Abstract
The last decade has witnessed a multistep evolution in the understanding of the natural history, clinical manifestations, and some of the molecular mechanisms that underlie the ineffective hematopoiesis and leukemic transformation in the myelodysplastic syndrome (MDS). The international prognostic scoring system, FAB, and WHO classifications have helped define specific subgroups with their characteristic cytogenetic, molecular and immunological abnormalities. Until recently the mainstay of the treatment has been entirely supportive with blood and platelet transfusions. What is increasingly manifest now is the considerable excitement generated by the emergence of novel therapeutic strategies based on painstaking research findings from the laboratories.
In Section I, Dr. Alan List reviews the therapeutic strategies with the specific emphasis on the relevance of molecular mechanism of apoptosis and targeted therapies using small molecules. Of particular interest is the excitement surrounding the clinical benefit obtained from potent immunomodulatory derivative (IMiD) of thalidomide CC5013. The review provides an update of the role of small molecule inhibitors of VEGF receptor tyrosine kinase, arsenic trioxide, oral matrix metalloprotease inhibitors, farnesyl transferase inhibitors, and imatinib mesylate in the treatment of MDS subgroups.
In Section II, Dr. Steven Gore describes the results of clinical trials of inhibitors of DNA methylation such as 5 azacytidine (5 AC) and 5-aza 2-deoxycytidine (Decitabine). The review also provides an update on the rationale and results obtained from the combination therapy using histone deacetylases (HDAC) and DNA methyltransferase inhibitors in the treatment of MDS.
In Section III, Professor Ghulam Mufti and Dr. Aloysius Ho describe the role of bone marrow transplantation with particular emphasis on recent results from reduced-intensity conditioned transplants, exploiting the graft versus leukemia effect without significant early treatment-related mortality. The section provides an update on the results obtained from the manipulation of the host’s immune system with immunosuppressive agents such as ALG and/or cyclosporine A.
I. Crippling the Clone
Alan F. List, MD*
Arizona Cancer Center, 1515 North Campbell, Room 3945, PO Box 245024, Tucson AZ 95724-5024
Enthusiasm for participation in clinical trials and expectations for benefit have never been greater for patients with myelodysplastic syndrome (MDS). Advancements in the development of novel therapeutics have been accelerated by elucidation of molecular targets integral either to propagation of the malignant clone, disease progression or disease-specific survival signals. Established management strategies for MDS emphasize the use of recombinant growth factors such as erythropoietin, granulocyte-colony stimulating factor (G-CSF) and granulocyte/macrophage-CSF (GM-CSF) to extend survival of erythroid and myeloid progenitors, supplemented by blood product transfusion and iron chelation therapy as necessary. Although improvement in neutrophil production is common, expectation for sustained erythroid response and to a greater extent, amelioration of thrombocytopenia, is limited. Availability of treatments to the practicing physician which offer the prospect to meaningfully alter quality of life or the natural history of disease for affected individuals is severely lacking. Preliminary experience with the most promising new therapeutics suggests that some may indeed for the first time offer sustained benefit and raise hopes that the relentless progression of disease and its complication may be curtailed. These agents can be segregated into two broad categories based upon their intended target of interaction, i.e., (1) survival signals, and (2) genetic integrity (Table 1 ).
Survival Signals
Antiangiogenic agents
Evidence to date indicates that clonal expansion and apoptotic response in MDS arises from an interaction between the malignant clone and its microenvironment. Recent investigations have implicated autocrine production of angiogenic molecules in the self-renewal of myelomonocytic precursors while fostering the generation of aptogenic cytokines. Vascular endothelial growth factor-A (VEGF-A) in particular has emerged as an important diffusible effector of the pathobiology of MDS. This angiogenic molecule is over expressed and elaborated in concordance with its high affinity, Type III receptor tyrosine kinases (VEGFR-1 and/or VEGFR-2) by myeloblasts and monocytes derived from the malignant clone.1 Indeed, laboratory studies support an autocrine role for VEGF as a mitogenic cytokine supporting myeloblast self-renewal in primary MDS and leukemia specimens.1,2 Paracrine induction of inflammatory cytokines from receptor-competent endothelial, macrophage or stromal cells within the microenvironment potentiates ineffective hematopoiesis by suppressing formation of VEGF receptor-naï;ve primitive progenitors. Based upon these and other preclinical investigations,1– 4 small molecule inhibitors of angiogenic cytokines have emerged as a promising class of therapeutics for MDS.
Thalidomide (Thalomid™, Celgene Inc, Warren NJ), which displays both anti-angiogenic and TNFα inhibitory properties, represents the first agent investigated in this class of therapeutics.5–,8 In a Phase II trial of thalidomide performed at the Rush Presbyterian Cancer Institute,9 15 of 83 (18%) evaluable patients experienced either red blood cell transfusion independence or a > 50% decrease in transfusion burden, whereas improvement in non-erythroid lineages was uncommon. Dose escalation beyond 200 mg daily was limited by cumulative neurological toxicity, and is likely unnecessary. Indeed, the North Central Cancer Treatment Group study N998B which evaluated the tolerance and activity of an alternate schedule of 200 mg daily with escalation to a maximum daily dose of 1000 mg was compromised by excessive early attrition due to toxicity at a median interval ≤ 2.5 months.10 Prolonged drug treatment when tolerated appears necessary to maximize hematological benefit. Median interval to erythroid response was 16 weeks in the Rush trial (range, 12–20 weeks), with an erythropoietic response rate of 29% among the 51 patients completing a minimum of 12 weeks of study treatment. Subsequent institutional studies have confirmed the erythropoietic activity of thalidomide, which, given its necessity for prolonged administration, appears best suited for treatment of patients with lower risk disease.11,12 Investigation of the overall clinical benefit of low-dose thalidomide in MDS is nearing completion in a national randomized, placebo-controlled Phase III trial.
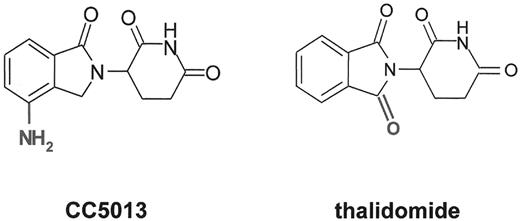
Novel, more potent thalidomide analogues with improved toxicity profiles recently entered clinical investigations.13–,15 CC5013 (Revimid™, Celgene Inc) is a more potent immunomodulatory derivative (IMiD) of thalidomide (Figure 1 ) that lacks the neurological toxicities of the parent compound.15,16 CC5013 inhibits trophic response to VEGF in myeloblasts and endothelial cells, while augmenting heterotypic adhesion of hematopoietic progenitors to bone marrow stroma to promote sustained growth arrest and preferential extinction of myelodysplastic clones.17 Among 25 MDS patients with symptomatic or transfusion-dependent anemia who completed 8 or more weeks of treatment with CC-5013, 16 (62%) experienced an erythroid response according to International Working Group (IWG) criteria, with 12 patients experiencing sustained transfusion independence or a 2 g/dL or greater rise in hemoglobin.16 Hematopoietic promoting activity was greater among patients with Low or Int-1 risk MDS, with 15 of 21 (71%) experiencing hematological benefit. Erythroid responses to CC5013 were associated with complete or partial (> 50%) reduction in the proportion of abnormal metaphases in 9 of 13 informative patients, as well as improved primitive progenitor outgrowth, and reduced grade of cytological dysplasia. Myeloid and platelet toxicity was dose limiting, but occurred at all dose levels depending upon cumulative drug exposure, necessitating either dose reduction or treatment interruption. CC5013 is a promising oral therapeutic that may secure a new position in the management of ineffective erythropoiesis in patients with MDS. CC1088, a member of a second functional class of analogs termed selective cytokine inhibitory drugs or SelCIDs, appears significantly less active in preliminary clinical studies in MDS.
Small molecule inhibitors of the VEGF receptor tyrosine kinases (RTK), which function as targetselective ATP mimetics to impair ligand-induced autophosphorylation, have had limited investigation in MDS. SU5416 (Sugen Inc, S. San Francisco, CA), represents the only agent of its class to complete Phase II investigation. Like most RTK antagonists, specificity is relative, with activity extending from VEGFR-1 and VEGFR-2 to other type III receptors such as those for the PDGFβ, FLT3, and c-kit.18,19 A multicenter trial involving patients with higher risk MDS or acute myeloid leukemia (AML) yielded minimal reduction in leukemia burden and corresponding hematological benefit despite increased apoptotic index in the myeloblast population.20,21 Clinical development of this agent was limited by its insolubility and requirement for twice weekly intravenous administration. Investigation of the orally bioavailable analogue SU11248 in patients with AML ended prematurely owing to limiting non-hematological organ toxicities.22 Despite the disappointing early results of this class of agents in myeloid malignancies, clinical investigation of potent and orally active receptor antagonists continues. The Cancer and Leukemia Group B is investigating PTK787 (Novartis, East Hanover, NJ) in patients with lower risk MDS using a once daily administration schedule, whereas a more potent RTK inhibitor developed by Agouron Pharmaceuticals (La Jolla, CA), AG13736, is entering Phase I and II investigation in patients with MDS or high risk AML.
Arsenic trioxide (Trisenox™, Cell Therapeutics Inc., Seattle, WA), approved by the Food and Drug Administration (FDA) for treatment of relapsed acute promyelocytic leukemia (APL), has broad biological properties that derive from its ability to covalently bind and deplete cellular sulfhydryl-rich proteins such as glutathione. Arsenic in its trivalent form inhibits glutathione peroxidase to potentiate peroxide generation, disrupt mitochondrial respiration and mitochondrial membrane integrity, repress antiapoptotic proteins and initiate caspase-mediated apoptotic response.23–,27 Higher arsenic trioxide (ATO) concentrations are necessary to induce apoptosis and suppress leukemia colony-forming capacity in non-APL AML cells.24,28 In MDS and AML, the anti-proliferative effects of ATO relate in part to its ability to suppress myeloblast elaboration of VEGF-A and its direct cytotoxicity to neovascular endothelium.29 Not surprisingly, bone marrow specimens from patients with MDS which natively harbor lower glutathione reserves compared to normal hematopoietic progenitors,30 also demonstrate increased apoptotic susceptibility to ATO that is enhanced by GM-CSF stimulation.31
Preliminary results of three clinical trials indicate that ATO has activity in both lower- and higher-risk MDS.32– 34 The doses and schedules applied in these studies varies, ranging from monthly cycles of two sequential weekly treatments of 0.25 mg/kg/day for 5 days followed by a 2 week treatment hiatus to a dose-intense induction with 0.30 mg/kg/day for 5 days followed by 0.25 mg/kg/day twice weekly maintenance for 15 weeks. Overall, approximately a third of patients have experienced hematological improvement, with few complete or partial remissions. Nonetheless, these initial experiences are encouraging, demonstrating the potential for trilineage hematological improvement with monotherapy that may be sustained for prolonged periods after treatment cessation. Given the manageable toxicity of ATO, combination trials have been initiated which offer the prospect to improve upon the results obtained with single agent therapy.
Other antiangiogenic agents investigated in MDS have demonstrated either a more limited toxicity: benefit profile for extended use or have not as yet completed clinical investigation. A double blind, randomized Phase II trial investigating two doses of the oral matrix metalloprotease (MMP) inhibitor AG3340 (Prinomastat™, Agouron) revealed erythropoietic activity of this novel compound that was limited to patients with lower risk MDS.35 The MMPs represent a family of zinc-dependent endopeptidases that function as effectors of the angiogenic response.36 MMPs are zymogens, which upon catalytic activation, degrade structural components of the extracellular matrix, disrupt integrin/stromal adhesion signals, and promote local VEGF, TNFα and soluble fas ligand liberation via proteolytic cleavage of proteoglycan- or membrane-bound forms.37–,39 AG3340 is a potent, orally bioavailable, selective inhibitor of MMPs 2 and 9 (gelatinases A and B), and MMP13 (collagenase-3), with weak inhibition of MMP1.40 Although arthralgias and joint stiffness were dose limiting at 15 mg dose versus 5 mg daily (63% vs. 33%), hematological activity was equivalent with 6 of 26 patients with Low or Int-I risk disease experiencing red blood cell transfusion-independence that was sustained beyond the 1 year of study treatment (median, 29+ weeks, maximum, 100+ weeks). Although this agent will not continue in clinical development, these data like those of CC5013 provide added proof that angiogenic molecule generation by the malignant clone contributes to ineffective erythropoiesis via the consequent impact on progenitor interaction with the extracellular matrix. Bevacizumab (Avastin™, Genentech, S. San Francisco, CA), a recombinant humanized monoclonal antibody which neutralizes VEGF-A in vivo, is currently completing Phase II investigation in MDS.
Farnesyl transferase inhibitors
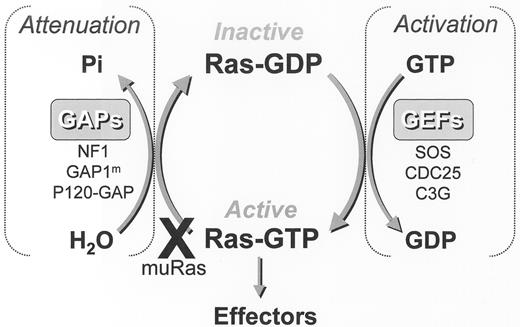
Activating point mutations of the RAS proto-oncogene are detected in fewer than 20% of unselected patients with MDS, but are common in chronic myelomonocytic leukemia (CMML).41–,44 The RAS gene superfamily encodes guanosine triphosphate hydrolases (GTPase) that serve as critical regulatory elements in signal transduction, cellular proliferation and maintenance of the malignant phenotype. The 3 RAS proto-oncogenes (H, N and K) encode 4 21-kd G-proteins including 2 alternatively spliced K-Ras products that are post-translationally modified before incorporation into the inner leaflet of the plasma membrane. Farnesylation of carboxy-terminal consensus sequences by farnesyl protein transferase (FPT) represents the first and rate limiting post-translational modification of Ras-GTPases that is requisite for membrane association and transforming activity.45 FTP catalyzes the transfer of a 15-carbon farnesyl group from farnesyl diphosphate to the C-terminal tetra-peptide CAAX (C, cysteine; A, any aliphatic amino acid; X, any amino acid) sequence of the Ras protein. These G-proteins normally cycle between two conformations induced by binding of either guanosine diphosphate (GDP) or GTP, the rate of which is controlled by GTPase regulatory proteins (Figure 2 ). GTPase activating proteins (GAP) accelerate GTP hydrolysis, whereas guanosine exchange factors (GEF) promote GDP dissociation from the G-protein. Point mutations in the RAS proto-oncogenes occur at critical regulatory sites (e.g., codons 12, 13, and 61) which inactivate the GTPase response normally stimulated by GAP binding, thereby extending the half-life of the Ras-GTP bound mutant.
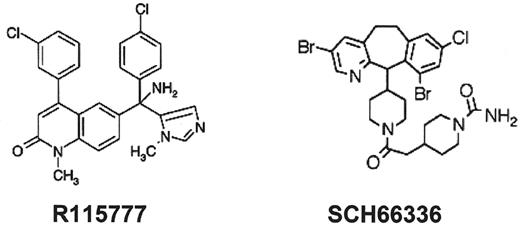
The farnesyl protein transferase inhibitors (FTI) represent a novel class of potent, orally bioavailable inhibitors of Ras activation and other prenylation-dependent molecules. These agents are able to modulate multiple signaling pathways that have been implicated in the pathobiology or progression of CMML and MDS in the absence of salvage isoprenylation pathways. Preliminary results of Phase I/II studies in MDS and CMML indicate promising hematopoietic promoting activity that extends to non-erythroid lineages.46–,49 R115777 or tipifarnib (Zarnestra™; Janssen Pharmaceuticals, Beerse, Belgium and Spring House, PA) and lonafarnib (SCH66336 or Sarasar™; Schering-Plough Research Institute, Kenilworth, NJ) are the leading non-peptide, heterocyclic oral FTIs that have completed Phase I and II clinical studies in hematological malignancies46–,51 (Figure 3 ). Using a 21-day treatment schedule, an initial Phase I study involving 35 patients with either high-risk acute myeloid or lymphoid leukemia or CML blast phase, R115777 yielded a 50% or greater reduction in blast percentage in 29% of patients with complete remissions in 6%, with all responses occurred in patients lacking demonstrable N-RAS mutations.46 In a subsequent Phase II study performed in poor risk AML or MDS, treatment with 600 mg bid every 4 to 6 weeks, yielded responses in 12 of 30 patients (8 CR, 2PR) with stabilization of disease in 12 patients. Three of 4 patients with trisomy 8 achieved CR after 1 cycle of therapy.52 In a Phase I trial performed exclusively in patients with MDS, 6 of 20 evaluable patients, including 2 of 4 patients with RAS mutations, experienced hematological improvement or a partial remission using the 3:1 week syncopated schedule. Myelosuppression and fatigue were dose limiting, with the maximum tolerated dose defined as 400 mg/m2 twice daily.47 A concurrent Phase II trial performed by MD Anderson investigators using a more dose-intensive and protracted schedule (600 mg twice daily for 4 of each 6-week cycles) yielded an unacceptably high frequency of early treatment withdrawal due to drug intolerance (41%). Two complete remissions and 1 partial remission (19%) were reported in patients lacking mutant RAS alleles among the 16 patients completing 2 or more cycles of therapy.48 Phase II studies in patients with myeloproliferative disorders including CMML are in progress, but it is evident from the data generated to date that the activity of these agents cannot be ascribed to the inhibition of farnesylation of constitutively active RAS proteins alone.
In a Phase I study of lonafarnib administered in a continuous schedule in patients with advanced CML, MDS, CMML or acute leukemia, clinical benefit or hematological improvement according to IWG criteria was observed in 5 (29%) of 17 evaluable patients, which included red blood cell transfusion independence, reduction in monocytosis and major platelet responses in 3 of 5 patients with CMML.50 Diarrhea and hypokalemia were limiting with continuous twice daily dosing at 300 mg. Trough plasma concentrations of lonafarnib exceeded 1000 ng/ml at the 200 mg dose level and were sufficient to inhibit FPT in vivo. An expanded Phase II trial was recently completed which included each of the 5 diagnostic groups included in the Phase I study.51 Fifteen to 30 patients were accrued to each cohort using a Simon two-stage design. Among the initial 12 patients with CMML enrolled, control of monocytosis was achieved in 6 (50%) patients associated with a high frequency of platelet response, whereas 3 (33%) of 15 patients with RAEB or RAEB-t experienced hematological improvement. A Phase III randomized trial is planned to investigate the clinical benefit and frequency of platelet response to lonafarnib in patients with CMML or advanced MDS with severe thrombocytopenia. Interestingly, at least 2 patients with CMML experienced rapid and sustained leukocytosis, which in 1 patient was complicated by pulmonary infiltrates that resolved after study drug withdrawal and treatment with dexamethasone.50 The latter findings appear analogous to the leukemia differentiation syndrome reported with retinoid therapy of APL and may be linked to the unique ability of lonafarnib to activate β-1 and β-2 integrins and promote both heterotypic and homotypic adhesion of CMML cells.53 Although initially intended to interrupt mutant RAS induced constitutive signaling, it is clear from the trials completed to date that clinical benefit is independent of mutation status, indicating that inhibition of wild type RAS or other farnesylated molecules may be of greater relevance to the activity of the FTIs in MDS and CMML.
Imatinib (Gleevec™)
Constitutive Ras/mitogen-activated protein kinase (MAPK) activation is demonstrable in 40%–60% of CMML cases, resulting either from mutations within RAS alleles or from reciprocal translocations deregulating receptor tyrosine kinases.43,44,54 In the absence of mutations, sustained activation of the Ras/MAPK cascade may occur through a constitutive upstream signal. Perhaps the most important therapeutic discovery in the management of CMML in recent years is the activity of imatinib in patients harboring a reciprocal chromosome translocation involving chromosome 5q33. Although a number of chromosomes and genes may partner in the gene rearrangements (Table 2 ), the clinical phenotype is distinct, recognized by the WHO classification as CMML with eosinophilia (CMML–Eos), but arising from the generation of novel fusion genes involving the PDGFβ receptor with constitutive RTK signaling.55–,59 Transgenic mouse models have shown that these novel RTK fusion genes are singularly responsible for the generation of these myeloproliferative disorders and are selectively responsive to PDGF-Rβ kinase inhibitors.56,60 Imatinib binds to the ATP-binding pocket of the PDGFβ receptor analogous to its interaction with BCR/ABL to act as a potent inhibitor of receptor kinase activity.60 Among 5 patients reported to date, each achieved rapid hematological control and sustained complete cytogenetic remission with imatinib monotherapy.61
Genetic Integrity
The limited life expectancy of patients with higher risk disease has justifiably placed treatment with cytotoxics in the forefront of management strategies for patients with advanced MDS, albeit with less optimistic expectations for sustained disease control. Chemotherapeutics employed to date range from single agent topoisomerase I inhibitors, to traditional AML chemotherapy combinations that routinely employ DNA targeted agents anchored by cytarabine and either topoisomerase I or II interactive agents.64–,66 A recently completed Phase III trial and a retrospective analysis of the MD Anderson experience indicate that the topotecan and cytarabine combination popularized in recent years offers no significant advantage over standard anthracycline-containing regimens, and may have inferior remission durability.63,64 Despite a comparatively high remission rate and reduced induction mortality in the older population compared to historical experiences, fewer than 10% of patients can be expected to remain in remission beyond 2 years. Efforts to improve specificity of cytotoxic therapy using the antibody conjugate gemtuzumab ozogamicin (Mylotarg™, Wyeth) have shown minimal clinical benefit in monotherapy trials involving patients with high-risk MDS.65,66
Strategies targeting biological features that confer native chemotherapy resistance have emerged as a promising approach to improve the results of standard induction chemotherapy. P-glycoprotein (Pgp) is a highly conserved plasma membrane glycoprotein encoded by the MDR1 gene that functions as an ATP-dependent multidrug exporter with broad specificity for natural product-derived antineoplastics and an inhibitor of caspase-dependent programmed cell death.67–,71 Pgp is natively overexpressed by myeloblasts in RAEB-t and secondary AML, and is associated with a higher probability of induction failure and inferior disease-free survival.72–,74 A randomized Phase III trial performed by the Southwest Oncology Group showed that concurrent treatment with the Pgp inhibitor cyclosporine-A (CsA) and infusional daunorubicin following cytarabine significantly reduces induction resistance in high-risk patients and significantly prolongs duration of remission and extends overall survival.77 Patients with RAEB-t or secondary AML experienced the greatest benefit, with an overall survival (CsA, 28% versus control, 0%) and disease-free survival (CsA, 60% versus control, 0%) at 2 years that has been unmatched in other studies. Similar trends were reported in a French cooperative group study that employed quinine as a Pgp modulator,76 indicating that this targeted induction strategy may significantly extend survival for selected individuals who are candidates for more intensive therapy. Additional clinical studies using CsA and newer generations of Pgp antagonists are in progress,77 raising hope that this early lead may give rise to a significant advance in the treatment of MDS and associated leukemias.
II. Molecularly Targeted Therapy for Myelodysplastic Syndrome
Steven D. Gore, MD*
Sidney Kimmel Cancer Center at Johns Hopkins, 1650 Orleans Street, Baltimore MD 21231
The development of molecularly targeted therapies for the treatment of MDS has been hampered by the lack of understanding of the fundamental genetic and biological abnormalities in MDS progenitor cells, as well as the biological abnormalities which lead to characteristic phenotypic abnormalities in more differentiated cells. Despite this major barrier, several new classes of drugs with reasonable biochemical rationale have proved very promising in early clinical development and promise to alter the current standard of care for patients with MDS. Absence of useful in vitro or in vivo models of MDS has required that most preclinical development of new agents targeting MDS utilize AML cell lines. In fact, an increasing number of early phase clinical trials do not distinguish between high-grade MDS and poor-risk AML. The similar response rate in these 2 patient subsets (see farnesyl transferase inhibitors below) emphasizes the biologic continuum of these disease states, and lends support to the World Health Organization (WHO) reclassification of high-grade MDS and acute leukemias which moves away from arbitrary cutoffs of blast percentages to subset these patients.1 The recognition that MDS most commonly progresses gradually, with increasing percentages of blasts over time, rather than acutely “transforming” from an indolent state to a fundamentally more acute state, justifies drug development in combined cohorts of high-grade MDS and high-risk AML (in particular, AML progressed from MDS).
Therapies Targeting Epigenetic Changes
Recent interest in the treatment of neoplastic cells by targeting epigenomic changes to restore normal gene transcription has been intense. Unlike genetic changes (mutations, deletions), which are irreversible outside of the introduction of new genetic information, epigenetic changes represent potentially reversible modifications to DNA and chromatin that are transmitted from parent cell to daughter cell and lead to altered gene expression. The potential reversibility of the epigenetic changes makes them attractive targets for cancer therapeutics.2
DNA methyltransferase inhibitors
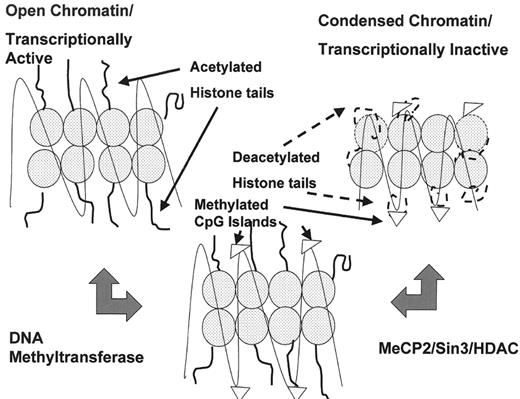
Abnormalities of cytosine methylation constitute some of the best characterized and most common epigenetic changes in cancer. The DNA of neoplastic cells may be characterized by global hypomethylation, dysregulation of DNA methyltransferase I, a key protein for the accurate maintenance of DNA methylation patterns in daughter cells, and regional hypermethylation of CpG dinucleotides in gene promoter regions. These CpG clusters, known as CpG islands, are normally protected from methylation in normal cells, with important exceptions including regions of X chromosome inactivation and imprinted genes. In contrast, CpG islands are often highly methylated in cancer cells.2 The methylated promoters are bound by specific proteins, such as MeCP2, which recruit transcriptional corepressors (Figure 4 ).3 The corepressor complexes lead to transcriptional silencing, at least in part through remodeling of chromatin into conformations that are prohibitive for transcription. This chromatin remodeling is due in part to histone deacetylases (HDAC, see below); however, the transcriptional state of chromatin is regulated by a complex histone code (see below).4
Extensive studies have demonstrated promoter methylation of a wide variety of cell regulatory genes in many cancers associated with silencing of those genes. These epigenomic changes are associated with phenotypic abnormalities in the malignant cells. Because promoter methylation is relatively “cancer-specific,” DNA methylation is an attractive therapeutic target. In malignant myeloid cells, the most widely reported methylated gene is the cyclin-dependent kinase inhibitor p15INK4B.5–,9 Methylation of the p15 promoter has been demonstrated in 68% of primary AML samples,10 and 35% of MDS11; the frequency of methylation increases with MDS disease progression. Methylation of p15 does not have independent prognostic significance in MDS when blast percentage is included as a variable.8 A variety of other genes are methylated in myeloid neoplasms. These include E-cadherin,12 p73, and RARβ.13
Interest in the DNA methyltransferase inhibitors 5-azacytidine (5AC) and 5-aza-2′-deoxycytidine (decitabine, DAC) for the treatment of AML and MDS significantly antedates the current focus on epigenetics. Clinical interest in 5-azacytidine and DAC date back to early studies of Jones and Taylor who demonstrated that these analogs induced cellular differentiation in association with demethylation of DNA.14 Early clinical studies of 5AC in leukemia approached it as a classical cytotoxic nucleoside.15 Subsequent studies, led by Silverman et al at Mt. Sinai and the Cancer and Leukemia Group B (CALGB), used lower doses of 5AC, initially intravenously and then subsequently subcutaneously, to treat patients with MDS.16,17
These studies culminated in the Phase III trial conducted in the CALGB, which randomized MDS patients between subcutaneous 5AC and observation.17,18 Patients in the treatment group received 5AC 75 mg/m2/day subcutaneously for 7 days on a 28-day schedule. A minimum of 4 cycles of therapy was planned. The dose was increased by 33% in patients who demonstrated no benefit by day 57. Patients on the observation/supportive care arm could cross over to 5AC if disease was worsening after a minimum of 4 months. Responding patients received 3 additional cycles after complete response; those with less than complete response continued 5AC until complete response or relapse.
This study definitively established the activity of 5AC in this patient population. The overall hematologic response rate to 5AC was 60%, including complete remissions in 7%, 16% partial responses, and 37% hematologic improvement. Trilineage responses were reported in 23% of patients. In contrast, the “response rate” in the observation arm was only 5%, limited to hematologic improvement. Further substantiation of the activity of 5AC stemmed from the observation of a similar response rate in the patients who crossed over from observation to 5AC treatment (47%). Median duration of response was 15 months. A controversial aspect of this study derived from the reporting of an increase in median time to pathological development of acute leukemia in the 5AC arm (21 months versus 12 months). No differences in the presenting features of the patients in the 2 arms have been identified to explain this effect; moreover, the changes in blast percentages upon the diagnosis of “acute leukemia” were substantial, suggesting that this difference in disease progression was not merely cosmetic. Landmark analysis of patients who remained on study for 6 months showed that median survival was superior for patients receiving 5AC from initial randomization (18 months) compared with patients who crossed over from supportive care (14 months) or those who crossed over after 6 months or never crossed over (11 months). A companion quality-of-life study demonstrated significantly greater improvement in fatigue, shortness of breath, physical functioning, affect, and psychological distress in patients receiving 5AC compared with supportive care.18
Investigation of sequential cytogenetic changes using both conventional and interphase cytogenetics in a large cohort of patients treated with 5AC at Mt Sinai showed that 8% of patients with clonal cytogenetic abnormalities developed cytogenetic remission; whereas 36% of patients presenting with no cytogenetic abnormalities developed cytogenetic abnormalities while on treatment. This number was similar to the frequency of such events in patients receiving other treatments for MDS. Thirteen percent of patients with clonal abnormalities developed additional abnormalities. Sixty percent of patients with clonal abnormalities developed hematologic improvement despite persistence of the abnormal cytogenetic clone.19
Confidence in the activity of DNA methyltransferase inhibitors in the treatment of MDS is increased by reports of similar response rates utilizing DAC. 5AC is a pro-drug for DAC; 5AC undergoes conversion to DAC before incorporation into DNA where it binds DNA methyltransferase irreversibly. DAC has been studied in a variety of Phase II trials as an intravenous formulation. Of 29 patients with high-risk MDS, 15 responded to continuous infusion DAC (40–50 mg/m2/day for 3 days), although 5 patients expired due to complications of cytopenias.20 Subsequently, 66 patients with MDS were treated with 45 mg/m2/day given as 3 4-hour infusions per day for 3 days. Courses were repeated every 6 weeks. Patients received no more than 6 cycles of therapy. Thirty-four patients improved, including 13 complete responses and 9 trilineage responses. This schedule of DAC administration has been associated with a delayed onset of cytopenias, particularly in the first cycle of therapy. Counts may drop as late as 6 weeks following the administration of DAC in this dose and schedule. An 8% toxic death rate was observed. Median response duration was 31 weeks. This shorter progression-free survival compared to 5AC may be due to the decision to give no more than 6 cycles of therapy in the DAC study.21 Importantly, 16 of 50 patients with clonal cytogenetic abnormalities who were evaluable for cytogenetic response to DAC developed normal karyotypes, with a median duration of cytogenetic response of 7.5 months.22 Jean-Pierre Issa and colleagues23 at the MD Anderson Cancer Center have explored schedules of DAC administration that provide prolonged exposure to lower doses, primarily in patients with acute leukemia. Such schedules (for example, 15 mg/m2/day for 10 days) have been well-tolerated, and responses have been observed. Phase II studies of such schedules of administration will be required to fully assess the response rate and relative toxicity of lower dose DAC. To date, no study of subcutaneous DAC has been reported.
The dose-equivalence of the most commonly studied dose and schedules of 5AC and DAC is difficult to evaluate. In vitro, DAC is 10-fold more potent in inhibition of DNA methytransferase.14 Pharmacokinetic studies are not available for either compound. The routes of administration differ. Toxicities appear similar, although DAC may be associated with greater myelosuppression in doses and schedules published to date. Current published data suggest that the 2 drugs as currently studied are equivalently effective in the treatment of MDS.
The relationship of DNA methyltransferase inhibition to the clinical activity of 5AC and DAC in the treatment of MDS remains a critical question. Lübbert and colleagues reported reversal of p15 methylation, associated with reexpression of p15 protein, in a subset of patients treated with DAC.24 The frequency of this reversal of methylation and gene expression cannot be estimated based on current data; nor can the association of changes in methylation with clinical response be ascertained; reversal of p15 methylation did not appear to be required for hematologic response in Lübbert’s study.24 Such studies will be important in the further development of this class of compounds, alone and in combination with other drugs.
Histone deacetylase inhibitors
The transcriptional state of chromatin depends on the translation of a complex set of posttranslational modifications to histone lysine tails including acetylation, methylation, and phosphorylation, referred to as the histone code.4,25 Modifications of specific residues on histones are recognized by specific binding proteins that impact chromatin conformation and transcription. These specific modifications are local; that is, histones are modified in the regions of specific gene promoters, inducing local remodeling of chromatin which impacts the expression of the specific genes. In general, acetylation of lysine residues on histone tails is associated with transcriptionally active chromatin (euchromatin), whereas deacetylated histones are associated with transcriptionally inactive chromatin (heterochromatin). Methylation of lysine 9 on histone H3 is associated with heterochromatin, whereas methylation of lysine 4 on that histone is associated with euchromatin. It is highly likely that as these epigenetic changes are better characterized, many of the enzymes which mediate these changes, such as histone methyltransferases, will be targeted in cancer therapeutics.
The earliest described histone modification known to be associated with transcriptional regulation was acetylation of specific lysine residues in the tails of histones H2, H3, and H4. Histones are acetylated by enzymes which contain histone acetyltransferase activity; in contrast, deacetylation is mediated by histone deacetylases (HDAC). Eleven human HDACs have been identified to date (reviewed by de Ruijter et al26). These include 2 major classes of HDACs (Class I and Class II); class I HDACs are almost exclusively nuclear, while class II HDACs shuttle in and out of the nucleus in response to specific cellular signals. In addition, a third class of HDACs known as the SIR2 family includes enzymes with NAD-dependent HDAC activity; unlike the other 2 classes, these are not inhibited by trichostatin A. HDACs are associated with specific chromatin loci in pairs and triplets.
A variety of HDAC inhibitors (HDACi) are under clinical investigation. Interest in the use of HDACi for the treatment of myeloid malignancies dates back to the recognition of the activity of butyrate derivatives and polar planar compounds to induce differentiation. Certain AML fusion genes, such as AML1-ETO, PML-RARα, and PLZF-RARα specifically recruit nuclear corepression complexes which include HDAC, thereby silencing expression of genes downstream from the promoters bound by the fusion proteins (reviewed by Gore and Carducci27). In such leukemias, HDACi may be utilized to specifically reverse the transcriptional repression induced by the fusion proteins. No such fusion proteins have been identified for most cases of MDS; hence, in this group of diseases, the impact of HDACi is more likely related to changes in gene expression altered through other epigenetic mechanisms.
Small-chain fatty acids:
A variety of small-chain fatty acids inhibit HDAC activity at submillimolar concentrations. These include sodium and arginine butyrate, sodium phenylbutyrate, and valproic acid. Although butyrate was successfully used to induce terminal differentiation in a child with AML28 and has been successfully used to induce expression of fetal hemoglobin in patients with sickle cell anemia29 and thalassemia,30 a subsequent Phase II study of butyrate in AML was without clinical activity.30 Formal pharmacokinetic/pharmacodynamic studies of butyrate have not been performed. Sodium phenylbutyrate (PB) was selected for development in part because of the potential for development of an oral formulation. PB induces histone acetylation, expression of p21WAF1/CIP1, induction of G1 cell cycle arrest, and induction of CD11b expression in myeloid leukemia cells.32 Concentrations that are not clinically achievable induce apoptosis. Phase I studies of continuous infusion PB demonstrated that the drug was well-tolerated; dose-limiting toxicity was a reversible encephalopathy due to accumulation of phenylacetate.33,34 PB was administered for 7/28 days, 7/14 days, and 21/28 days. Lineage responses were achieved in several patients with MDS and AML. At the maximum tolerated dose (375 mg/kg/day), sustained plasma concentrations ranged from 0.3 to 0.5 mM; in vitro, induction of acetylation of histones in hematopoietic cells requires approximately 0.25 mM.35 Higher peak plasma concentrations of PB have been achieved in solid tumor patients receiving short-term infusions of PB (M.A. Carducci, personal communication). An oral formulation has been studied which can supply similar plasma concentrations; however, the daily dosing requires as many as 75 pills per day.36 Despite the difficulty with delivery of concentrations of PB with HDAC inhibitory activity, PB remains extremely well-tolerated.
Recent data demonstrating that valproic acid has similar HDAC inhibitory activity has raised hopes that oral formulations of this drug, which is commonly used for neuropsychiatric disorders, could be used to modulate gene expression.37 Valproate is used clinically at submillimolar concentrations; in vitro acetylation changes have been demonstrated at 0.5 mM. The pharmacodynamic impact of valproate on leukemic cells is similar to PB. Valproate is currently being investigated in monotherapy and in combination with other drugs in MDS and in AML.
SAHA and FK228:
The prototype HDAC inhibitor used for in vitro study, trichostatin, is an hydroxamic acid. To date, the only hydroxamic acid under clinical investigation is suberoylanilide hydroxamic acid (SAHA). SAHA has been developed in both intravenous and oral formulations.38 Clinical administration has been associated with induction of histone acetylation. No data are yet available detailing the impact of SAHA on myeloid malignancies.
FK228 (depsipeptide) is a cyclic peptide whose HDAC inhibitory activity appears to be due in great part to a reduced metabolite. FK228 appears to be specific for Class I HDAC. FK228 has been studied in Phase I clinical trials including 1 in AML.39 Tumor lysis syndrome has been induced by FK228, indicating significant cytotoxicity to myeloid cells; however, no sustained responses were seen in the Phase I trial. Asthenia appears to be dose limiting.
MS-275:
MS-275 is a benzamide HDAC inhibitor undergoing Phase I investigation in MDS and AML. Similar to FK228, fatigue seems to be a major toxicity of MS275. MS275 has a half-life of greater than 24 hours; changes in histone acetylation have persisted for several weeks following the administration of MS-275. No sustained responses have been reported to date (JE Karp, personal communication).
Integration of HDAC inhibitors into the treatment of MDS
The recognition that HDAC recruitment accounted at least in part for the silencing of genes with methylated promoters led to the demonstration that optimal reexpression of such genes required sequential exposure to a methyltransferase inhibitor followed by an HDACi. While definitive demonstration of a correlation between methyltransferase inhibition and clinical activity of methyltransferase inhibitors in MDS has yet to be provided, a great deal of interest remains in augmenting the clinical activity of 5AC and DAC through the addition of an HDACi. To date, data are only available from a dose-finding study of 5AC followed by PB. In this study, a variety of doses and schedules of 5AC have been administered, followed by a 7-day continuous infusion of PB.40 The combination has been well-tolerated, and significant sustained clinical responses have been achieved. Changes in methylation, histone acetylation, and gene reexpression are being monitored. Similar studies are planned combining DAC plus valproic acid.
HDACi synergize with retinoids in a variety of systems.35,41 This has led to the concept of potentially combining these classes of agents to build on the modest single activity of retinoids in MDS as single agents or in combination with growth factors.42 Studies combining all trans-retinoic acid with PB and with valproate are ongoing. Since RARβ is methylated and silenced in a variety of cancers, including myeloid malignancies, some rationale exists to study the combination of a methyltransferase inhibitor, HDACi, and retinoid.
Farnesyl Transferase Inhibition
Interest in inhibition of farnesyl transferase in myeloid malignancies derived from the observation that ras mutations were common in these leukemias. Tipifarnib has undergone Phase I testing in AML, and a Phase II trial is ongoing. In the Phase I trial, significant clinical responses were seen at all dose levels tested, including 2 complete remissions. Interestingly, no patients in that initial trial were found to have mutations of ras. Inhibition of farnesyl transferase was demonstrated, beginning at the dose level of 300 mg BID; farnesylation of target proteins was inhibited at 600 mg BID. In the Phase II trial, patients with high-risk AML (age > 60, known adverse cytogenetics, therapy- or MDS-related AML) and high-grade MDS, have been treated with tipifarnib 600 mg BID for 21/28 days. An overall response rate of 37% has been observed to date (J.E. Karp, personal communication). In a separate Phase I trial of tipifarnib in MDS, Kurzrock and colleagues demonstrated inhibition of farnesyl transferase; clinical responses were seen, including a complete response.43
Another farnsesyl transferase inhibitor, lonafarnib, has undergone early Phase I and II studies in patients with hematologic malignancies.44,45 Two patients with MDS were treated on the Phase I, and 15 on the Phase II trial. Five patients with CMML were treated on the Phase I trial, and 12 on the Phase II trial. Of the 5 CMML patients on the Phase I trial, 3 developed hematologic improvement. Of the 15 MDS patients on the Phase II study, 3 developed hematologic improvement, while 6 of the 12 patients with CMML normalized monocytes counts. Preliminary evidence of clinical activity of this second compound increases confidence that farnesyl transferase inhibition is a valid target for MDS therapy.
Novel Differentiation Approaches
Most agents that have been used to induce terminal differentiation in vitro are antiproliferative; they share in common induction of p21WAF1/CIP1 and concomitant cell cycle arrest.46 In vitro data suggest that the pairing of such cytostatic agents with myeloid growth factors might lead to the predominance of differentiating activity of the growth factor over the proliferative impact of the growth factor.47,48 One combination which appears particularly promising is the combination of the protein kinase C activator bryostatin with GM-CSF. In AML cell lines, this combination leads to suppression of clonogenic cell growth in association with induction of markers of terminal myeloid differentiation. A Phase I trial of this combination is ongoing in patients with AML and MDS; responses have included improvement in neutrophil counts and decreases in bone marrow blast percentage (BD Smith, personal communication). A similar concept was tested using the combination of all trans-retinoic acid plus erythropoietin. Thirteen of the 27 patients developed erythroid responses. Neutrophil and platelet responses were seen in 5 of 12 and 5 of 9 evaluable patients, respectively. Three trilineage responses developed on this study.42
Future Directions
The emergence of drugs with activity in myelodysplastic syndrome provides the opportunity for deeper understanding of the biology of these diseases. While DNA methyltransferase inhibitors demonstrate the highest single agent response rates reported, their mechanisms of action against MDS remain speculative. It will be critical to demonstrate the correlation (or lack thereof) between clinical response and methyltransferase inhibition or downstream changes in methylation. Expression of which genes correlate with response? Does methylation of any specific genes predict response? Similarly, while it appears that farnesyl transferase inhibitors are active against MDS, their activity is not exclusively or selectively due to inhibition of ras per se. Is the clinical activity due to inhibition of farnesylation; if so, which farnesyl-dependent targets are required for response? Does this class of drugs possess biological activity separate from its targeted inhibitory activity? By studying pathways highlighted by these active drugs’ clinical activities, and potentially by studying congeners which differ significantly in targeted activity, it is likely that greater understanding of the pathobiology of MDS will emerge.
III. Results of Transplantation and Immunosuppressive Therapy in MDS
Ghulam J. Mufti, MB, DM, FRCP, FRCPath,* and Aloysius Y.L. Ho, MBBS, MMed, MRCP, MRCPath
Kings College Hospital, Department of Haematology, Denmark Hill, London SE5 9RS, UK
Hematopoietic Stem Cell Transplantation for Myelodysplastic Syndrome
Autologous and allogeneic hematopoietic stem cell transplantation (HSCT) has been extensively investigated in MDS. Autologous HSCT in MDS is theoretically feasible only in a small proportion of patients who achieve a complete remission following induction chemotherapy and in whom a suitable autologous harvest can be collected. A successful autograft is restricted by a limited potential for peripheral blood stem cell (PBSC) collection,1 graft contamination,2 delayed engraftment,3 and a high relapse risk of up to 72%, with a 2-year disease-free survival of only 25% for patients 40 to 63 years of age.4 There is a suggestion, however, from Registry data that some patients over the age of 40 may benefit from an autograft, with a 3-year disease-free survival (DFS) of approximately 33% if autografted in first remission.5 Nevertheless, enthusiasm for this approach is limited.
Although conventional myeloablative allogeneic HSCT has a significantly lower relapse rate than autografts at 28% to 48%, the transplant-related mortality (TRM) can be substantial at up to 39% to 54%6–,9 Transplant-related complications including graft-versus-host disease (GVHD) increases in frequency and severity with advancing age.10 There is extensive registry data available on patients who have undergone standard myeloablative allografts over the years. Analysis of IBMTR data on 452 recipients of matched-sibling allografts, with a median age of 38 years (range, 2–64 years), conditioned with varying “standard” intensity conditioning regimens, revealed a 3-year TRM of 37%, with DFS of 40% and an overall survival (OS) of 42%.11 This is similar to data from the EBMT database of 1378 patients transplanted between 1983 and 1998, which showed a 3-year DFS for matched-sibling donor recipients of approximately 36% and for VUD recipients 25%. TRM was 58% for unrelated donors, more than double that of 25% for autografts.5
The NMDP database for 510 patients with MDS receiving VUD allografts between 1988 and 1998 showed that for patients with a relatively young median age of 38 years (<1–62 years), the DFS at 2 years was 29% with a TRM of 54%. Conditioning regimens were variable, but were all of “standard intensity.” TRM occurred within the first 100 days in 69% of the patients, with regimen-related toxicity and GVHD accounting for 34% of overall mortality.9
Multivariate analysis shows that age and disease stage were independent prognostic variables for TRM, DFS, and OS, with patients transplanted early in the disease course having a significantly lower relapse risk independent of the donor source, and with older patients faring worse than younger.5 The risk of relapse was higher in patients transplanted with higher blast counts and IPSS scores, and in T-cell depleted transplants.11
Allogeneic HSCT is the only therapeutic modality at present that may be delivered with curative intent in MDS. Allogeneic HSCT replaces recipient dysplastic hemopoiesis with healthy donor hemopoiesis and immune system with an attendant graft-versus-leukemia (GVL) effect. Its applicability, however, is limited by the availability of a suitable HLA-matched donor and by the toxicity of the conditioning regimens, which is directly proportional to the age of the recipient.8,12 As the majority of patients with MDS are of advanced age, with only about 25% of patients younger than 60 years,13,14 often with concurrent medical conditions that effectively preclude standard conditioning for allogeneic HSCT, various strategies have been adopted in order to attempt to reduce the toxicities associated with the transplant procedure. At present, there is considerable interest in novel conditioning regimens.
The use of targeted-dose busulfan in a conditioning regimen of “standard” intensity together with cyclophosphamide has improved the TRM and DFS. Deeg et al6 initially allografted 50 MDS patients (13 RA, 19 RAEB, 16 RAEB-t/AML, 2 CMML) with a median age of 58.8 years (range, 55.3–66.2 years) using 5 “standard intensity” conditioning regimens. A targeted-dose busulfan (to plasma levels of 600–900 ng/mL) protocol was used in 1 cohort of 16 patients, which produced a significantly higher relapse-free and overall survival compared with the other 4 protocols. An extension of this study included 109 patients (69 RA/RARS, 24 RAEB, 10 RAEB-t/AML, 6 others) with a median age of 46 years (range, 6–66 years). All were conditioned with cyclophosphamide and targeted-dose busulfan. The 100-day TRM was 12–13%. At 3 years, the estimated TRM was 28–30% and DFS 56–59%. The outcome correlated inversely with the IPSS and cytogenetic risk scores and treatment-related MDS did poorly.15
Other novel transplant conditioning regimens are in evolution. Within the last 4–5 years, it has been demonstrated that reduced-intensity or “nonmyeloablative” conditioning can result in stable donor hemopoietic engraftment, without the toxicity associated with conventional HSCT.16,17 Disease eradication and control is afforded by the graft-versus-malignancy (GVM) effect and to a lesser extent by transplant conditioning. Reduced-intensity conditioning regimens which avoid toxic myeloablative chemotherapy and which rely upon adoptive immunotherapy are extremely attractive, especially in the setting of indolent disease in a minimal residual disease state where the GVM effect has an opportunity to develop.
These regimens all induce profound immunosuppression in order to permit donor engraftment and donor-recipient tolerance. Donor-recipient chimera are created, which eventually shift toward full donor hematopoiesis spontaneously, or with escalating doses of donor T cells.17 The essential element of these protocols involves intensive immunosuppression with combinations of low-dose total body irradiation (TBI), Fludarabine, antithymocyte globulin (ATG) and/or alemtuzumab (Campath-1H), followed by infusion of unmanipulated peripheral blood stem cells or bone marrow. Postallograft, only short-term immunosuppression with cyclosporine, tacrolimus, with or without mycophenolate mofetil is delivered.
In some protocols, however, the initial incidence of severe (Grade III–IV) acute graft-versus-host disease (aGVHD) was significant at up to 38%–60%.17–,21 Alemtuzumab (Campath-1H) is a monoclonal anti-CD52 antibody. Its use as an in vivo T-cell depletion agent has dramatically reduced the incidence of severe aGVHD22–,25 in reduced intensity protocols. The long half-life of alemtuzumab also results in the depletion of donor CD52 positive cells including circulating antigen-presenting dendritic cells26–,28 following infusion into the recipient. Although the incidence and morbidity associated with GVHD may be significantly reduced, the disadvantage of in vivo purging with alemtuzumab may be a clinically significant reduction in the GVM effect due to prolonged immunosuppression associated with an increased risk of disease relapse.29,30
The data on reduced-intensity allografts for MDS are still relatively immature, with relatively short follow-ups and with a multitude of differing conditioning regimens used by different investigators. Although RIC has been demonstrated to be safe and feasible as an alternative to standard conditioning, earlier publications have included a heterogeneity of disease states that often included lymphoid and other nonmyeloid conditions, making it impossible to focus on the follow-up and outcome of MDS patients in these series. A summary of these studies is shown in Table 2 .
One of the few RIC reports including patients with only myeloid malignancies included 37 patients, 17 with AML and 20 with MDS. Conditioning was made up of fludarabine (150 mg/m2) and busulfan (10 mg/kg), with cyclosporine and short-course methotrexate as GVHD prophylaxis. With an advanced median age of 57 years (range, 22–66 years) and a median follow-up of 297 days, the estimated TRM at 1 year was 5% and progression-free survival 66%. Most important, but not surprising, the estimated DFS in patients with GVHD was 58% compared with 13% for those without GVHD.36
Conditioning with fludarabine (150 mg/m2), busulfan (8 mg/kg), and Campath-1G or alemtuzumab (Campath-1H) (100 mg) was used in a series of 23 MDS patients with a median age of 48 years. The estimated TRM at 2 years was 31% with death predominantly due to opportunistic infections. The 2-year DFS was 39% and OS 48%. A direct comparison with a historical group of MDS patients who received “standard” conditioning was made, with the proviso that the 2 groups of patients are fundamentally different medically, especially with respect to age and the presence of comorbid conditions. Reduced-intensity conditioned patients had significantly reduced duration of aplasia, less mucositis, neutropenic fever, antibiotic, analgesia, TPN use, acute and chronic GVHD, and lower early TRM compared with the standard conditioning group, which had a TRM of 50%, and a DFS and OS of 44%.34
Our current experience with RIC allografts (HLA-matched siblings and VUD) in MDS and AML with trilineage dysplasia using fludarabine, busulfan, and alemtuzumab (Campath-1H) now extends to 75 patients with a median follow-up of 358 days (range, 32–1495 days). The actuarial OS, DFS, and NRM at 1 year were 69%, 60%, and 18% respectively for all disease groups. Patients in the IPSS-Int-1 group had a DFS at 1 year of 83%; IPSS-H, 31%; and TLD-AML, 56% (unpublished data).
While it has been generally accepted that patients with acute myeloid leukemia (AML) with or without multilineage dysplasia receive induction chemotherapy prior to transplant conditioning, there has been little consensus and opinions vary in patients with MDS. The role of “debulking” prior to transplant conditioning has not been well defined with some studies showing no statistical difference between the outcomes of patients who received induction chemotherapy and those who underwent allografting directly,37,38 but with other studies demonstrating that the absence of complete remission at transplant was associated with a poor outcome.38,39
Although RIC allografts compare extremely favorably early posttransplant, late events impact upon the TRM and DFS such that the OS, TRM, and DFS survival curves do not begin to plateau until after day 200,40 with a suggestion that the curves may merge after 3 to 4 years. The majority of these events in the RIC setting appear to be infective in origin, reflecting intense and prolonged immunosuppression. In contrast, the early events in standard conditioning are related to conditioning-induced toxicity—veno-occlusive disease, sepsis, and aGVHD.
The omission of high-dose myeloablative chemoradiotherapy as conditioning markedly reduces the early morbidity hitherto ubiquitously associated with allogeneic transplantation. Reduced-intensity procedures are therefore more tolerable and are safely applicable to patients who have been previously ineligible for allogeneic HSCT because of age, preexisting infection, organ dysfunction, or other comorbidity. However, regardless of the intensity of conditioning, DFS following allografting still correlates strongly with the MDS subtype, disease duration, the IPSS scores, and cytogenetic abnormalities.7,34,41–,44 While with standard allograft conditioning the TRM has been demonstrated conclusively to be associated with age and HLA matching,7–,9,41–,49 the impact of these in RIC allografts is less clear, with some suggestion that HLA mismatches are much better tolerated.22,39,50
While RIC regimens are associated with a different spectrum of posttransplant complications, the data at present confirm that this approach can safely permit donor engraftment and eradication of recipient hemopoiesis with an intermediate-term outcome that is not inferior to conditioning regimens of standard intensity. Longer follow-up and further accrual into RIC HSCT studies for MDS is warranted to determine if it will result in improved disease- and event-free survivals particularly for the low- and intermediate-risk MDS patients with long untreated median survivals and low risk of disease evolution. We eagerly await the maturation of data from the many studies of reduced-intensity conditioning allografts.
Chimerism Following Reduced-Intensity Allografts
Chimerism studies and the kinetics of donor engraftment are fundamental to our understanding of the mechanisms for the eradication of recipient hemopoiesis and immune competent cells and the engraftment and proliferation of donor hemopoiesis and recovery of immune function including the development of GVHD and the invaluable GVL effects. The outcome of the allograft is entirely dependent upon donor engraftment and the appropriate balance between donor and recipient chimerism as well as recovery of the immunological functions associated with transplantation.51– 55
Various methodologies have been used to assess quantitatively the relative contributions of donor and recipient hemopoiesis postallografting, including fluorescent analysis of variable number tandem repeat (VNTR), short tandem repeat (STR) sequences, and single nucleotide polymorphisms (SNPs)56–,58 with varying sensitivities, which appear to be in the order of approximately 5%. A recent technique using real-time quantitative PCR quotes a reproducible sensitivity of 1:1000 cells.59 However, the use of STRs or VNTRs is currently the standard method applicable to most patients.
Increasingly sophisticated cell separation techniques have allowed for detailed assessments of chimerism within specific cell lines. Particular interest has focused upon peripheral blood derived T cells, NK cells, myeloid cells, and dendritic cells (especially DC2). In its simplest form, the appearance of increasing recipient chimerism within the pathological cell lines involved, e.g., increasing recipient CD34+ cells in AML predicted for relapse.30,60– 62
The kinetics of engraftment within each cell lineage, however, appears to depend upon the conditioning regimen used, the source of hematopoietic stem cells, and posttransplant immunosuppression.32,63–,66 High NK cell numbers in the graft are associated with high donor T-cell chimerism.67
Studies have demonstrated that DC2 chimerism after HSCT affects the development of GVHD, with DCs in patients developing cGVHD being exclusively of donor origin.68,69
The persistence of a large proportion of recipient T cells is predictive of poor engraftment61 and loss of donor T cells has been associated with subsequent loss of the graft.70 Full T-cell engraftment appears to precede myeloid engraftment, the development of GVHD, and the GVL effect.32 However, full donor T and NK engraftment may be associated with a GVL effect in the absence of GVHD.71 T-cell engraftment, however, is not necessarily synonymous with development of T-cell function.
In T-cell depleted standard myeloablative allografts for myeloma, TCR Vβ repertoire complexity was demonstrated to improved more rapidly following donor lymphocyte infusion (DLI). DLI was also associated with increased numbers of TCR rearrangement excision circles (TRECs) in CD3+ T cells and with conversion to complete donor hemopoiesis.72 This observation is not universal, with potential interactions between several peritransplant variables such as conditioning regimens, type of T-cell depletion, loss of thymic function in adults, exposure to infectious agents, GVHD, and immunosuppressive treatment contributing to potential delays in the recovery of the T-cell repertoire.73
The kinetics of engraftment following alemtuzumab (Campath-1H)-containing conditioning regimens appears to differ from other T-cell depleted allografts possibly because of its anti-DC effect.26,27 We have noted 4 possible temporal patterns of peripheral blood myeloid (CD15+) and T-cell chimerism (CD3+):
initial full donor chimerism in both lineages at day 28, and continued stable full donor chimerism.
initial full donor engraftment but loss of T-cell engraftment followed by loss of myeloid engraftment over 2 to 3 months in the absence of cytogenetic or morphological features of relapse.
initial full myeloid engraftment, but incomplete T-cell engraftment followed by loss of T cells and myeloid engraftment over 2–3 months, in the absence of cytogenetic or morphological features of relapse.
initial incomplete engraftment followed by full T-cell and myeloid engraftment with withdrawal of immunosuppression (unpublished data).
This differs from our experience with standard myeloablative conditioning in which T-cell and myeloid engraftment are completely donor by day 28, and remain stable in the absence of relapse.
Chimerism assessments, especially cell lineage-specific chimerism, have developed into an important research and clinical tool, assisting in the understanding of the kinetics of engraftment in the posttransplant setting, guiding the withdrawal of posttransplant immunosuppression and the appropriate timely use of DLI in order to achieve full donor chimerism, stimulate the GVL effect, and ultimately prevent relapse. There is, however, room for significant advances, and the current recommendations from the recent Tandem workshop of the IBMTR and ASBMT should be helpful in achieving these aims.74
In summary, the recommendations are that chimerism analysis be performed with sensitive and informative methodologies; that peripheral blood cells may be more useful than bone marrow; that lineage-specific chimerism should be the assay of choice in reduced-intensity conditioning allografts; that chimerism analysis should be performed at 1, 3, 6, and 12 months in T-cell depleted, reduced-intensity conditioned, or in regimens incorporating novel GVHD prophylaxis regimens as DLI may depend on chimerism status. In reduced-intensity transplantation, the early patterns of chimerism may predict either GVHD or graft loss and more frequent (every 2–4 weeks) peripheral blood chimerism may be indicated.74
Immunomodulatory Agents—ATG and Cyclosporine
While MDS is a distinct pathophysiological entity from aplastic anemia, hypoplastic MDS which is characterized by cytopenias, bone marrow dysplasia, and marrow hypocellularity, has been difficult to distinguish from aplastic anemia.75–,80 The pathophysiology of the cytopenia associated with marrow failure in the 2 conditions, specifically the T-cell mediated immune suppression of hemopoiesis, may be similar or even identical.81– 83 The immunologically mediated marrow abnormalities of MDS, however, extend beyond “hypoplastic MDS” into refractory anemia and some cases of RAEB.
Evidence of immune dysfunction in MDS include abnormal CD4:CD8 ratios, increased activated cytotoxic T-cells as evidenced by a higher percentage of CD8+CD28– and CD8+CD28–CD57+ cells in AA and MDS patients compared with controls and skewing of the T-cell receptor Vβ complimentarity-determining region 3 (CDR3) patterns which would be consistent with T-cell oligoclonal expansion.84– 89 Immunotherapeutic agents that inhibit these immune mechanisms play an important role in the management of the immune-mediated marrow failure syndromes in MDS.
Antithymocyte globulin (ATG) derived from the immunization of horses or rabbits with human thoracic duct lymphocytes suppresses T lymphocytes.90 It has been demonstrated to produce clinically meaningful responses in patients with aplastic anemia and MDS. Approximately 34–44% of unselected patients with RA or RAEB can be expected to become transfusion independent within 8 months of a single 4-day course of ATG 40 mg/kg/day. A higher response rate in patients with RA compared with patients with RAEB (64% vs 33%) was observed. In 81% of these, transfusion independence was maintained for a median of 36 months (range, 3–72 months). Of patients with severe thrombocytopenia, 47.5% responded with sustained platelet count improvements and 55% of patients with severe neutropenia had sustained neutrophil counts of > 1 × 109/L. Responses were associated with a statistically significant survival advantage.78,80 A later study of similar size but in patients with less than 10% blasts demonstrated similar results with 50% achieving transfusion independence and a median duration of response of 15.5 months (range, 2–42 months). Of RA patients, 62% responded.88
Nonclonal hemopoiesis, as demonstrated by a nonclonal X-chromosomal inactivation pattern inferring the absence of a predominant dysplastic/malignant clone, was associated with a favorable response to ALG/ATG in 4 female patients with low-risk MDS. Three of the 4 had a demonstrable nonclonal XCIP which did not change significantly after treatment with ATG/ALG.92
Analysis of T-cell repertoires of patients with MDS before and after ATG by T-cell receptor V-β (TCR-Vβ) spectratype analysis has demonstrated significantly skewed spectratypes, representative of clonal or oligoclonal T-cell populations between MDS patients and healthy controls. Responders to ATG lost prominent skewed peaks on their spectratypes which suggests a loss of or reduction in overrepresented clonal T-cell populations, which is also associated with a loss of inhibition of colony-forming units granulocyte-macrophage (CFU-GM).77,93
The presence of a paroxysmal nocturnal hemoglobinuria (PNH) phenotype, i.e., CD55 and CD59 negative granulocytes and erythrocytes, is of pathogenetic and prognostic importance in MDS. A significant increase of PNH-type cells has been detected in 17.6% of patients with RA. These patients may exhibit distinct clinical features, such as “less-pronounced” red cell dysplasia, more severe thrombocytopenia, a lower incidence of clonal cytogenetic abnormalities (4.8% vs 32.8%) and a lower incidence of progression to acute leukemia (0% vs 6.2%). They also demonstrated a higher probability of responding to cyclosporine therapy (77.8% vs 0%) and have a higher prevalence of the HLA-DR15 allele (90.5% vs 18.5%).94 The presence of a PNH clone has been independently demonstrated to be highly predictive of hematologic improvement following the administration of anti-thymocyte globulin.95
HLA-DR2 and HLA-DR15 frequencies have been shown to be higher in patients with both RA and aplastic anemia.96,97 Both appear to be associated with clinically significant positive responses to ATG or cyclosporine. Patients positive for HLA-DR15 were 8.53 times more likely to respond, with 68% of HLA-DR15 positive and 59.6% of HLA-DR15 negative patients responding to a combination of ATG and cyclosporine. Among the DR15-positive patients, responders had a higher frequency of a PNH clone and were more likely to be younger than 60 years of age. DR15 positive responders were more likely to have a marrow cellularity of < 30%. Other pretreatment variables of statistical significance include a shorter duration of red cell transfusion dependence and a younger age.98
The administration of cyclosporine alone in patients with hypoplastic myelodysplastic syndrome has resulted in prolonged partial hematological improvements.99,100 In vitro cyclosporine significantly decreased the numbers of interferon-γ expressing CD4 cells, but not Fas-ligand production, with 8 of 11 patients with hypoplastic MDS responding.101
The significant toxicity profile of ATG, however, mandates that potential patients be carefully selected to maximize the probability of response102 and appropriately supported to minimize the morbidity associated with the administration of ATG, particularly that of severe thrombocytopenia, immunosuppression, the risk of opportunistic infections, and serum sickness syndromes. At present, the evidence appears to support its use in refractory anemia patients less than 60 years of age who are HLA-DR15 positive with a bone marrow cellularity of < 30%, a detectable PNH cone, and who do not have evidence of clonal hemopoiesis by conventional cytogenetics or clonality studies.
Targeting Tumor Necrosis Factor
TNFα is an immunomodulatory cytokine that potentiates the effects of gamma-interferon (γ-IFN) and induces Fas and γ-IFN receptor expression in hemopoietic cells.103,104 TNFα has potent inhibitory activity towards hematopoiesis. TNFα levels have been demonstrated to be elevated in patients with MDS and have been shown to play a major role in the apoptosis of hemopoietic cells in MDS.105– 111 The inhibition of TNFα therefore appears to be a legitimate target for directed therapy. At present, there are 2 anti-TNFα agents available for clinical use, with limited data available in MDS.
Etanercept is a soluble recombinant TNF-receptor fusion protein which binds and eliminates biologically active TNFα in vivo. While it has excellent clinical activity in rheumatoid arthritis,112 its clinical activity in MDS appears to be low with little demonstrable correlation of efficacy with pretherapy TNFα levels.113– 115
Infliximab is an IgG1κ chimeric anti-TNFα monoclonal antibody composed of human constant and murine variable regions that bind specifically to TNFα. It has impressive activity in the inflammatory arthropathies.116,117 In MDS, however, it has only been evaluated in 2 patients with low- and intermediate-risk MDS who had elevated TNFα levels and a high apoptotic index. Infliximab therapy resulted in a major and a minor erythroid response, with a remarkable decrease in the percentage of apoptotic marrow stem cells.115 A series of further studies is currently under way and the results of these are eagerly awaited.
Novel therapeutics categorized by pharmacologic class and target of interaction.
| Mechanism Modulated (Pharmacologic Class) . | Agent . |
|---|---|
| Survival Signals | |
| Antiangiogenic | thalidomide (Thalomid™) CC5013 (Revimid™), CC1088 bevacizumab (Avastin™) arsenic trioxide (Trisenox™) |
| Receptor tyrosine kinase inhibitors | SU5416, SU11248 PTK787 AG13736 imatinib mesylate (Gleevec™) |
| Protein kinase C inhibitor | PKC412 |
| Matrix metalloprotease inhibitor | AG3340 (Prinomastat™) |
| Farnesyl transferase Inhibitors | R115777 (Zarnestra™) SCH66336 (lonafarnib; Sarasar™) |
| Genetic Integrity | |
| Immunoconjugate | gemtuzumab ozogamicin (Mylotarg™) |
| P-glycoprotein antagonist | cyclosporine-A quinine sulfate LY335979 (zosuquidar trihydrochloride) |
| Mechanism Modulated (Pharmacologic Class) . | Agent . |
|---|---|
| Survival Signals | |
| Antiangiogenic | thalidomide (Thalomid™) CC5013 (Revimid™), CC1088 bevacizumab (Avastin™) arsenic trioxide (Trisenox™) |
| Receptor tyrosine kinase inhibitors | SU5416, SU11248 PTK787 AG13736 imatinib mesylate (Gleevec™) |
| Protein kinase C inhibitor | PKC412 |
| Matrix metalloprotease inhibitor | AG3340 (Prinomastat™) |
| Farnesyl transferase Inhibitors | R115777 (Zarnestra™) SCH66336 (lonafarnib; Sarasar™) |
| Genetic Integrity | |
| Immunoconjugate | gemtuzumab ozogamicin (Mylotarg™) |
| P-glycoprotein antagonist | cyclosporine-A quinine sulfate LY335979 (zosuquidar trihydrochloride) |
Tyrosine kinase fusion genes in chronic myelomonocytic leukemia (CMML) with eosinophilia.
| Fusion Gene . | Translocation . |
|---|---|
| ETV6 (TEL)-PDGFRB | t(5;12)(q33;p13) |
| HIP1-PDGFRB | t(5;7)(q33;q11) |
| H4-PDGFRB | t(5;10)(q33;q21) |
| RAB5-PDGFRB | t(5;17)(q33;p13) |
| Fusion Gene . | Translocation . |
|---|---|
| ETV6 (TEL)-PDGFRB | t(5;12)(q33;p13) |
| HIP1-PDGFRB | t(5;7)(q33;q11) |
| H4-PDGFRB | t(5;10)(q33;q21) |
| RAB5-PDGFRB | t(5;17)(q33;p13) |
Summary of selected reduced-intensity allograft studies that include patients with myelodysplastic syndrome (MDS).*
| Author (Study) # Patients . | Disease . | Median Age . | Conditioning & Stem Cell Source . | Median Follow-Up . | GVHD . | NRM . | DFS . | OS . | Comments . |
|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: aGVHD, acute graft-versus-host disease (grade in parentheses); cGVHD, chronic GVHD; ecGVHD, extensive cGVHD; ATG, antithymocyte globulin; NRM, nonrelapse mortality; CDA, 2-chloro-deoxyadenosine; DFS, disease-free survival; OS, overall survival, MTX, methotrexate | |||||||||
| * Number of patients in each diagnostic group is in parentheses | |||||||||
| Giralt (1997)31 15 patients | AML (13); MDS (2) | 59 years (27–71) | fludarabine 120 mg/m2, idarubicin 12 mg/m2, cytarabine 8 g/m2 or melphalan 140 mg/m2; OR cda 60mg/m2, cytarabine 5g/m2 [HLA identical related donor, or 1Ag mismatch] | 100 days (34–175) (survivors) | aGVHD (I) 7%; aGVHD (II) 14%; cGVHD 0% | 33% at reporting | — | — | Median survival 78 days (0–175) |
| Slavin (1998)17 26 patients | MDS (1); AML (8) | 34 years (1–61) | fludarabine 180 mg/m2, busulphan 8 mg/kg, ATG 80 mg/kg; cyclosporine [HLA-matched siblings] | 8 months | aGVHD (III–IV) 25% | 15% (8 months) | 80.7% (8 months) | 85% (8 months) | 2 AML died of GVHD |
| Childs (1999)32 15 patients | RAEB-t (2); CMML (1); non-MDS (12) | 50 years (23–68) | fludarabine 125 mg/m2, cyclophosplamide 120 mg/kg, cyclosporine; [HLA-matched siblings] | 200 days (121–409) (survivors) | aGVHD (II–IV) 60%; cGVHD 27% | 14% (at median follow-up) | 40% (at median follow-up) | 53% (at median follow-up) | 1 MDS patient in CR at median follow-up |
| McSweeney (2001)33 45 patients | RAEB-t (1); AML (10) | 56 years (31–72) | TBI 200 cGy; cyclosporine; mycophenolate [HLA-matched siblings] | 417 days (310–759) (survivors) | aGVHD (II–III) 47% | 6.7% (at median follow-up) | 53% (at median follow-up) | 66.7% (at median follow-up) | RAEB-t progressive disease; 4:10 AML in CR |
| Martino (2001)1976 patients | MDS (12) | 53 years (18–66) | fludarabine 150 mg/m2; melphalan 140 mg/m2 or busulphan 10 mg/kg; cyclosporine, short course MTX [HLA-matched siblings] | 287 days for MDS | aGVHD (II–IV) 32% (100day); ecGvHD 43% (1yr) | 1:12 MDS patients at reporting | 6:12 MDSpatients atreporting | 11:12 MDS patients at reporting | |
| Corradini (2002)18 45 patients | AML (5); RAEB-t (6); | 49 years (20–68) | thiotepa 20 mg/kg; cyclophosphamide 60 mg/kg; fludarabine 60 mg/m2; cyclosporine; [HLA-matched siblingsor 1Ag mismatched related] | 385 days (24–820) | aGVHD (II–IV) 47%; aGVHD (III–IV) 13%; | 13% | — | 53% | 3 AML and 2 RAEB-t in CR at reporting |
| Parker (2002)34 23 patients | RA (6); RAEB (6); RAEB-t (!); MDS-AML (6); t-MDS (4) | 48 years (25–63) | fludarabine 150 mg/m2, busulfan 8 mg/kg, CAMPATH 100 mg, cyclosporine; [7 HLA matched sibling; 16 VUD] | 10 months (4–24) | aGVHD (II–IV) 17% cGVHD 15% | 31% (2 years) | 39% (2 years) | 48% (2 years) | |
| Feinstein (2003)35 18 patients | de novo AML (13); 2oAML (5) | 59 years (36–73) | TBI 2 Gy; OR TBI 2Gy, fludarabine 90 mg/m2; cyclosporine, mycophenolate [HLA-matched siblings] | 766 days (188–1141) (survivors) | aGVHD (II–IV) 44% | 0% (D+100); 17% (1 year) | 42% (1 year) | 54% (1 year) | 2 rejections in TBI-only group |
| Author (Study) # Patients . | Disease . | Median Age . | Conditioning & Stem Cell Source . | Median Follow-Up . | GVHD . | NRM . | DFS . | OS . | Comments . |
|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: aGVHD, acute graft-versus-host disease (grade in parentheses); cGVHD, chronic GVHD; ecGVHD, extensive cGVHD; ATG, antithymocyte globulin; NRM, nonrelapse mortality; CDA, 2-chloro-deoxyadenosine; DFS, disease-free survival; OS, overall survival, MTX, methotrexate | |||||||||
| * Number of patients in each diagnostic group is in parentheses | |||||||||
| Giralt (1997)31 15 patients | AML (13); MDS (2) | 59 years (27–71) | fludarabine 120 mg/m2, idarubicin 12 mg/m2, cytarabine 8 g/m2 or melphalan 140 mg/m2; OR cda 60mg/m2, cytarabine 5g/m2 [HLA identical related donor, or 1Ag mismatch] | 100 days (34–175) (survivors) | aGVHD (I) 7%; aGVHD (II) 14%; cGVHD 0% | 33% at reporting | — | — | Median survival 78 days (0–175) |
| Slavin (1998)17 26 patients | MDS (1); AML (8) | 34 years (1–61) | fludarabine 180 mg/m2, busulphan 8 mg/kg, ATG 80 mg/kg; cyclosporine [HLA-matched siblings] | 8 months | aGVHD (III–IV) 25% | 15% (8 months) | 80.7% (8 months) | 85% (8 months) | 2 AML died of GVHD |
| Childs (1999)32 15 patients | RAEB-t (2); CMML (1); non-MDS (12) | 50 years (23–68) | fludarabine 125 mg/m2, cyclophosplamide 120 mg/kg, cyclosporine; [HLA-matched siblings] | 200 days (121–409) (survivors) | aGVHD (II–IV) 60%; cGVHD 27% | 14% (at median follow-up) | 40% (at median follow-up) | 53% (at median follow-up) | 1 MDS patient in CR at median follow-up |
| McSweeney (2001)33 45 patients | RAEB-t (1); AML (10) | 56 years (31–72) | TBI 200 cGy; cyclosporine; mycophenolate [HLA-matched siblings] | 417 days (310–759) (survivors) | aGVHD (II–III) 47% | 6.7% (at median follow-up) | 53% (at median follow-up) | 66.7% (at median follow-up) | RAEB-t progressive disease; 4:10 AML in CR |
| Martino (2001)1976 patients | MDS (12) | 53 years (18–66) | fludarabine 150 mg/m2; melphalan 140 mg/m2 or busulphan 10 mg/kg; cyclosporine, short course MTX [HLA-matched siblings] | 287 days for MDS | aGVHD (II–IV) 32% (100day); ecGvHD 43% (1yr) | 1:12 MDS patients at reporting | 6:12 MDSpatients atreporting | 11:12 MDS patients at reporting | |
| Corradini (2002)18 45 patients | AML (5); RAEB-t (6); | 49 years (20–68) | thiotepa 20 mg/kg; cyclophosphamide 60 mg/kg; fludarabine 60 mg/m2; cyclosporine; [HLA-matched siblingsor 1Ag mismatched related] | 385 days (24–820) | aGVHD (II–IV) 47%; aGVHD (III–IV) 13%; | 13% | — | 53% | 3 AML and 2 RAEB-t in CR at reporting |
| Parker (2002)34 23 patients | RA (6); RAEB (6); RAEB-t (!); MDS-AML (6); t-MDS (4) | 48 years (25–63) | fludarabine 150 mg/m2, busulfan 8 mg/kg, CAMPATH 100 mg, cyclosporine; [7 HLA matched sibling; 16 VUD] | 10 months (4–24) | aGVHD (II–IV) 17% cGVHD 15% | 31% (2 years) | 39% (2 years) | 48% (2 years) | |
| Feinstein (2003)35 18 patients | de novo AML (13); 2oAML (5) | 59 years (36–73) | TBI 2 Gy; OR TBI 2Gy, fludarabine 90 mg/m2; cyclosporine, mycophenolate [HLA-matched siblings] | 766 days (188–1141) (survivors) | aGVHD (II–IV) 44% | 0% (D+100); 17% (1 year) | 42% (1 year) | 54% (1 year) | 2 rejections in TBI-only group |
Chemical structure of CC5013 and thalidomide.
CC5013 differs from its parent compound by the removal of a ketone and addition of an amino group to the glutamate aromatic ring.
Chemical structure of CC5013 and thalidomide.
CC5013 differs from its parent compound by the removal of a ketone and addition of an amino group to the glutamate aromatic ring.
Ras activation cycle.
Trophic signals activate guanine exchange factors (GEFs) such as SOS and CDC25 to accelerate the rate of guanosine diphosphate (GDP) dissociation, stabilize the Ras protein in its nucleotide free state, and facilitate guanosine triphosphate (GTP) binding. GTPase activating proteins (GAP) accelerate GTP hydrolysis thereby inactivating Ras. Sustained Ras activation may occur in mutant Ras proteins in which GTPase response to GAP binding is inactivated, or by mutational inactivation of GAPs.
Ras activation cycle.
Trophic signals activate guanine exchange factors (GEFs) such as SOS and CDC25 to accelerate the rate of guanosine diphosphate (GDP) dissociation, stabilize the Ras protein in its nucleotide free state, and facilitate guanosine triphosphate (GTP) binding. GTPase activating proteins (GAP) accelerate GTP hydrolysis thereby inactivating Ras. Sustained Ras activation may occur in mutant Ras proteins in which GTPase response to GAP binding is inactivated, or by mutational inactivation of GAPs.
Chemical Structure of the farnesyl protein transferase (FPT) inhibitors R115777 (tipifarnib) and SCH66336 (lonafarnib).
Chemical Structure of the farnesyl protein transferase (FPT) inhibitors R115777 (tipifarnib) and SCH66336 (lonafarnib).
Impact of chromatin remodeling on gene transcription.
Methylated cytosine residues in CpG dinucleotides in promoter regions of genes recruit transcriptional repression complexes including histone deacetylases. The deacetylated lysine tails of the histones interact tightly with DNA, rendering the chromatin transcriptionally inactive.
Impact of chromatin remodeling on gene transcription.
Methylated cytosine residues in CpG dinucleotides in promoter regions of genes recruit transcriptional repression complexes including histone deacetylases. The deacetylated lysine tails of the histones interact tightly with DNA, rendering the chromatin transcriptionally inactive.