patients who completed one year of therapy, the beneficial effect against bacterial infections was more striking (P = .001). There were no significant differences in the incidence of viral and fungal infections, and no survival difference between the two groups.13
The use of IVIG is costly.118 It has been estimated that six million dollars would be needed to achieve 1 quality-adjusted life-year without an increase in life expectancy.118 Several investigators have examined the use of lower doses of IVIG in an attempt to improve the cost/benefit ratio.119,120 Gamm et al119 compared two doses of IVIG in 34 patients: 250 or 500 mg/kg every month for 1 year. There was no significant difference in the incidence of infections between the two groups. However, given the number of patients treated, this study lacked the power to detect significant differences. Molica et al.120 performed a crossover study involving 42 patients with CLL and hypogammaglobulinemia (IgG < 600 mg/dL) and/or a history of a severe infection in the preceding 6 months. A protective effect against infections for patients completing either 6 or 12 months of therapy with IVIG 300 mg/kg was evident. However, even a low-dose regimen of IVIG was not cost-effective in preventing infections in CLL. Using an even lower dose of IVIG (10 g every 3 weeks), Jurlander et al121 demonstrated a reduction in total number of infections in 15 patients during the duration of therapy compared to a similar time period before the initiation of IVIG. The number of antibiotic prescriptions, febrile episodes, and hospital admissions related to infection was reduced. The reductions in febrile episodes and hospital admissions were statistically significant (P = .004 and P = .047, respectively).
The use of IVIG is associated with a reduced incidence of bacterial infections in patients with CLL. However, the high cost and limited availability of IVIG, coupled with the potential of less expensive methods of prevention such as antibiotics, limit the role of IVIG in patients with CLL. The use of IVIG in patients with low IgG levels and recurrent sino/pulmonary bacterial infections may be warranted.
Active immunization
Viral respiratory infections including those caused by influenza are a significant cause of morbidity in the older population. Vaccination of patients with CLL is hampered by their inability to mount a significant immune response. Gribabis et al122 examined the immunologic response to influenza vaccine in 43 patients with CLL. Antibody production against at least one antigen (titer > 1:20) 15 days after immunization was seen in 81% of patients; this response was maintained on days 30 and 60 in 23 patients (63%). Patients with IgG levels < 700 mg/dL were less likely to respond than those with an IgG level > 700 mg/dL. In a similar study, Bucalossi et al123 determined the immunologic response to influenza vaccine in 30 patients with CLL and 10 matched controls. Patients were vaccinated on days 1 and 31. A significant antibody response was observed in 16 (53%) of 30 patients and in all controls. Five of 16 responders only had a protective titer after the second immunization, and in eight other patients, increases in the titer were noted after repeat vaccination. There were also significant differences in the response between patients with low and normal immunoglobulin levels (P < .05); a correlation between response to vaccination and disease stage (P < .05) was also seen.128 Thus, effective antibody responses to influenza vaccine may occur in some patients with CLL, usually those with less advanced disease and an IgG level > 700 mg/dL. However, these responses may not be sustained, and repeat immunization is required.
Responses to a variety of other vaccines have also been examined. Shaw et al14 reported suboptimal responses to typhoid, diphtheria, influenza, and mumps vaccines in patients with CLL regardless of the serum immunoglobulin level. Larson and Tomlinson124 could not detect an antibody response against a pneumococcal polysaccharide vaccine in 8 of 9 patients. Jacobson et al125 found that antibody titers against pneumococcal vaccine were lower in patients with CLL than in healthy controls. More recently, Sinisalo et al126 evaluated responses to vaccination against pneumococcal polysaccharide, Haemophilus influenzae b (Hib) conjugate and tetanus toxoid antigens in 31 patients with CLL and 25 controls. All antibody responses were highly significant in the control group; in the patients with CLL evidence of responsiveness was detected only in the case of Hib polysaccharide antigen. Certain patient subgroups showed low reactivity against tetanus toxoid antigen. Thus plain polysaccharide vaccines appeared to be ineffective in patients with CLL. Conjugate vaccines may offer protection against infections caused by encapsulated bacteria in these patients.
The effects of immunization against VZV in patients with CLL has not been examined. However, HSV vaccines contain live, attenuated virus, and the significant immunosuppression in these patients may render such vaccines unsafe. Redman et al used heat inactivation of live varicella vaccine in patients post BMT.127 Seventy-five patients were randomized to receive vaccine or no intervention. The incidence of herpes zoster was similar: 23% in vaccinated patients versus 20% in unvaccinated patients. However, postherpetic neuralgia occurred in 4 of 5 unvaccinated patients compared to 0 of 5 patients receiving vaccination.127 Disease severity associated with VZV reactivation was dramatically decreased in vaccinated patients given three doses (P = .007).
The role of immunization in patients with CLL is not established, and no specific recommendations with regard to vaccination can be made. However, if considered, vaccination should be given before the initiation of chemotherapy and a booster immunization provided, based on the level of protective antibodies. When healthy household members are immunized against polio, the inactive polio vaccine should be chosen to avoid the potential for infection associated with the use of a live-virus vaccine in immunocompromised patients.
Preemptive therapy
A supply of amoxicillin/clavulanate for immediate use in the setting of fever and chills may reduce the morbidity of bacterial infections. Similarly, a supply of acyclovir or valacyclovir that can be started at the first sign of oral or skin lesions may ameliorate herpetic infections and possibly decrease the incidence of post-herpetic neuralgia.
Hematopoietic growth factors
Although mechanisms contributing to the increased incidence of infections in patients with CLL are complex, neutropenia is likely to have a major role. As discussed earlier, the development of neutropenia is multifactorial, with marrow infiltration by leukemic cells and the effects of cytotoxic chemotherapy as the main predisposing factors. The use of hematopoietic growth factors to reverse granulocytopenia may be beneficial in CLL although no randomized trials have been conducted. Itala et al128 treated 12 patients with advanced CLL with COP (cyclophosphamide, vincristine, prednisone) chemotherapy; prophylactic GM-CSF was administered during every other cycle. Median neutrophil counts 2 and 3 weeks after chemotherapy were significantly higher during courses utilizing growth factor support (P < .02 and P < .002, respectively). Eosinophil counts were also significantly increased by GM-CSF. There were no significant differences in the number of febrile days, days of intravenous antibiotics, or days of hospitalization in this small study. The same authors also reported on the effects of GM-CSF in eight patients with CLL and chronic neutropenia.129 They noted an increase in neutrophil count by a median of 6.6-fold in all patients. Improved neutrophil function as measured by chemiluminescence, random migration, and formyl methionyl leucyl phenylalanine (fMLP)-stimulated chemotaxis was observed. At M.D. Anderson, 25 previously treated patients with Rai stage 3 or 4 disease received monthly courses of fludarabine followed by granulocyte colony-stimulating factor.130 There was a significant reduction in the incidence of neutropenia (absolute neutrophil count < 1,000 cells/mL) in the growth factor–treated group compared with matched historical controls (45% versus 79%; P = .002). This translated into a reduction in the incidence of delay in therapy (P = .005) and the incidence of pneumonia (8% in the treated group versus 37% in the controls). The incidence of other infections (sepsis, fever of unknown origin, and minor infections) was similar in the two groups.130 Thus, several small single arm trials indicated that growth factor therapy is effective in reducing the length and severity of neutropenia associated with disease progression and its treatment, and may be beneficial in reducing the severity of infections in patients with CLL.
Management of Infections
General considerations
The management of infections in patients treated with purine analogs or alemtuzumab relies on the identification of the patients at high-risk for life-threatening infections, the initiation of appropriate diagnostic tests, and prompt antimicrobial therapy. This therapy should be adjusted according to a patient’s number and type of risk factors for infection and the site of infection (pneumonia, meningitis, mucositis, etc.).
In most patients initiation of a broad spectrum antibiotic alone will be acceptable initial therapy for infection. In patients who are more seriously ill, a second antibiotic such as trimethoprim-sulfa is a reasonable addition because of its broad-spectrum activity and specific activity against atypical pathogens that may be seen in the setting of CLL such as PCP and Listeria. Patients who have mucocutaneous abnormalities should be treated with therapeutic doses of acyclovir or an equivalent. Patients with pneumonia, particularly in the setting of hypoxemia, should be treated empirically with high-dose trimethoprim-sulfa (15–20 mg/kg per day) to cover for PCP initially until results of bronchoscopy is can be obtained. High dose trimethoprim-sulfa should also be used empirically in patients with central nervous system symptoms. In patients allergic to sulfa, ampicillin would be an alterative.
Persistent fever not responding to broad-spectrum antibiotics raises the question of fungal or viral infection. In patients recently treated with aletuzumab, the suspicion for CMV would be high. In some areas CMV testing using an antigen assay or PCR may not be unavailable; empiric use ganciclovir or foscarnet should be initiated. The choice between the two may be based on the status of the patient in terms of their current blood counts and renal function. In patients who are myelosuppressed, initiation of therapy with foscarnet would be preferable and in patients who have renal insufficiency treatment should be initiated with ganciclovir. Patients with long standing disease, particularly in the setting of prolonged neutropenia, may benefit from empiric initiation of antifungal therapy if still febrile on broad-spectrum antibiotics. If chest x-ray is negative, the use of chest CT can be helpful in identifying typical fungal lesions. Relying on cultures in this setting is not useful since it is often difficult to grow mold from sputum.
Conclusion
With continued progress in treating hematological malignancies using more effective and more myelosuppressive and immunosuppressive agents, the role of supportive care has become more prominent. Nevertheless, most recommendations for prophylaxis are empiric because there are very few controlled trials evaluating such strategies. Positive data from pilot studies need to be confirmed in larger trials. In addition, analyses identifying prognostic factors for specific infections may identify a high-risk population and allow prophylactic strategies to be more cost-effective by avoiding treatment of patients who are unlikely to suffer such complications.
Severe complications of mucosal injury.
Gram-positive infections
|
Gram-negative bacillary infections
|
Anaerobic infections
|
Neutropenic Enterocolitis
|
Candida infections
|
| Acute graft-versus-host disease |
| Systemic inflammatory response syndrome |
| Malnutrition |
Gram-positive infections
|
Gram-negative bacillary infections
|
Anaerobic infections
|
Neutropenic Enterocolitis
|
Candida infections
|
| Acute graft-versus-host disease |
| Systemic inflammatory response syndrome |
| Malnutrition |
Palliative therapy for mucosal barrier injury.
| Oral Mucositis . | Gut Mucosal Injury . |
|---|---|
| Abbreviation: G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage CSF | |
| Current | Current |
| Chlorhexidine | Glutamine supplement |
| Antimicrobial agents | Proton-block inhibitors |
| Sucralfate | Vitamins |
| Narcotic analgesics | Antioxidants |
| Topical analgesics | Narcotic analgesics |
| Oral hygiene | |
| Investigational | Investigational |
| Amifostine | Transforming growth factors |
| Keratinocyte growth factor | Interleukin-11 |
| Transforming growth factors | Trefoils |
| Laser therapy | Probiotics |
| GM-/G-CSF | Lactoferrin |
| Pentoxifylline | Glucagon-like peptides |
| Oral Mucositis . | Gut Mucosal Injury . |
|---|---|
| Abbreviation: G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage CSF | |
| Current | Current |
| Chlorhexidine | Glutamine supplement |
| Antimicrobial agents | Proton-block inhibitors |
| Sucralfate | Vitamins |
| Narcotic analgesics | Antioxidants |
| Topical analgesics | Narcotic analgesics |
| Oral hygiene | |
| Investigational | Investigational |
| Amifostine | Transforming growth factors |
| Keratinocyte growth factor | Interleukin-11 |
| Transforming growth factors | Trefoils |
| Laser therapy | Probiotics |
| GM-/G-CSF | Lactoferrin |
| Pentoxifylline | Glucagon-like peptides |
Incidence of fungal infections in patients with hematologic cancer after the introduction of triazole prophylaxis.
| . | . | Aspergillus . | Candida . | |
|---|---|---|---|---|
| Disease . | Incidence . | . | After Triazoles . | Before Triazoles . |
| Abbreviations: AlloBMT, allogeneic bone marrow transplantation; Auto, autologous; PBSCT, peripheral blood stem cell transplant. | ||||
| Modified with permission from Thomson Current Drugs and Tahsine Mahfouz and Elias Anaissie, Prevention of fungal infections in the immunocompromised host. Current Opinion in Investigational Drugs. 2003 ;4 (8):974 –990.12 | ||||
| AlloBMT, PBSCT | 15–25% | 10–20% | < 5% | 15–25% |
| Acute myelogenous leukemia | 10–15% | 10% | < 5% | 15–20% |
| Acute lymphocytic leukemia | 5–10% | ~ 5% | < 5% | 10% |
| Auto-BMT, PBSCT | 2–6% | ≤ 2% | < 5% | 10% |
| . | . | Aspergillus . | Candida . | |
|---|---|---|---|---|
| Disease . | Incidence . | . | After Triazoles . | Before Triazoles . |
| Abbreviations: AlloBMT, allogeneic bone marrow transplantation; Auto, autologous; PBSCT, peripheral blood stem cell transplant. | ||||
| Modified with permission from Thomson Current Drugs and Tahsine Mahfouz and Elias Anaissie, Prevention of fungal infections in the immunocompromised host. Current Opinion in Investigational Drugs. 2003 ;4 (8):974 –990. | ||||
| AlloBMT, PBSCT | 15–25% | 10–20% | < 5% | 15–25% |
| Acute myelogenous leukemia | 10–15% | 10% | < 5% | 15–20% |
| Acute lymphocytic leukemia | 5–10% | ~ 5% | < 5% | 10% |
| Auto-BMT, PBSCT | 2–6% | ≤ 2% | < 5% | 10% |
Risk factors for invasive fungal infections in patients with hematologic malignancies.
| Risk Factor . | Infection . | Population . |
|---|---|---|
| Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; CLL, chronic lymphocytic leukemia; allo BMT, allogeneic bone marrow transplantation; aspergillosis, invasive Aspergillus infection; candidiasis, invasive candidiasis; CVC, central venous catheter; PBSCT, peripheral blood stem cell transplantation | ||
| † Profound and persistent: < 100 cells/microliter, ≥ 10 days. | ||
| Modified with permission from Thomson Current Drugs and Tahsine Mahfouz and Elias Anaissie, Prevention of fungal infections in the immunocompromised host. Current Opinion in Investigational Drugs. 2003 ;4 (8):974 –990. | ||
| Prior colonization/Infection | ||
| Colonization | ||
| Gut mucosa | Invasive candidiasis | AML, autoBMT, hematological malignancies |
| Airways | Aspergillosis | Hematological malignancies |
| Skin | Fusariosis | Hematological malignancies |
| Prior infection | ||
| Aspergillosis | AML | |
| Fusariosis | AML, ALL, lymphomas | |
| Blastomycosis, Coccidioidomycosis | Lymphomas | |
| Net state of immunosuppression | ||
| Immunosuppression may be limited to one arm of the immune system (e.g., neutropenia), or broad (neutropenia, lymphopenia, asplenia and others). | ||
| Broad immunosuppression | ||
| Older age | Yeast and mould infections | Allo, autoBMT, PBSCT |
| Refractory malignancy | Yeast and mould infections | Acute leukemias |
| Myeloablative chemotherapy | Yeast and mould infections | Allo, autoBMT, PBSCT |
| Low CD 34+ dose (≤ 2 x 106/kg) | Yeast and mould infections | Allo, autoBMT, PBSCT |
| Stem cell manipulation | Various infections | Hematological malignancies |
| Histo-incompatibility | Aspergillosis | AlloBMT |
| Extensive prior chemotherapy | Hematological malignancies | |
| Neutropenia, profound, persistent † | Aspergillosis, Invasive candidiasis, Fusariosis | AML |
| Lymphopenia and CD4 cytopenia | ||
| All causes | Cryptococcosis | AlloBMT |
| Aspergillosis | Therapy with nucleoside analogs | |
| Adrenal corticosteroids | Aspergillosis, invasive candidiasis | AlloBMT, PBSCT |
| 1 to 2 mg/kg/day ≥ 3 to 5 weeks | ||
| Invasive candidiasis | Leukemias | |
| Cryptococcosis | Hematological malignancies | |
| Nucleoside analogs | Yeast and mould infections | CLL, lymphoma |
| Campath-1 H | Yeast and mould infections | CLL |
| Interleukin-2 therapy | Aspergillosis | Several patient populations |
| Infliximab (anti TNF-alpha) | Aspergillosis | AlloBMT |
| Splenectomy | Yeast and mould infections | Allo, autoBMT |
| Organ dysfunction | ||
| Pulmonary | All infections (fungal & other) | Cancer |
| Gut | Invasive candidiasis > other | Mucositis post-chemotherapy and post-radiotherapy |
| Invasive candidiasis | AlloBMT | |
| Skin | Cryptococcosis, Candidiasis | Sezary syndrome, CVC |
| Fusariosis | Hematological malignancies | |
| Aspergillosis and infection with Malassezia spp | Hematological malignancies, trauma (CVC site) | |
| Risk Factor . | Infection . | Population . |
|---|---|---|
| Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; CLL, chronic lymphocytic leukemia; allo BMT, allogeneic bone marrow transplantation; aspergillosis, invasive Aspergillus infection; candidiasis, invasive candidiasis; CVC, central venous catheter; PBSCT, peripheral blood stem cell transplantation | ||
| † Profound and persistent: < 100 cells/microliter, ≥ 10 days. | ||
| Modified with permission from Thomson Current Drugs and Tahsine Mahfouz and Elias Anaissie, Prevention of fungal infections in the immunocompromised host. Current Opinion in Investigational Drugs. 2003 ;4 (8):974 –990. | ||
| Prior colonization/Infection | ||
| Colonization | ||
| Gut mucosa | Invasive candidiasis | AML, autoBMT, hematological malignancies |
| Airways | Aspergillosis | Hematological malignancies |
| Skin | Fusariosis | Hematological malignancies |
| Prior infection | ||
| Aspergillosis | AML | |
| Fusariosis | AML, ALL, lymphomas | |
| Blastomycosis, Coccidioidomycosis | Lymphomas | |
| Net state of immunosuppression | ||
| Immunosuppression may be limited to one arm of the immune system (e.g., neutropenia), or broad (neutropenia, lymphopenia, asplenia and others). | ||
| Broad immunosuppression | ||
| Older age | Yeast and mould infections | Allo, autoBMT, PBSCT |
| Refractory malignancy | Yeast and mould infections | Acute leukemias |
| Myeloablative chemotherapy | Yeast and mould infections | Allo, autoBMT, PBSCT |
| Low CD 34+ dose (≤ 2 x 106/kg) | Yeast and mould infections | Allo, autoBMT, PBSCT |
| Stem cell manipulation | Various infections | Hematological malignancies |
| Histo-incompatibility | Aspergillosis | AlloBMT |
| Extensive prior chemotherapy | Hematological malignancies | |
| Neutropenia, profound, persistent † | Aspergillosis, Invasive candidiasis, Fusariosis | AML |
| Lymphopenia and CD4 cytopenia | ||
| All causes | Cryptococcosis | AlloBMT |
| Aspergillosis | Therapy with nucleoside analogs | |
| Adrenal corticosteroids | Aspergillosis, invasive candidiasis | AlloBMT, PBSCT |
| 1 to 2 mg/kg/day ≥ 3 to 5 weeks | ||
| Invasive candidiasis | Leukemias | |
| Cryptococcosis | Hematological malignancies | |
| Nucleoside analogs | Yeast and mould infections | CLL, lymphoma |
| Campath-1 H | Yeast and mould infections | CLL |
| Interleukin-2 therapy | Aspergillosis | Several patient populations |
| Infliximab (anti TNF-alpha) | Aspergillosis | AlloBMT |
| Splenectomy | Yeast and mould infections | Allo, autoBMT |
| Organ dysfunction | ||
| Pulmonary | All infections (fungal & other) | Cancer |
| Gut | Invasive candidiasis > other | Mucositis post-chemotherapy and post-radiotherapy |
| Invasive candidiasis | AlloBMT | |
| Skin | Cryptococcosis, Candidiasis | Sezary syndrome, CVC |
| Fusariosis | Hematological malignancies | |
| Aspergillosis and infection with Malassezia spp | Hematological malignancies, trauma (CVC site) | |
Properties of systemically available antifungal agents.
| Drug . | Formulation . | Bioavailability . | Safety . | Drug-Drug Interaction . | Cost . | Dosing/Day . |
|---|---|---|---|---|---|---|
| Polyenes | ||||||
| D-AmB | IV | NA | Poor | Good | $ | ≤1† |
| LAMB | IV | NA | Good | Good | $$$$ | ≤1† |
| ABCD | IV | NA | Fair | Good | $$$ | ≤1† |
| ABLC | IV | NA | Fair | Good | $$$ | ≤1† |
| Triazoles | ||||||
| Fluconazole | PO, IV | Excellent | Excellent | Good | $(po), $$ (iv) | 1 |
| Itraconazole | PO, IV | Fair/Good | Good | Fair | $$(po), $$$(iv) | 1–2 |
| Voriconazole | PO, IV | Good/excellent* | Good* | Fair* | $$(po), $$$(iv) | 2 |
| Flucytosine | PO, ?IV | Excellent | Fair†† | Good | $ po | 4 |
| Caspofungin | IV | NA | Good* | Good* | $$$$ | 1 |
| Abbreviations: D-AmB, Amphotericin B deoxycholate; LAMB, liposomal Amphotericin B; ABCD, Amphotericin B colloidal dispersion; ABLC, Amphotericin B lipid complex; NA, not applicable | ||||||
 Itraconazole solution has better bioavailability than the capsule form
* Limited clinical data
$ inexpensive, $$ moderately expensive, $$$ expensive, $$$$ very expensive
† IV Amphotericin B products may be given daily or every other day after completion of induction therapy
†† Requires close monitoring of serum levels and clinical toxicity (especially in patients with renal dysfunction) to avoid accumulation and resulting in gut and bone marrow toxicity Itraconazole solution has better bioavailability than the capsule form
* Limited clinical data
$ inexpensive, $$ moderately expensive, $$$ expensive, $$$$ very expensive
† IV Amphotericin B products may be given daily or every other day after completion of induction therapy
†† Requires close monitoring of serum levels and clinical toxicity (especially in patients with renal dysfunction) to avoid accumulation and resulting in gut and bone marrow toxicity | ||||||
| Modified with permission from Thomson Current Drugs and Tahsine Mahfouz and Elias Anaissie, Prevention of fungal infections in the immunocompromised host. Current Opinion in Investigational Drugs. 2003 ;4 (8):974 –990. | ||||||
| Drug . | Formulation . | Bioavailability . | Safety . | Drug-Drug Interaction . | Cost . | Dosing/Day . |
|---|---|---|---|---|---|---|
| Polyenes | ||||||
| D-AmB | IV | NA | Poor | Good | $ | ≤1† |
| LAMB | IV | NA | Good | Good | $$$$ | ≤1† |
| ABCD | IV | NA | Fair | Good | $$$ | ≤1† |
| ABLC | IV | NA | Fair | Good | $$$ | ≤1† |
| Triazoles | ||||||
| Fluconazole | PO, IV | Excellent | Excellent | Good | $(po), $$ (iv) | 1 |
| Itraconazole | PO, IV | Fair/Good | Good | Fair | $$(po), $$$(iv) | 1–2 |
| Voriconazole | PO, IV | Good/excellent* | Good* | Fair* | $$(po), $$$(iv) | 2 |
| Flucytosine | PO, ?IV | Excellent | Fair†† | Good | $ po | 4 |
| Caspofungin | IV | NA | Good* | Good* | $$$$ | 1 |
| Abbreviations: D-AmB, Amphotericin B deoxycholate; LAMB, liposomal Amphotericin B; ABCD, Amphotericin B colloidal dispersion; ABLC, Amphotericin B lipid complex; NA, not applicable | ||||||
 Itraconazole solution has better bioavailability than the capsule form
* Limited clinical data
$ inexpensive, $$ moderately expensive, $$$ expensive, $$$$ very expensive
† IV Amphotericin B products may be given daily or every other day after completion of induction therapy
†† Requires close monitoring of serum levels and clinical toxicity (especially in patients with renal dysfunction) to avoid accumulation and resulting in gut and bone marrow toxicity Itraconazole solution has better bioavailability than the capsule form
* Limited clinical data
$ inexpensive, $$ moderately expensive, $$$ expensive, $$$$ very expensive
† IV Amphotericin B products may be given daily or every other day after completion of induction therapy
†† Requires close monitoring of serum levels and clinical toxicity (especially in patients with renal dysfunction) to avoid accumulation and resulting in gut and bone marrow toxicity | ||||||
| Modified with permission from Thomson Current Drugs and Tahsine Mahfouz and Elias Anaissie, Prevention of fungal infections in the immunocompromised host. Current Opinion in Investigational Drugs. 2003 ;4 (8):974 –990. | ||||||
Dosing schedule for antifungal prophylaxis and therapy.
| . | Daily Dosing . | |
|---|---|---|
| Agent . | Prophylaxis . | Treatment . |
| For prophylaxis, consider oral route when applicable. For preemptive, empirical therapy and therapy of established infection, start with IV route and shift to oral route when applicable. | ||
| * Itraconazole: 400 mg bid po (capsule or solution) or 200 mg bid IV. Use the IV formulation if severe mucositis or achlorhydria occurs. Capsule and solution should be taken with a cola beverage. Capsule is better absorbed with fat- and protein-rich meals, whereas the solution should be taken without meals. | ||
| † these doses effective for prevention of candidiasis. | ||
| § Voriconazole: 1-day loading: 400 mg bid po; 6 mg/kg bid IV. For prophylaxis against aspergillosis, consider higher doses. | ||
| ** For invasive aspergillosis use 3–5 mg/kg/day. The 1-mg/kg/day dose should only be used for candidal infections. Prophylaxis not supported by randomized trials. | ||
| ‡ Caspofungin: loading dose of 70 mg IV once. | ||
| Modified with permission from Thomson Current Drugs and Tahsine Mahfouz and Elias Anaissie, Prevention of fungal infections in the immunocompromised host. Current Opinion in Investigational Drugs. 2003 ;4 (8):974 –990. | ||
| Fluconazole | 200 to 400 mg po | 400 to 800 mg iv → po |
| Itraconazole* | 200 mg iv or 400 mg po bid | 200 mg iv or 400 mg po bid |
| Voriconazole § | Not tested | 3 to 4 mg/kg iv → po bid |
| Amphotericin B | 0.2 mg/kg/day or 0.5 mg/kg × 3/wk† | 1 to 1.5 mg/kg |
| ABCD (Amphotec) | Not tested | 3 to 5 mg/kg |
| ABLC (Abelcet) | Not tested | 5 mg/kg |
| LAMB** | 1 mg/kg or 2 to 3 mg/kg × 3/wk | 1 to 5 mg/kg |
| Caspofungin‡ | Not tested | 50 mg iv |
| . | Daily Dosing . | |
|---|---|---|
| Agent . | Prophylaxis . | Treatment . |
| For prophylaxis, consider oral route when applicable. For preemptive, empirical therapy and therapy of established infection, start with IV route and shift to oral route when applicable. | ||
| * Itraconazole: 400 mg bid po (capsule or solution) or 200 mg bid IV. Use the IV formulation if severe mucositis or achlorhydria occurs. Capsule and solution should be taken with a cola beverage. Capsule is better absorbed with fat- and protein-rich meals, whereas the solution should be taken without meals. | ||
| † these doses effective for prevention of candidiasis. | ||
| § Voriconazole: 1-day loading: 400 mg bid po; 6 mg/kg bid IV. For prophylaxis against aspergillosis, consider higher doses. | ||
| ** For invasive aspergillosis use 3–5 mg/kg/day. The 1-mg/kg/day dose should only be used for candidal infections. Prophylaxis not supported by randomized trials. | ||
| ‡ Caspofungin: loading dose of 70 mg IV once. | ||
| Modified with permission from Thomson Current Drugs and Tahsine Mahfouz and Elias Anaissie, Prevention of fungal infections in the immunocompromised host. Current Opinion in Investigational Drugs. 2003 ;4 (8):974 –990. | ||
| Fluconazole | 200 to 400 mg po | 400 to 800 mg iv → po |
| Itraconazole* | 200 mg iv or 400 mg po bid | 200 mg iv or 400 mg po bid |
| Voriconazole § | Not tested | 3 to 4 mg/kg iv → po bid |
| Amphotericin B | 0.2 mg/kg/day or 0.5 mg/kg × 3/wk† | 1 to 1.5 mg/kg |
| ABCD (Amphotec) | Not tested | 3 to 5 mg/kg |
| ABLC (Abelcet) | Not tested | 5 mg/kg |
| LAMB** | 1 mg/kg or 2 to 3 mg/kg × 3/wk | 1 to 5 mg/kg |
| Caspofungin‡ | Not tested | 50 mg iv |
Antifungal triazoles active against moulds: practical tips for use.
| Property Required /Variable Present . | Select Itraconazole . | Select Voriconazole . | |
|---|---|---|---|
| Broad spectrum/potency | + | + | |
Good bioavailability , rapid time to steady state , rapid time to steady state | + | ||
| Higher central nervous system drug exposure | + | ||
| Tolerability of oral formulation | + | ||
| Once a day dosing | + | ||
| Safety profile† | ? | ? | |
| Patient receiving warfarin* | + | ||
| Patient receiving benzodiazepines* | + | ||
| Patient of Japanese/Chinese descent§ | + | ||
| Triazoles and gut dysfunction: Always use itraconazole and voriconazole IV in the presence of significant gut dysfunction (mucositis, nausea and vomiting, gut graft versus host disease, severe diarrhea, ileus, other) | |||
| IV triazoles and renal failure: Cyclodextrins (carrier of IV itraconazole and voriconazole) are excreted unchanged via kidneys with a potential for accumulation in patients with renal dysfunction. Avoid IV formulation for patients on renal dialysis and those with creatinine clearance (CrCl) < 50 mL/min (voriconazole) or <30 mL/min (itraconazole). Use oral therapy instead. | |||
 PO Triazole and bioavailability: Always take with a Coke. For itraconazole capsule: take with meals; for itraconazole solution: take away from meals. PO Triazole and bioavailability: Always take with a Coke. For itraconazole capsule: take with meals; for itraconazole solution: take away from meals. | |||
| † Voriconazole precautions: Instruct patients taking voriconazole to avoid driving at night or exposing himself or herself to strong and direct sunlight. | |||
| * Drug Interactions: Voriconazole has higher likelihood of drug-drug interaction with cyclosporine, tacrolimus, warfarin, or rifampin compared to a higher likelihood of an itraconazole drug-drug interaction with benzodiazepines* | |||
| §Voriconazole and CYP 2C19: Voriconazole is metabolized by CYP isoenzymes including CYP2C19, which exhibits genetic polymorphism. An individual’s ability to metabolize a CYP2C19 substrate is genetically determined as homozygous extensive metabolizer (EM), heterozygous EM and poor metabolizer. Significantly higher voriconazole drug exposure occurs in heterozygous EM and homozygous poor metabolizers (below). | |||
| CYP 2C19 Genotype | Caucasian/Negroid | Japanese/Chinese | Voriconazole Concentration |
| Homozygous Extensive | ~75% | ~35% | 1 X |
| Heterozygous Extensive | ~23% | ~45% | ~ 2 X |
| Homozygous Poor | ~2% | ~20% | ~ 4 X |
| Property Required /Variable Present . | Select Itraconazole . | Select Voriconazole . | |
|---|---|---|---|
| Broad spectrum/potency | + | + | |
Good bioavailability , rapid time to steady state , rapid time to steady state | + | ||
| Higher central nervous system drug exposure | + | ||
| Tolerability of oral formulation | + | ||
| Once a day dosing | + | ||
| Safety profile† | ? | ? | |
| Patient receiving warfarin* | + | ||
| Patient receiving benzodiazepines* | + | ||
| Patient of Japanese/Chinese descent§ | + | ||
| Triazoles and gut dysfunction: Always use itraconazole and voriconazole IV in the presence of significant gut dysfunction (mucositis, nausea and vomiting, gut graft versus host disease, severe diarrhea, ileus, other) | |||
| IV triazoles and renal failure: Cyclodextrins (carrier of IV itraconazole and voriconazole) are excreted unchanged via kidneys with a potential for accumulation in patients with renal dysfunction. Avoid IV formulation for patients on renal dialysis and those with creatinine clearance (CrCl) < 50 mL/min (voriconazole) or <30 mL/min (itraconazole). Use oral therapy instead. | |||
 PO Triazole and bioavailability: Always take with a Coke. For itraconazole capsule: take with meals; for itraconazole solution: take away from meals. PO Triazole and bioavailability: Always take with a Coke. For itraconazole capsule: take with meals; for itraconazole solution: take away from meals. | |||
| † Voriconazole precautions: Instruct patients taking voriconazole to avoid driving at night or exposing himself or herself to strong and direct sunlight. | |||
| * Drug Interactions: Voriconazole has higher likelihood of drug-drug interaction with cyclosporine, tacrolimus, warfarin, or rifampin compared to a higher likelihood of an itraconazole drug-drug interaction with benzodiazepines* | |||
| §Voriconazole and CYP 2C19: Voriconazole is metabolized by CYP isoenzymes including CYP2C19, which exhibits genetic polymorphism. An individual’s ability to metabolize a CYP2C19 substrate is genetically determined as homozygous extensive metabolizer (EM), heterozygous EM and poor metabolizer. Significantly higher voriconazole drug exposure occurs in heterozygous EM and homozygous poor metabolizers (below). | |||
| CYP 2C19 Genotype | Caucasian/Negroid | Japanese/Chinese | Voriconazole Concentration |
| Homozygous Extensive | ~75% | ~35% | 1 X |
| Heterozygous Extensive | ~23% | ~45% | ~ 2 X |
| Homozygous Poor | ~2% | ~20% | ~ 4 X |
Important drug interaction with triazoles: effect of azoles on other agents.
| . | . | Pharmacokinetics of Azoles Affecting other Drugs . | ||
|---|---|---|---|---|
| Effect of Azoles on other agents . | Azole Used . | Effect of Azole on Drug Exposure . | Adjust Dose of or Discontinue Concomitant Drug . | Monitoring . |
| Abbreviations: AUC, area under the curve | ||||
| Antineoplastic | ||||
| Busulfan | Itraconazole | 25% ↑ | Avoid azole | Monitor for toxicity |
| Voriconazole | ↑ | |||
| Vinca alkaloids | Itraconazole | ↑ | ||
| Voriconazole | ↑ | |||
| Docetaxel | Itraconazole | ↑ | ||
| Voriconazole | ↑ | |||
| Cyclophosphamide | Itraconazole | ↑ | ||
| Immunosuppressives | ||||
| Methylprednisolone | Itraconazole | 2–3 x ↑ | Change to dexamethasone | |
| Voriconazole | ↑? | |||
| Cyclosporine | Itraconazole | 50% ↑ | Decrease cyclosporine dose by 50% and/or according to levels and clinical toxicity | Monitor immunosuppressant level daily |
| Voriconazole | 2 x ↑ | |||
| Fluconazole | 50% ↑ | |||
| Tacrolimus | Itraconazole | 5 x ↑ | Decrease tacrolimus dose by 50 % and/or according to levels and clinical toxicity | |
| Voriconazole | 3 x ↑ | |||
| Fluconazole | ↑ | |||
| Sirolimus | Ketoconazole | 11 x ↑AUC | Azoles contraindicated | |
| Itraconazole | ↑↑↑ | |||
| Voriconazole | ↑↑↑ | |||
| Fluconazole | ↑↑ | Decrease sirolimus dose | Decrease sirolimus level | |
| Digoxin | Itraconazole | 68% ↑ | Decrease digoxin dose according to digoxin level | Monitor digoxin level daily, EKG |
| Fluconazole | ↑ | |||
| Warfarin | Itraconazole | ↑ | Decrease warfarin dose according to INR | Monitor INR |
| Voriconazole | ↑ | |||
| Fluconazole | 70% ↑ | |||
| Benzodiazepines | ||||
| Midazolam | Itraconazole | 2 x ↑ | Avoid agent, decrease dose by 50%, or use oxazepam instead | Monitor for central nervous system toxicity (drowsiness, sleepiness) |
| Voriconazole | ↑? | |||
| Fluconazole | 2 x ↑ | |||
| Triazolam | Itraconazole | 2 x ↑ | ||
| Voriconazole | ↑? | |||
| Fluconazole | 2 x ↑ | |||
| Diazepam | Itraconazole | 35% ↑ | ||
| Voriconazole | ↑? | |||
| Zolpidem | Voriconazole | ↑? | ||
| Proton pump inhibitors | Voriconazole | ↑ | Reduce proton pump inhibitor dose by 50% | |
| Fluconazole | ↑ | |||
| Phenytoin | Itraconazole | ↑↑ AUC | Avoid triazole | Monitor phenytoin level |
| Voriconazole | ↑ 80 | |||
| Fluconazole | ↑↑ AUC | |||
| Rifampin | Itraconazole | ↑ AUC | Monitor for rifampin toxicity | |
| Voriconazole | ↑ AUC | Avoid voriconazole | ||
| Fluconazole | ↑ AUC | |||
| Rifabutin | Voriconazole | ↑↑↑AUC | Avoid voriconazole, fluconazole | |
| Fluconazole | ↑↑ | |||
| Oxybutynin | Itraconazole | 2 x ↑AUC | Avoid itraconazole | |
| Sulfonylurea | Voriconazole | ↑ | Adjust dose according to blood sugar | Monitor glucose concentration |
| Fluconazole | ↑ | |||
| HMG-CoA Reductase Inhibitors | ||||
| Lovastatin/Simvastatin | Itraconazole | 10–20 x ↑ | Avoid using simvastatin, lovastatin, atorvastatin. May use pravastatin or fluvastatin | Monitor for rhabdomyolysis |
| Voriconazole | ↑↑ | |||
| Fluconazole | ↑↑ | |||
| Atorvastatin | Itraconazole | 3 x ↑ | ||
| Voriconazole | ↑↑ | |||
| Fluconazole | ↑↑ | |||
| Ergot Alkaloids | Voriconazole | ↑ | Avoid concomitant use | |
| Buspirone | Itraconazole | 19 x ↑ | Avoid itraconazole, voriconazole | |
| Voriconazole | ↑? | |||
| . | . | Pharmacokinetics of Azoles Affecting other Drugs . | ||
|---|---|---|---|---|
| Effect of Azoles on other agents . | Azole Used . | Effect of Azole on Drug Exposure . | Adjust Dose of or Discontinue Concomitant Drug . | Monitoring . |
| Abbreviations: AUC, area under the curve | ||||
| Antineoplastic | ||||
| Busulfan | Itraconazole | 25% ↑ | Avoid azole | Monitor for toxicity |
| Voriconazole | ↑ | |||
| Vinca alkaloids | Itraconazole | ↑ | ||
| Voriconazole | ↑ | |||
| Docetaxel | Itraconazole | ↑ | ||
| Voriconazole | ↑ | |||
| Cyclophosphamide | Itraconazole | ↑ | ||
| Immunosuppressives | ||||
| Methylprednisolone | Itraconazole | 2–3 x ↑ | Change to dexamethasone | |
| Voriconazole | ↑? | |||
| Cyclosporine | Itraconazole | 50% ↑ | Decrease cyclosporine dose by 50% and/or according to levels and clinical toxicity | Monitor immunosuppressant level daily |
| Voriconazole | 2 x ↑ | |||
| Fluconazole | 50% ↑ | |||
| Tacrolimus | Itraconazole | 5 x ↑ | Decrease tacrolimus dose by 50 % and/or according to levels and clinical toxicity | |
| Voriconazole | 3 x ↑ | |||
| Fluconazole | ↑ | |||
| Sirolimus | Ketoconazole | 11 x ↑AUC | Azoles contraindicated | |
| Itraconazole | ↑↑↑ | |||
| Voriconazole | ↑↑↑ | |||
| Fluconazole | ↑↑ | Decrease sirolimus dose | Decrease sirolimus level | |
| Digoxin | Itraconazole | 68% ↑ | Decrease digoxin dose according to digoxin level | Monitor digoxin level daily, EKG |
| Fluconazole | ↑ | |||
| Warfarin | Itraconazole | ↑ | Decrease warfarin dose according to INR | Monitor INR |
| Voriconazole | ↑ | |||
| Fluconazole | 70% ↑ | |||
| Benzodiazepines | ||||
| Midazolam | Itraconazole | 2 x ↑ | Avoid agent, decrease dose by 50%, or use oxazepam instead | Monitor for central nervous system toxicity (drowsiness, sleepiness) |
| Voriconazole | ↑? | |||
| Fluconazole | 2 x ↑ | |||
| Triazolam | Itraconazole | 2 x ↑ | ||
| Voriconazole | ↑? | |||
| Fluconazole | 2 x ↑ | |||
| Diazepam | Itraconazole | 35% ↑ | ||
| Voriconazole | ↑? | |||
| Zolpidem | Voriconazole | ↑? | ||
| Proton pump inhibitors | Voriconazole | ↑ | Reduce proton pump inhibitor dose by 50% | |
| Fluconazole | ↑ | |||
| Phenytoin | Itraconazole | ↑↑ AUC | Avoid triazole | Monitor phenytoin level |
| Voriconazole | ↑ 80 | |||
| Fluconazole | ↑↑ AUC | |||
| Rifampin | Itraconazole | ↑ AUC | Monitor for rifampin toxicity | |
| Voriconazole | ↑ AUC | Avoid voriconazole | ||
| Fluconazole | ↑ AUC | |||
| Rifabutin | Voriconazole | ↑↑↑AUC | Avoid voriconazole, fluconazole | |
| Fluconazole | ↑↑ | |||
| Oxybutynin | Itraconazole | 2 x ↑AUC | Avoid itraconazole | |
| Sulfonylurea | Voriconazole | ↑ | Adjust dose according to blood sugar | Monitor glucose concentration |
| Fluconazole | ↑ | |||
| HMG-CoA Reductase Inhibitors | ||||
| Lovastatin/Simvastatin | Itraconazole | 10–20 x ↑ | Avoid using simvastatin, lovastatin, atorvastatin. May use pravastatin or fluvastatin | Monitor for rhabdomyolysis |
| Voriconazole | ↑↑ | |||
| Fluconazole | ↑↑ | |||
| Atorvastatin | Itraconazole | 3 x ↑ | ||
| Voriconazole | ↑↑ | |||
| Fluconazole | ↑↑ | |||
| Ergot Alkaloids | Voriconazole | ↑ | Avoid concomitant use | |
| Buspirone | Itraconazole | 19 x ↑ | Avoid itraconazole, voriconazole | |
| Voriconazole | ↑? | |||
Important drug interactions with azoles: effect of other agents on azoles.
| . | . | Pharmacokinetics of Other Drugs Affecting Azoles . | ||
|---|---|---|---|---|
| Effect of Other Agents on Azoles . | Azole Used . | Effect on Azole . | Adjust Dose of Azole or Avoid Concomitant Drug . | Monitoring . |
| Abbreviations: AUC, area under the curve | ||||
| Rifabutin, rifabutin | Itraconazole | ↓↓ AUC | Avoid rifampin/rifabutin in patients receiving triazoles | Monitor azole level |
| Voriconazole | ↓↓↓ AUC | |||
| Fluconazole | ↓ AUC | |||
| Phenytoin | Itraconazole | ↓↓↓↓ AUC | Avoid concomitant use | |
| Voriconazole | ↓ AUC | Double voriconazole dose | ||
| H2-blockers | Itraconazole | ↓ AUC 30–50% | Use with cola, increase dose | |
| Proton pump inhibitors | capsule | ↓ AUC 64% | ||
| Anti-retroviral agents | ||||
| Didanosine | Itraconazole | ↓ AUC | Adjust azole dose according to azole level | Monitor azole level |
| Nevirapine | Voriconazole | ↓? AUC | ||
| Delavirdine | ↑? AUC | |||
| Phenobarbital | Itraconazole | ↓↓↓↓ AUC | Avoid concomitant use | Monitor azole level |
| Carbamazepine | Itraconazole | |||
| Voriconazole | ||||
| Isoniazid | Itraconazole | |||
| . | . | Pharmacokinetics of Other Drugs Affecting Azoles . | ||
|---|---|---|---|---|
| Effect of Other Agents on Azoles . | Azole Used . | Effect on Azole . | Adjust Dose of Azole or Avoid Concomitant Drug . | Monitoring . |
| Abbreviations: AUC, area under the curve | ||||
| Rifabutin, rifabutin | Itraconazole | ↓↓ AUC | Avoid rifampin/rifabutin in patients receiving triazoles | Monitor azole level |
| Voriconazole | ↓↓↓ AUC | |||
| Fluconazole | ↓ AUC | |||
| Phenytoin | Itraconazole | ↓↓↓↓ AUC | Avoid concomitant use | |
| Voriconazole | ↓ AUC | Double voriconazole dose | ||
| H2-blockers | Itraconazole | ↓ AUC 30–50% | Use with cola, increase dose | |
| Proton pump inhibitors | capsule | ↓ AUC 64% | ||
| Anti-retroviral agents | ||||
| Didanosine | Itraconazole | ↓ AUC | Adjust azole dose according to azole level | Monitor azole level |
| Nevirapine | Voriconazole | ↓? AUC | ||
| Delavirdine | ↑? AUC | |||
| Phenobarbital | Itraconazole | ↓↓↓↓ AUC | Avoid concomitant use | Monitor azole level |
| Carbamazepine | Itraconazole | |||
| Voriconazole | ||||
| Isoniazid | Itraconazole | |||
Empirical/pre-emptive therapy: Agents of choice.
| . | . | Beneficial effect: Rate (interval), P value . | |
|---|---|---|---|
| Study . | Agents and Population . | Fungal Infections . | Mortality . |
| Abbreviations: LAMB, liposomal amphotericin B; AmB-D, amphotericin B; pts, patients; ABCD, amphotericin B colloidal dispersion; NS, not significant. | |||
| Bow, 200035 | Empirical, Drug vs AmB-D: 2756 cancer patients | ||
| Azoles vs. AmB-D | 1.15 (0.76–1.74) NS | 1.18 (0.47–2.99) NS | |
| LAMB vs. AmB-D | 1.11 (0.7–1.75) NS | 0.35 (0.14–0.87) LAMB ↓ | |
| Johansen, 200236 | Empirical, Polyenes: 1790 cancer patients | ||
| LAMB vs. AmB-D 1149 pts | 0.63 (0.39–1.01) P = .053 LAMB ↓ IFI | 0.74 (0.52–1.07), P = .11 Trend to ↓ with LAMB | |
| Intralipid AmB vs. AmB-D 379 pts | 0.67 (0.12–3.75) NS 0.53 (0.2–1.4), NS | ||
| ABCD vs. AmB-D 262 pts | 1.21 (0.28–5.16) NS | 1.19 (0.61–2.34), NS | |
| Gotzsche, 200233 | Prophylaxis/empirical therapy: Drug more effective than none = 4094 patients | ||
| AmB-D, LAMB | 0.39 (0.2–0.76), P = .01 | 0.73 (0.5–1.03), P = .08 | |
| Itraconazole | 0.51 (0.27–0.96), P = .04 | 0.88 (0.58–1.34), P = .6 | |
| . | . | Beneficial effect: Rate (interval), P value . | |
|---|---|---|---|
| Study . | Agents and Population . | Fungal Infections . | Mortality . |
| Abbreviations: LAMB, liposomal amphotericin B; AmB-D, amphotericin B; pts, patients; ABCD, amphotericin B colloidal dispersion; NS, not significant. | |||
| Bow, 200035 | Empirical, Drug vs AmB-D: 2756 cancer patients | ||
| Azoles vs. AmB-D | 1.15 (0.76–1.74) NS | 1.18 (0.47–2.99) NS | |
| LAMB vs. AmB-D | 1.11 (0.7–1.75) NS | 0.35 (0.14–0.87) LAMB ↓ | |
| Johansen, 200236 | Empirical, Polyenes: 1790 cancer patients | ||
| LAMB vs. AmB-D 1149 pts | 0.63 (0.39–1.01) P = .053 LAMB ↓ IFI | 0.74 (0.52–1.07), P = .11 Trend to ↓ with LAMB | |
| Intralipid AmB vs. AmB-D 379 pts | 0.67 (0.12–3.75) NS 0.53 (0.2–1.4), NS | ||
| ABCD vs. AmB-D 262 pts | 1.21 (0.28–5.16) NS | 1.19 (0.61–2.34), NS | |
| Gotzsche, 200233 | Prophylaxis/empirical therapy: Drug more effective than none = 4094 patients | ||
| AmB-D, LAMB | 0.39 (0.2–0.76), P = .01 | 0.73 (0.5–1.03), P = .08 | |
| Itraconazole | 0.51 (0.27–0.96), P = .04 | 0.88 (0.58–1.34), P = .6 | |
The pattern of oral mucositis in time scored by 4 different grading systems.
1. Kolbinson et al8 2. Weisdorf et al6 3. Rocke et al7 4. Donnelly et al.4
This is an unpublished figure from JP Donnelly 2003
Example of lactulose/rhamnose (L/R) ratio pattern after myeloablative conditioning.
L/R ratio of 6 recipients of an allogeneic hematopoietic stem cell (HSC) transplant after conditioning with idarubicin, cyclophosphamide and total body irradiation (TBI).
Example of lactulose/rhamnose (L/R) ratio pattern after myeloablative conditioning.
L/R ratio of 6 recipients of an allogeneic hematopoietic stem cell (HSC) transplant after conditioning with idarubicin, cyclophosphamide and total body irradiation (TBI).
Biological model of oral mucositis. The 4 different phases of oral mucositis. Adapted from Sonis et al.24
Biological model of oral mucositis. The 4 different phases of oral mucositis. Adapted from Sonis et al.24
Mucosal barrier injury of the gastrointestinal tract.
Mucosal barrier injury occurs in 4 phases. The first phase is the inflammatory/vascular phase and is characterised by the induction of proinflammatory cytokines interleukin (IL)-1, tumor necrosis factor (TNF)-alpha, and interferon (IFN)-gamma by cytotoxic drugs and irradation while the epithelial cells are still intact. The second phase is the epithelial phase when cells cease dividing and die. This coincides with neutropenia. The third phase is when necrosis and ulceration occur and is when the resident microbial flora and their products, e.g., endotoxin, translocate into the bloodstream. Moreover, impaired local defenses and lower levels of secretory IgA may allow local infection to develop. The final phase is when healing takes place. It involves the action of naturally occurring substances including trefoils, epithelial growth factors (EGFs), and transforming growth factors (TGFs). The events that take place in the gut are almost certainly more complicated than those occurring in the oral cavity since the gastrointestinal tract is intrinsically more complex in terms of its function. It possesses the specialized gastrointestinal-associated lymphoid tissue (GALT) system, and its resident microflora are more numerous and varied.
Blijlevens et al.17
Mucosal barrier injury of the gastrointestinal tract.
Mucosal barrier injury occurs in 4 phases. The first phase is the inflammatory/vascular phase and is characterised by the induction of proinflammatory cytokines interleukin (IL)-1, tumor necrosis factor (TNF)-alpha, and interferon (IFN)-gamma by cytotoxic drugs and irradation while the epithelial cells are still intact. The second phase is the epithelial phase when cells cease dividing and die. This coincides with neutropenia. The third phase is when necrosis and ulceration occur and is when the resident microbial flora and their products, e.g., endotoxin, translocate into the bloodstream. Moreover, impaired local defenses and lower levels of secretory IgA may allow local infection to develop. The final phase is when healing takes place. It involves the action of naturally occurring substances including trefoils, epithelial growth factors (EGFs), and transforming growth factors (TGFs). The events that take place in the gut are almost certainly more complicated than those occurring in the oral cavity since the gastrointestinal tract is intrinsically more complex in terms of its function. It possesses the specialized gastrointestinal-associated lymphoid tissue (GALT) system, and its resident microflora are more numerous and varied.
Blijlevens et al.17
Preventing invasive candidiasis and aspergillosis.
Modified with permission from
Preventing invasive candidiasis and aspergillosis.
Modified with permission from
Role of surgery in pulmonary aspergillosis in patients with hematologic cancer.
Role of surgery in pulmonary aspergillosis in patients with hematologic cancer.