Abstract
Major advances have occurred in our understanding of the biology, immunology, and opportunities for treatment of chronic lymphocytic leukemia (CLL) in recent times. Surface antigen analysis has helped us define classical CLL and differentiate it from variants such as marginal zone leukemia, mantle cell leukemia, and prolymphocytic leukemia. An important observation has been that the B-cells in indolent types of CLL, which do not require therapy, have undergone somatic hypermutation and function as memory B-lymphocytes whereas those more likely to progress have not undergone this process.
Section I by Dr. Nicholas Chiorazzi encompasses emerging elements of the new biology of CLL and will address the types of somatic hypermutation that occur in CLL cells and their correlation with other parameters such as telomere length and ZAP70 status. In addition he addresses the concept of which cells are proliferating in CLL and how we can quantitate the proliferative thrust using novel methods. The interaction between these parameters is also explored.
Section II by Dr. Thomas Kipps focuses on immune biology and immunotherapy of CLL and discusses new animal models in CLL, which can be exploited to increase understanding of the disease and create new opportunities for testing the interaction of the CLL cells with a variety of elements of the immune system. It is obvious that immunotherapy is emerging as a major therapeutic modality in chronic lymphocytic leukemia. Dr. Kipps addresses the present understanding of the immune status of CLL and the role of passive immunotherapy with monoclonal antibodies such as rituximab, alemtuzumab, and emerging new antibodies. In addition the interaction between the CLL cells and the immune system, which has been exploited in gene therapy with transfection of CLL cells by CD40 ligand, is discussed.
In Section III, Dr. Michael Keating examines the question “Do we have the tools to cure CLL?” and focuses on the fact that we now have three distinct modalities, which are able to achieve high quality remissions with polymerase chain reaction (PCR) negativity for the immunoglobulin heavy chain in CLL. These modalities include initial chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab, the use of alemtuzumab for marrow cytoreduction in minimal residual disease and allogeneic bone marrow transplants. The emergence of non-ablative marrow transplants in CLL has led to the broadening of the range of opportunities to treat older patients. The addition of rituximab to the chemotherapy preparative regimens appears to be a significant advance.
The combination of our increased understanding of the biology, immune status, and therapy of CLL provides for the first time the opportunity for curative strategies.
I. Unraveling the Biology of Chronic Lymphocytic Leukemia
Nicholas Chiorazzi, MD,* Bradley Messmer, PhD, Rajendra N. Damle, PhD, Steven L. Allen, MD, Kanti R. Rai,MB,BS, and Manlio Ferrarini, MD
North Shore – LIJ Research Institute, 350 Community Drive, Manhasset, NY 11030 and Departments of Medicine, North Shore University Hospital, and NYU School of Medicine, Manhasset and New York, NY
In recent years, our view of the biological features classically ascribed to the leukemic B cells of chronic lymphocytic leukemia (B-CLL) has changed. B-CLL cells were once assumed to derive from immature immunologically incompetent B lymphocytes and to behave as inert cells that divided minimally, died rarely, and thereby passively accumulated to numbers that eventually compromised the patient.1 It now appears that B-CLL cells derive from mature, antigenically experienced, immunologically competent B lymphocytes and that the leukemic cells from most patients turn over at definable rates suggesting that a genetic abnormality in apoptosis that cannot be overridden by an exogenous signal may not exist. These findings impute that B-CLL is a dynamic, not passive, accumulative disease.2
This Section reviews recently defined and currently unfolding aspects of the biology of B-CLL cells and then proposes a model describing the path a normal B cell could traverse to become a leukemic cell of either increasing or stable degrees of aggressivity. Key concepts underlying this model include roles for (1) antigen selection of precursor B cells, (2) maturational responses to antigen by the precursor clones, (3) a slow but progressive development and selection for genetic lesions that continues after the leukemic state is established, and (4) an appreciable and occasionally sizeable level of leukemic cell turnover.
B-CLL Cells Derive From Mature Immunocompetent B Lymphocytes
The following sections discuss information, derived from various groups, indicating that B-CLL cells evolve from immunologically competent B lymphocytes and that B-CLL cells themselves retain certain key immune functions that affect their in vivo biology and the clinical course and outcome of individual patients.
Selection of B-CLL precursor cells by antigen
Multiple laboratories have analyzed the IgVH genes expressed by B-CLL cells in hopes of identifying a relationship between the structure of the B-cell receptor for antigen (BCR) and this disease. Such a relationship could support a role for antigen selection in disease development. It also might help identify causative antigens.
Antigen selection of specific B-lymphocyte clones can be inferred if the structure of the BCR of the B-cell clones that expand in a disease differs from the anticipated random display or the display observed in normal individuals. This skewing results from more effective binding of antigen by cells with a BCR of complementary structure thereby enabling cell triggering and clonal expansion.
If clonal expansion is induced randomly (Figure 1, left-hand panel; see Appendix, page 617), then all B-cell clones, irrespective of BCR structure and antigen-binding capacity, will expand to some degree. If, however, clonal expansion is limited to those B-cells that have BCRs complementary to a selecting antigen (Figure 1, right-hand panel; see Appendix, page 617), then only certain clones will amplify (clones B, D, E, and G). Other clones with inappropriately conformed BCRs will remain inactive because they will not receive stimulatory signals (clones A, C, and F).
The primary structure of the antigen-binding site of a B-lymphocyte’s BCR is determined by the DNA sequence of its variable (V) domains. These are comprised of recombined VH, D, and JH segments in the heavy (H) chains and VL and JL segments in the light (L) chains. Additional diversity is provided by the insertion or deletion of nucleotides at the joints of recombination.
If we assume that these recombination events are random (which may be an oversimplification), then the likelihood that a particular VH gene will be expressed in an individual B-lymphocyte is 1/44, since there are approximately 44 functional germline VH genes. Therefore, finding an individual VH gene in a B-cell population at a frequency significantly greater than 1/44 would suggest that a nonstochastic selective process led to the expansion of the B-cells using that particular VH gene in their BCRs. Similarly, a specific D segment would randomly occur in 1/25 B cells and a particular JH gene in 1/6 B-cells. Therefore, similar criteria for selection can be used when evaluating the use of these gene segments. Finally, the likelihood that specific VH, D, and JH genes would be used in the same VHDJH rearrangement is 1/6600 (1/44 × 1/25 × 1/6), for a specific VL and JL gene is 1/200 (1/40 × 1/5) or 1/124 (1/31 × 1/4) for a κ versus a λ rearrangement, respectively. Only 1/1,320,000 B-cells would be predicted to randomly express the same VH, D, JH, Vκ, and Jκ segments in its BCR.
The frequencies at which specific VH, D, and JH gene segments are used to construct the BCRs of B-CLL cells are different from what would be expected from random assortment and from what is found in normal peripheral blood CD5+ and CD5− B-lymphocytes.3 The VH genes most commonly used in B-CLL cells are 1-69, 3-07, and 4-34.
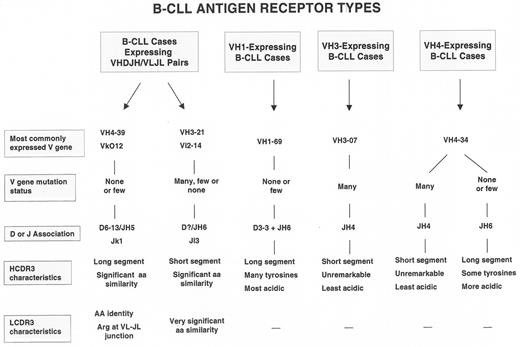
Furthermore, the processes that occur in normal B-lymphocytes at the time of VHDJH rearrangement lead to major differences in structural features of the third complementarity determining region (CDR3) of both the Ig H and L chains. These regions of the V domains are the most critical for binding the majority of antigens. The B-CLL cases that use the most common VH genes in their VHDJH rearrangements can also use specific D and JH segments and hence have characteristic HCDR3s.3,4 These VH gene and HCDR3 associations describe 3 prototypic B-CLL BCRs (Figure 2 ).
These BCR prototypes are built primarily around rearranged H chain features; however, in addition, remarkably similar BCR structures involving both the H and L chain V domains exist among subsets of cases (Figure 2 ). These latter cases represent the clearest examples of selection for specific BCR structures among B-CLL precursors. For example, a group of IgG+ B-CLL cases have an H chain V region comprised, in each instance, of VH 4-39/D6-13/JH5, and an L chain V region consisting of VLO12/Jκ1.5–,8 As mentioned above, the likelihood of these specific VH, D, JH, VL, and JL segments occurring in the same B-CLL cell by chance alone is 1 in 1.32 million. These rearranged gene segments are almost identical to the germline segments and therefore the amino acid sequences of the BCRs of each case are virtually identical. Furthermore, the LCDR3 of these cells, in every instance, display an arginine at the VLJL junction. Since this amino acid is created by diverse genetic mechanisms, a role for it in antigen binding and selection seems very likely. Another unique subset of cases expresses a VH-VL pair involving the VH3-21/JH6 and Vλ2-14/Jλ3 genes. In almost every instance, the HCDR3 segments of these cases are remarkably short and of very similar amino acid sequence.9,10 Recently, additional examples of B-CLL cases that use similar rearranged H and L chain V region genes were reported,11 suggesting that this phenomenon may be more frequent than previously appreciated.
Accumulation of Ig V gene mutations in precursors of B-CLL cells
Selection for B-cell clones that bind specific antigens and thereby respond and expand is a feature of competent immunocytes. If one accepts that specific B-cell clones cannot expand unless signaled by antigen through engagement of the BCR, then the studies discussed above indicate that the precursor cells that became leukemic were immunocompetent. The following section outlines information indicating that, after antigen binding and clonal expansion, some B-CLL precursor cells accumulate somatic mutations, another feature of competent B-lymphocytes.
The somatic hypermutation process requires BCR cross-linking by antigen, cellular activation, cooperation of T lymphocytes and other cells, and involvement in the germinal center reaction. It was originally presumed that B-CLL cells would lack V gene mutations because they express the CD5 antigen, a molecule that marks a murine B-cell subset that does not accumulate such mutations to an appreciable degree. It is now clear, however, that the Ig V genes of ~50% of randomly chosen IgM-expressing cases and ~75% of isotype-switched cases exhibit somatic mutations.3,5,12– 15 In general, these mutations fulfill the criteria for selection by antigen, i.e., there are more replacement mutations in the CDRs and less in the framework regions (FRs), which permits the development of a more specific antigen-binding site while maintaining the necessary supporting scaffold of the BCR.
One can divide B-CLL cases into 2 groups, mutated and unmutated, using an arbitrary limit for variation from germ line V genes (≥ 2% differences from germ line = mutated; < 2% difference = unmutated3). These 2 mutation-defined subgroups also differ in specific VH gene use and HCDR3 features (Figure 2 ) and have clinical relevance: the cases in the unmutated group have a much more aggressive course than those in the mutated group (Table 1 14,16).
B-CLL cells express an activated, antigen-experienced cell surface phenotype
The development of Ig V gene mutations requires a series of active cellular processes initiated by BCR cross-linking by antigen. Therefore, those B-CLL cases that exhibit V gene mutations must have come from immunologically competent B-cells. However, using this parameter as the sole definition of immune competence, B-CLL cells without V gene mutations could derive from immature, antigen naïve incompetent cells. Nevertheless, since the absence of V gene mutations does not necessarily indicate the absence of prior antigenic stimulation, these cases could derive from competent B cells that had been antigen stimulated but did not develop mutations. The following surface membrane phenotypic studies, using immunofluorescent microscopy and monoclonal antibodies to defined markers, suggest that antigen stimulation, and response to it, are prerequisites for the development of B-CLL, even in those cases that do not exhibit Ig V gene mutations.17
When compared with age-matched normal subjects, B-CLL cells overexpress activation markers (CD23, CD25, CD69, and CD71) and underexpress markers downregulated by cell activation (CD22, CD79b, and IgD). In addition, the leukemic cells uniformly express CD27, identifying them as B cells that were triggered and had entered the memory cell pool. Gene expression profiling studies support this interpretation.18,19 When the surface membrane phenotypes of B-CLL cases with and without Ig V gene mutations are compared, the 2 subgroups differ in the percentages of cells expressing some of these markers.17 Based on the reciprocal relationship of markers that indicate early versus late cellular activation, the unmutated cases resemble B cells that are temporally more proximal to an inductive stimulus than the mutated cases.
Telomere shortening as an indicator of prior cell cycling of B-CLL cells and their precursors
Telomeres shorten with each round of cell division. Therefore, telomere length measures the proliferative history of a cell. Telomeres of B-CLL cells are much shorter than those in B-cells of age-matched normal donors. Surprisingly, telomere lengths of unmutated B-CLL cases are much shorter than those of mutated cases,20,21 suggesting that unmutated leukemic cells have a more extensive history of cell division than mutated cases. These cases also have significantly higher telomerase activity which may compensate for the extremely short telomeres in these cells.20
Can B-CLL cells still respond to antigen stimulation?
Collectively, the studies discussed above provide compelling evidence that B-CLL cells emerge from a population of antigen-selected, mature, immunocompetent B-lymphocytes that have carried out typical functions of normal B-cells. However, these studies do not address directly whether or to what extent B-CLL cells themselves are immunocompetent. Data derived from in vivo and in vitro studies suggest that, for certain key immune parameters, B-CLL cells are competent or can be made to function competently.
For example, in vivo, some B-CLL cells spontaneously differentiate into plasma cells,22 undergo isotype class switching,23–,25 develop new Ig V gene mutations,26 and express27–,29 and upregulate or downregulate the activation-induced cytidine deaminase (AID)29 that is essential for the somatic mutation process.30 Many of these same phenomena can be induced by appropriate stimuli in vitro.23,26,29,31,32
In addition to these functions, B-CLL cells from certain cases maintain the ability to signal through the BCR.33,34 This ability is manifested by tyrosine phosphorylation of key signaling proteins, Ca++ mobilization, expression of genes reminiscent of BCR signaling,18 and the prevention or induction of apoptosis in vitro, following BCR cross-linking.33–,35 The latter findings are especially significant since they challenge the contention that B-CLL cells have an inherent defect in programmed cell death that cannot be altered by external signals. Other studies, demonstrating that cell-cell contact can inhibit spontaneous apoptosis in vitro, support the conclusion that an obligate apoptotic defect does not necessarily exist in B-CLL.36–,42 Interestingly, those cases that retain BCR signaling capacity can be distinguished from those that do not by the presence of CD38,33,43 or ZAP-7044 or the absence of Ig V gene mutations,34 all markers of poor prognosis, although these correlations are not absolute. This suggests that the ability of the B-CLL cell to retain signaling capacity in some way affects clonal survival and expansion and impacts negatively on a patient’s clinical course.
Oligoclonal B-cell expansions in normal aging individuals and family members of patients with B-CLL
As normal humans and mice age, their lymphocyte repertoires switch from a random distribution to a relatively restricted set of clones.45,46 This narrowing of the cellular repertoire can be ascribed to either antigen selection or a genetic diathesis independent of antigen selection or both. As discussed above, the easiest approach to identify the diversity of lymphocyte clones within an individual is to determine the distribution of the clonally restricted antigen receptors expressed within the cell population by DNA sequencing of the antigen receptor genes used by individual lymphocytes. A less precise, but technologically more convenient, method uses flow cytometry and monoclonal antibodies specific for individual antigen receptors or for characteristic subset specific surface markers.
Using such an immunofluorescence approach, Rawstron et al47 identified small collections of B-lymphocytes that bear characteristic cell surface markers of B-CLL cells (CD19+, 20+, CD5+, CD79blow) circulating in the blood of ~3% of randomly chosen normal individuals of 40 years of age or older. Although the total number of these cells in a given individual is quite small, the absence of these cells in the majority of normal subjects suggests that they represent a clonal amplification of a selected set of B-lymphocytes. The monotypic expression of L chain isotypes (κ or λ) and an apparent restriction in IgVH gene use among these cells support this concept. Of note, cells with this phenotype are twice as frequent in men as women and among subjects of ≥ 60 years of age.
Support for an inherited susceptibility to develop clonal expansions of this phenotype comes from studies of the circulating B-cells of healthy first-degree relatives of patients with B-CLL.48 The blood of these apparently normal individuals contains these populations at significantly increased frequency (median ~13.5% of family members) and in similar numbers to those found in randomly chosen normal subjects from families apparently without B-CLL. With few exceptions, the presence of these cells among individuals and the levels of these cells within individuals were relatively constant. These cells, whether derived from families with or without B-CLL, expressed little or no CD38, a cell surface phenotype of cases that frequently follow an indolent course.16
B-CLL Cells Progressively Develop Genetic Lesions Over Time
The concept that clonal evolution can lead to more dangerous clonal variants is well established in oncology. In B-CLL, this principle is manifest by the development of single or complex abnormal genetic karyotypes over time. These abnormalities are rarely evident at clinical presentation and therefore represent prime examples of clonal evolution. Although specific DNA segment gains do occur in B-CLL (e.g., partial or complete trisomy 12), the disease is primarily characterized by DNA deletions. Deletions at 13q14, 11q22-q23, 17p13, and 6q21 are the most common.49 The precise genes targeted by these deletions that lead to disease progression are not defined in several instances, although for 17p13 it appears to be p53 and for 11q22-q23 occasionally is ATM.
However, the relatively specific and restricted location of these more common chromosomal abnormalities may have caused more diverse, widespread genomic variability to go unnoticed. Genome-wide screening studies have found alterations of relatively small chromosomal regions spread throughout the leukemic cell genome.50–,52 These alterations consist of clonal monoallelic and biallelic losses as well as gains such as duplications, amplifications, and trisomies. Most interesting is that in several instances such losses or gains were seen at the subclonal level indicating the presence and possible emergence of clonal variants that differed at several loci.51 If B-CLL cells commonly generate such diverse genetic alterations, then a wide array of functional variants that might arise could be selected to avoid clonal restraint and death.
The mechanism(s) that lead to the chromosomal alterations classically associated with B-CLL and to the apparently more widespread genomic changes are unknown. However, two features of B-CLL cells should be remembered: shortened telomere length and AID expression. Significant shortening of telomeres can lead to telomere dysfunction which, in turn, can lead to genome instability.53 Although non-reciprocal chromosomal rearrangements usually result from telomere dysfunction, chromosomal losses and regional amplifications and deletions also occur with very short telomeres. The fact that poor outcome patients (i.e., those with unmutated Ig V genes and with large numbers of CD38+ cells) have uniformly short telomeres20,21 and are enriched for chromosomal aberrations13–,15,52,54 and p53 dysfunction,54 suggests a link between telomere dysfunction and widespread genomic abnormalities. In addition, the somatic hypermutation process appears to be ongoing in B-CLL cases.26 If this process is mistargeted, as occurs in certain lymphomas,55 then mutations or deletions could occur at various sites in the cellular DNA. Furthermore, the AID gene, which is used by normal B cells to create Ig V gene mutations and to delete DNA segments during isotype switching, can be expressed in B-CLL cells.27–,29 Because AID is expressed intermittently in only a small fraction of the B-CLL clone,29 new genetic defects developing through the action of this enzyme would be diverse in nature and would exist only among certain clonal members. However, it should be clear that at this juncture there are no firm data to implicate AID gene expression with ongoing SHM or genome instability.
Clinical Implications of These Findings
Some patients with B-CLL survive for decades after initial diagnosis and never require therapy; others succumb rapidly to the disease despite therapy. To deal with this heterogeneity in clinical course and outcome, Rai et al56 and subsequently Binet et al57 created staging systems based on clinical symptoms, physical signs, and laboratory values. These systems currently represent the gold standards for patient evaluation and treatment decisions. Some of the findings mentioned above have led to the recognition of subgroups of patients, at the laboratory level, that are proving to be valuable adjuncts to the Rai and Binet staging systems.
Ig V gene mutation status is currently the most accurate discriminator of clinical outcome in several retrospective studies.14–,16,54,58– 60 Patients with few mutations succumb to the disease at least 3 times faster than those with significant mutations (approximate median survivals < 8 years versus ≥ 24 years).
Two protein markers that are easier to measure in the clinical laboratory that have prognostic value are intracellular ZAP-7044,61,62 and cell surface CD38.16,59,63 An inverse correlation exists between each of these markers and clinical course and survival. Since CD38 levels may change over time in some patients15,59,63,64 and may correlate with increased disease aggressiveness,64 this marker may also be helpful in determining a worsening of clinical course. At the present time, it appears that ZAP-70 correlates well with Ig V gene mutations and therefore may be a convenient clinical surrogate for V gene mutation status.61 B-CLL cells in both the high-risk Ig V gene subgroup and the CD38 subgroup13–,15,52,54 are more likely to express ominous cytogenetic abnormalities (e.g., trisomy 12, 11q-, and 17p-).
Level of Leukemia Cell Turnover In Vivo
The rate at which any biological system evolves is determined by the frequency that variants are produced and the relative selective advantages that the variants possess. For newly arisen subclones to become a significant proportion of the total population, they must either arise very early in the life of the clone or have a selective advantage. That selective advantage must result in an increased growth or accumulation rate that may be due to faster division, decreased death or clearance, or both. If a B-CLL clone was simply dividing slowly with little loss of cells, it would be difficult for novel variants to catch up with the already existing massive number of clonal members. However, with substantial turnover of the leukemic cells, novel variants could more quickly enrich to a level that could become clinically significant.
We have measured leukemic cell turnover in vivo65 using a non-radioactive, stable isotopic labeling technique that utilizes heavy water (D2O) to mark the DNA of newly generated cells.66 This approach has enabled us to follow in vivo the dynamics of B-CLL cell turnover during a 12-week labeling period followed by a washout period of 12 to 20 weeks. In most patients, incorporated label is observed in the CD19+CD5+ B-cell compartment after 2 weeks. However, in several patients there is a marked lag time before labeled cells appear and their relative abundance continues to increase well after week 12 when D2O intake ends. This suggests that the cells that are produced in solid compartments (bone marrow, lymph nodes, spleen) can be slowly released. Fitting a 2-compartment mixture model to the data produces cell turnover rate estimates between 1% and 10% per week for most patients studied.65
These unexpectedly high turnover rates have several implications. They indicate that the B-CLL clone is undergoing substantial replication. Since significant changes in peripheral lymphocyte counts did not occur in the patients studied, the high turnover rates also suggest that considerable cell death is occurring within the clone. This is consistent with in vitro studies indicating that B-CLL cells rapidly undergo spontaneous apoptosis that can be enhanced by BCR crosslinking by anti-IgM antibodies.34,35,67 This faster than anticipated turnover suggests that novel variants, with competitive advantages in proliferation and/or survival, could have the opportunity to become a significant proportion of the clone. It will be interesting to compare the turnover rates of the B-CLL cases that use mutated versus unmutated Ig V genes, since they differ so significantly in telomere lengths which can be an indicator of both past and present proliferation.
A Model for the Development and Evolution of B-CLL Cells
Collectively, the preceding data suggest that B-CLL develops from specific B-lymphocytes that express BCRs of restricted structure, implying that the BCR is linked, in some intimate fashion, to the pathogenesis of this disease. The most parsimonious explanation for this involvement would be that the BCRs of the B-lymphocytes that become leukemic capture and internalize the agent that leads to transformation. Although appealing, there is, as yet, no solid proof for this route of transformation. A currently more appealing model is that multiple antigens, that do not directly have transforming activity, select out B-cells for leukemic transformation by repetitively initiating cell signaling, DNA replication, and clonal expansion. This selection could cull out B-cells from the entire available BCR repertoire or from a BCR repertoire that is limited to a distinct subset of normal B-lymphocytes.2,5,8 Repetitive cell cycling then could serve as a promoting factor for the slow but progressive accumulation of DNA changes that would be necessary to confer leukemic properties on a member of the expanding normal B-cell clone.
These antigens could be foreign, autologous, or both. We prefer the last possibility for several reasons. First, many B-CLL BCRs are autoreactive.68–,70 This is consistent with finding the VH genes commonly expressed in B-CLL BCRs to be those associated with autoreactivity.71–,73 Second, susceptibility to autoreactivity and autoimmunity is frequently linked within families, which would be consistent with the apparent propensity of family members of B-CLL patients to develop clonal amplifications.47,48 Third, the structures of the B-CLL BCRs in certain instances are very reminiscent of antibodies that react with capsular polysaccharides of microorganisms,8,74,75 e.g., Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitides. Fourth, immune cross-reactivity between polysaccharide/carbohydrate antigens and autoantigens is not infrequent76 and anti-polysaccharide antibodies can be converted into autoantibodies (anti-dsDNA) by very minimal (1 amino acid) change in the Ig V region structure.77 The B-cell subpopulation that is especially important in anti-carbohydrate and anti-self (autoantibody) responses resides in the marginal zone (MZ) of solid lymphoid tissues.78 These MZ B-cells can display discrete BCRs comprised of very restricted V(D)J segments in animal systems and exhibit the presence and absence of Ig V gene mutations in both mice and humans. Based on these considerations, we proposed previously that B-CLL cells derive from MZ B cells that have been driven by foreign carbohydrate antigens and by autoantigens.2
How can we reconcile differences in BCR structure with clinical progression and disease outcome? If we assume that the prototypic B-CLL BCRs (Figure 2 ) were selected and driven by discrete types of antigens that differed in their capacity to induce Ig V gene mutations (i.e., foreign antigens that could induce mutations versus autoantigens that usually cannot induce mutations in the absence of overt autoimmunity), then unmutated B-CLL cells could have competitive advantage. This advantage might come about because of the availability of stimulatory autoantigens and the retained capacity to signal through the BCR once autoantigen engagement occurs. Indeed, those cases that retain this signaling property usually express molecular and cellular features seen in patients with the worst clinical outcomes (absence of V gene mutations, expression of ZAP-70 and CD38, expression of unfavorable genetic lesions, and p53 dysfunction). This would not preclude cases with mutated BCRs to also develop such advantages, if their BCRs, which have affinity for foreign antigens (e.g., bacterial polysaccharides), also cross-reacted with autoantigens. As mentioned, this type of antigen-binding cross-reaction or conversion is well documented. Alternatively, latent or persistent infection could provide the necessary foreign antigenic drive in these instances.
What then might be the competitive advantage that repetitive BCR signaling imparts to the leukemic subclone in vivo? The most unifying property would be the ability of such B-CLL cells to more easily and frequently enter the cell cycle and thereby generate more dangerous subclones that could convert a leukemic cell into a more aggressive variant (Figure 3; see Appendix, page 617). Favoring this hypothesis is the fact that the B-CLL cases with BCRs whose structural features most convincingly suggest antigen-binding and drive (VH4-39+, VH3-21+, and VH 1-69+ cases, Figure 2 ) appear to have the worst clinical courses. Significant genome instability would likely amplify or possibly result from the impact of such repetitive signaling. Because of this, we might consider that B-CLL is a disease phenotype, characterized by the clonal expansion of a subset of B-lymphocytes that eventually develops a series of genetic abnormalities based on alterations occurring throughout the genome. These abnormalities probably would occur stochastically in individual members of the clone and advantageous functional changes could be selected. Although we have focused on the role of the BCR in these processes, there is no reason to believe that other receptors (e.g., CD40, cytokine and chemokine receptors, and Toll-like receptors) could not render similar effects.
In such a model, B-CLL may not be defined by a single initiating genotypic abnormality (although a potential candidate for this central abnormality exists79), but rather by any one of a number of genetic changes that lead to clonal expansion in excess of clonal death. These paths could include heightened responses to signaling through various receptors, more effective or abundant adhesion to the endothelium or stroma, or homing receptors that could enhance trafficking to a microenvironment that would support expansion and survival, etc. When one considers clonal turnover rates of ~1%–10% per week, it becomes more reasonable that such adaptations would enable selected subclones to become a dominant fraction of the total. Figure 3 (see Appendix; page 617) depicts a model of these potential steps in the clonal development and evolution of B-CLL cells.
II. Immune Biology and Therapy of Chronic Lymphocytic Leukemia
Thomas J. Kipps, MD, PhD*
University of California at San Diego, Division of Hematology/Oncology, Department of Medicine, 9500 Gilman Drive, SCRB 102, La Jolla CA 92093-0663
Essential Monoclonal Lymphopathy
Flow cytometry can be used to monitor for lymphocytes that have phenotypic features in common with that of chronic lymphocytic leukemia (CLL) B cells, namely cells that co-express CD19 and CD5 and that have relatively low-level expression of CD20 and CD79b. This can be used as a sensitive test for minimal residual disease (MRD) following therapy.1 This method also can be used to examine the blood mononuclear cells of healthy subjects for cells that either are normal counterparts to the CLL B cell or possibly nascent CLL. One study evaluated first-degree relatives of CLL patients from 21 different families with 2 or more family members with CLL. This study found that approximately 8 (14%) of the 59 healthy first-degree relatives of patients with familial CLL who were tested had circulating B cells with these “CLL-B-cell” characteristics.2 The detection of such cells in the blood of healthy control subjects was significantly less frequent, suggesting that genetic factors might contribute to the relative abundance of these cells in the blood.
Conceivably, an excess of cells with these characteristics could presage development of CLL. Evaluation of the immunoglobulin genes used by such B cells from any one subject revealed evidence for oligoclonal (and in some cases monoclonal) gene rearrangements, suggesting an apparent excess of B cells belonging to one or a few different clones. Such clonal expansions of B cells were found more frequently in men than in women (with a male-to-female ratio of nearly 2:1) and more common in people ages 60 to 89 than in younger adults. Because these demographics correspond to the noted predominance of CLL in men and the aged, it seems reasonable to speculate that these clonal B-cell expansions represent cell populations that potentially could evolve into B-cell CLL. The noted frequency of finding such cells in the blood of healthy control subjects appears surprisingly high, with 3.5% of tested subjects (n = 910) having detectable blood lymphocytes with such characteristics.3 At this time, it is uncertain whether persons who have detectable clonal B-cell populations are at increased risk for developing CLL. Because the cause and clinical significance of such clonal, blood B-cell expansions is unknown, it appears appropriate to term this condition as “essential monoclonal lymphopathy.” Similar to the patients with essential monoclonal gammopathy who have an increased risk for developing plasma cell myeloma, patients with essential monoclonal lymphopathy could have a greater risk for developing CLL than the general population.4
Animal Models of CLL
Carlo Croce and associates developed an animal model for CLL that could allow for study of the critical events in leukemogenesis.5 Mice made transgenic for the human TCL1 gene under the control of a tissue-specific μ immunoglobulin enhancer (Eμ-TCL1) develop clonal B-cell expansions that are similar to those observed in patients with essential monoclonal lymphopathy. These animals develop detectable clonal expansions of CD5+ B-cell populations in the peritoneum at 2 months of age that become evident in the spleen by 3 to 5 months and then in the marrow by 5 to 8 months of age. Elder mice eventually develop a CLL-like disease, each animal developing a monoclonal outgrowth of B cells that share many features in common with that of the leukemia B cells of patients with CLL, including the coexpression of CD5, low-level surface immunoglobulin, and pan B-cell surface antigens.5 These cells infiltrate the blood and secondary lymphoid tissues, causing lymphocytosis, splenomegaly, and lymphadenopathy. The pathology of involved lymph nodes appears similar to that of patients with CLL.
Although the TCL1 gene is expressed at high levels by CLL B cells, overexpression of this gene that maps a 14q32.1 is not unique to this disease. Indeed, TCL1 initially was identified through analyses of translocations involving T-cell receptor genes of T-cell prolymphocytic leukemias.6 Furthermore, mice made transgenic for TCL1 using different tissue-specific promoters/enhancers do not develop a B-cell-CLL-like disease, but rather develop other types of lymphoid malignancies.7,8 As such, overexpression of the TCL1 gene per se does not cause a B-cell-CLL-like disease. Rather, overexpression of TCL1 at particular stages of B-cell development, combined with other factors, such as stimulation via surface immunoglobulin receptors and/or secondary mutations, appear required for development of a monoclonal B-cell-CLL-like disease in these animals. In this regard, it is noteworthy that the neoplastic B cells that develop in these animals appear to express a restricted repertoire of immunoglobulin genes, suggesting a potential role for stimulation via surface immunoglobulin receptors in the development of leukemia. Evaluation of such factors in this animal model could shed light on the mechanism(s) that contribute to leukemogenesis in patients with CLL.
Immunoglobulin Genes in CLL
The mutational status of the immunoglobulin genes expressed by CLL cells can be used to segregate patients into 2 subsets that have significantly different tendencies for disease progression. Patients with leukemia cells that express unmutated immunoglobulin heavy chain variable region genes (IgVH genes) have a greater tendency for disease progression than those who have leukemia cells that express IgVH genes with less than 96% nucleic acid sequence homology with their germ-line counterparts.9– 15 Generally, the IgVH genes expressed by any one leukemia-cell population do not show significant intraclonal diversity or tendency to accumulate additional somatic mutations over time. Coupled with the observation that patients with leukemia cells that express mutated Ig receptors generally have a more indolent clinical course than those with CLL cells that express unmutated Ig genes, it appears certain that leukemia cells that express mutated Ig genes do not evolve from cases that originally expressed unmutated Ig genes.
Because of this, some investigators have argued that the two subsets have, in fact, a distinct cytogenesis (reviewed by Naylor and Capra16). It was speculated that the subgroup of leukemia cells that express unmutated IgVH genes were derived from pregerminal center, or naïve, B cells. On the other hand, the leukemia cells that expressed mutated IgVH genes were presumably derived from postgerminal center, or memory-type, B cells.
However, these assumptions are not supported by the findings made from analyses of the IgVH genes expressed in CLL. Indeed, CLL cells that express unmutated Ig genes do not use a random assortment of IgVH genes that reflect those used by naïve B cells. For example, one particular IgVH gene, namely VH1-69, is used by about a fifth of such cases,17,18 but is infrequently used by CLL cells that express mutated IgVH genes or normal B cells. Moreover, there are several alleles of this gene that can be segregated into two types, based on differences in sequence encoding the second complementarity-determining region (CDR2), which can be distinguished using anti-idiotypic monoclonal antibodies (mAbs).19 Only one type of allele of VH1-69 is generally used in CLL. (Of note, this allele appears infrequently in populations at low risk for developing CLL, e.g., persons of Far East Asian ancestry). Furthermore, the antibody-heavy chains encoded by this allele in CLL have third complementarity-determining regions (CDR3) that are distinct from the CDR3 of antibody heavy chains expressed by normal B cells that use the same IgVH gene.18,20 Because the CDR2 and CDR3 fold together to form a major part of the antibody’s antigen-binding site, the discovery of such distinctive regions strongly suggests that the antibodies used by such CLL B cells are selected based on their capacity to bind some as yet undefined self or environmental antigen. As such, the CLL cells that use unmutated IgVH genes cannot be assumed to represent neoplastic naïve B cells.
Studies involving gene microarray analyses have provided further evidence for this. Indeed, regardless of whether CLL cells use unmutated or mutated Ig genes, they share common expression levels of many genes and have gene expression profiles that are distinct from that of other B-cell malignancies or normal, nonmalignant adult blood (or even neonatal core blood) B cells.21,22 Furthermore, the gene expression patterns observed in CLL appear to be most compatible with that of antigen-experienced, non-naïve B cells.
Zeta-Associated Protein of 70 kD (ZAP-70)
CLL cells that use unmutated IgVH genes can be distinguished from those that express mutated IgVH genes through the differential expression of a relatively small subset of genes. One of these genes encodes the zeta-associated protein of 70 kd, or ZAP-70. ZAP-70 is a 70-kd cytoplasmic protein tyrosine kinase that ordinarily is expressed only in natural killer (NK) cells and T cells, in which it originally was identified as being able to associate with the CD3 zeta chain (ζ-chain) of the T-cell-receptor complex. In contrast to CLL cells that have mutated Ig receptors, CLL cells that use unmutated IgVH genes express ZAP-70 RNA.21 Subsequent studies found that CLL B cells that had unmutated VH genes generally expressed levels of ZAP-70 protein that were comparable to those expressed by normal blood T cells.23 In contrast, CLL B cells that expressed mutated IgVH genes generally do not express detectable levels of ZAP-70 protein.
Expression of ZAP-70 has functional significance for the signaling capacity of the B-cell receptor (BCR) complex expressed in CLL. ZAP-70 is a protein tyrosine kinase (PTK) of T cells that is characterized by 2 tandem src homology (SH2) domains and a C-terminal catalytic domain.24,25 Following ligation of the T-cell receptor (TCR), there is activation of Src family PTK that in turn phosphorylates tyrosine-containing immunoreceptor tyrosine-based activation motifs (ITAMs) within the cytoplasmic tails of the accessory molecules of the TCR.26 ZAP-70 is recruited to the phosphylated ITAMs and subsequently becomes activated, in turn causing activation of Tec family PTKs and downstream signaling pathways, such as the phospholipase Cγ/Ca2+ signaling pathway and the Ras/mitogen-activated protein kinase (MAPK) pathway.27 B cells generally lack ZAP-70, but instead use another related PTK, p72syk, for signal transduction via the BCR complex.28 Similar to ZAP-70, p72syk is recruited to the phosphylated ITAMs of the activated BCR complex where it subsequently becomes activated. As such, ZAP-70 and p72syk play similar roles in membrane antigen-receptor signaling pathways.
It had been recognized that CLL B cells generally have a diminished response to ligation of the BCR complex.29–,31 The poor responsiveness of CLL B cells to BCR cross-linking had been argued secondary to low-level expression of surface Ig, inadequate levels or dysfunction of p72syk, a tyrosine kinase with homology to ZAP-70, or overexpression of an alternative transcript encoding a truncated form of CD79b (Igβ), a critical signaling molecule of the BCR complex. Even though most CLL cells generally express similar levels of p72syk, they vary in the level to which p72syk is phosphorylated following ligation of the BCR complex.29–,31 Moreover, the level of tyrosine-phosphorylation of p72syk appears associated with the ability of the leukemia cell to respond to cross-linking of its antibody receptors.31
However, more recent studies demonstrate that ligation of the BCR complex on CLL cells that express ZAP-70 induced significantly greater tyrosine-phosphorylation of cytosolic proteins, including p72syk, than did similar stimulation of CLL cells that did not express ZAP-70.23 Furthermore, the capacity to signal via the BCR for antigen was associated most closely with the expression of this kinase, even in unusual cases of CLL that expressed ZAP-70 but used mutated IgVH genes. Moreover, recent studies have identified unusual cases of CLL in which the leukemia cells express unmutated Ig receptors but fail to express ZAP-70. Like the cases of leukemia that have mutated Ig receptors and lack detectable expression of ZAP-70, these cases also fail to demonstrate receptor signaling via ligation of the BCR. Moreover, the average level of BCR-induced phosphorylation of p72syk and other signal-transducing proteins in such cases was significantly less than that observed in cases that expressed ZAP-70, regardless of the mutational status of the expressed IgVH genes. These observations suggest that ZAP-70 can enhance the signaling capacity of the BCR complex in CLL.
Ongoing studies are addressing whether the signaling capacity of the BCR is more tightly associated with clinical progression than expression of mutated versus unmutated IgVH genes in CLL. Indeed, competent BCR complex signaling might provide a growth factor stimulus to the leukemia B cells that is conducive to disease progression. If so, then analyses of the BCR signaling capacity might provide for a more reliable prognostic indicator in CLL than the mutational status of the expressed antibody VH genes or other markers, which are only associated indirectly with the biological factors governing disease progression.
Nurselike Cells
A small proportion of the mononuclear cells from the blood of patients with CLL can differentiate into large, round, adherent cells that attract CLL cells and protect them from undergoing spontaneous or drug-induced cell death. Because these cells share features in common with thymic nurse cells that nurture developing thymocytes, we designated these cells “nurselike cells,” or NLC.32 Although NLC apparently are derived from hematopoietic cells, these cells have features that are distinct from those of blood-derived monocytes, macrophages, or dendritic cells.33 Although NLC differentiate from blood mononuclear cells after several days in vitro, fully differentiated NLC can be found in the spleen and secondary lymphoid tissue of patients with CLL. There they might play a role in protecting CLL cells from apoptosis in vivo. This model implies that CLL cells are dependent upon specific extrinsic factors from NLC and other stromal elements for their survival.
Conceivably, CLL cells recirculate from the blood through secondary lymphoid tissues and back into the systemic circulation in response to certain chemokines, such as stromal-derived factor 1 (SDF-1), CCL21, and/or CCL19.34 Production of such chemokines as SDF-1 by NLC could recruit leukemia cells from the blood into secondary lymphoid tissues, where the leukemia cells in turn could receive survival stimuli from NLC and other stroma elements. Because these chemokine receptors are down modulated in response to the relevant chemokine, leukemia cells within lymphoid compartments potentially could be replaced by newly arriving leukemia cells and then reenter the systemic circulation. The leukemia cells in the blood that fail to reenter such protective compartments might undergo spontaneous cell death and account for the appearance of “smudge” cells that typically are found in the blood smears of patients with this disease. As such, the relative number and activity of such stromal elements might be a limiting factor governing tumor progression, particularly during early stages of the disease when the interdependency of leukemia cells with accessory cells seems most apparent.
NLC protect leukemia cells through a mechanism(s) that requires cell-cell contact. This implies that appropriate ligand-receptor interactions are critical for leukemia cell survival. Indeed, ligation of certain receptors can enhance leukemia cell survival in vitro. For example, CLL B cells resist spontaneous or drug-induced apoptosis in vitro when exposed to the fibronectin fragment H89, a ligand for α4β1 integrin expressed by the CLL cells of many patients.35 The engagement of this integrin on the leukemia cell surface by fibronectin on NLC could enhance the expression levels of antiapoptotic proteins of the bcl-2 family.
Another potentially important molecule is the chemokine SDF-1α. Because of its electrostatic charge at physiologic pH, this chemokine can bind the plasma membrane and form a concentration gradient surrounding the cells that produce this factor. The CLL cells from all patients tested express high levels of the receptor for SDF-1α (CXCR4).36 Exposure of CLL cells to SDF-1α triggers endocytosis of CXCR4, mobilization of calcium, actin polymerization, and chemotaxis in vitro.36 Similar to marrow stromal cells (MSC), NLC express high levels of SDF-1α and can attract leukemia cells via a CXCR4-dependent mechanism. In addition, SDF-1α can trigger CLL cells to activate p44/42 mitogen-activated protein-kinase (ERK 1/2),32 a key signaling pathway for promoting cell survival through transcription-dependent and transcription-independent mechanisms. This could account for the observation that neutralizing antibodies to SDF-1α can inhibit some of the protective activity of NLC on CLL cells in vitro.
The B-cell activating factor belonging to the tumor necrosis factor (TNF) family (BAFF) and its receptor, BAFF-R, also potentially factor in the protective-advantage afforded by NLC for CLL cells. CLL cells express BAFF-R,37,38 also known as the receptor for B-lymphocyte stimulatory factor (BlyS),39 TALL-1,40 zTNF4, or TANK.41 Ligation of BAFF-R by BAFF is essential for B-cell development because defects in either the ligand or the receptor arrest progression from immature to mature B cells. In vitro, BAFF increases B-cell survival and can induce proliferation of anti-IgM-stimulated blood B cells.42 One report indicated that CLL cells express RNA encoding BAFF,38 raising the prospect that BAFF could serve as an autocrine growth/survival factor in this disease. However, it has not been possible to detect expression of functional BAFF protein on leukemia cell surface membranes. NLC, on the other hand, express high levels of BAFF, which apparently contributes to their capacity to support leukemia cell survival. Agents that interfere with 1 or more of such important receptor-ligand interactions could prove useful in the treatment of patients with this disease.
Immune Therapy of CLL
Passive immune therapy
Approval of rituximab (anti-CD20) for treatment of follicular lymphoma ushered in the advent of passive immune therapy for CLL.43 Although CD20 is expressed at low levels by CLL B cells relative to the cells in follicular lymphoma, several clinical trials have demonstrated this mAb to have a therapeutic benefit in patients with CLL. When used as a single agent, rituximab generally can induce only partial responses,44,45 even at higher doses intended to overcome soluble inhibitors to the CD20 mAb that are found in the sera of most patients with CLL.46,47 However, rituximab apparently can improve the therapeutic outcome of patients when used concomitantly with other drugs that commonly are used in the treatment of CLL, such as fludarabine monophosphate (fludara) or fludara and cyclophosphamide.48
Campath-1H (alemtuzumab) has been approved for treatment of patients with CLL that is refractory to commonly used drugs, such as chlorambucil and/or fludara.49 This mAb has significant activity against leukemia cells in the blood and marrow, but appears less effective in clearing cells in secondary lymphoid tissues,50 where they might be protected from apoptosis by NLC and other stromal elements. Of particular interest, this antibody appears capable of clearing leukemia cells that lack p53,51 which typically are resistant to standard chemotherapy. For this reason, strategies incorporating use of alemtuzumab to treat patients with minimal residual disease following chemotherapy are being evaluated for their capacity to provide curative therapy for patients with this disease.52
Other agents for passive immune therapy of CLL are being evaluated. These include mAbs or immunotoxins specific for other antigens expressed by CLL cells (e.g., CD23 [IDEC-152], HLA-DR determinants [Hu1D10], and even CD25 [denileukin diftitox, OTAK]). Radiolabelled mAbs, such as zevalin, also are being evaluated for their capacity to provide a therapeutic benefit. The use of radiolabelled mAbs, however, is handicapped by the invariable infiltration of the marrow by CLL cells, making stem cell toxicity a significant concern.
Active immune therapy
Identification of leukemia-associated antigens could lead to development of vaccines for inducing active antileukemia immunity in patients with this disease. Microarray analyses of leukemia cells have identified many other genes that appear expressed at high levels in CLL B cells relative to that of other cell types or B-cell malignancies. Conceivably, some of these encode antigens that are expressed primarily by CLL B cells and not by other cell types. One antigen long-identified as restricted in its expression to the leukemia cell is that of the idiotype for the antibody expressed by the leukemia cell clone. Strategies similar to those used in the immune therapy of follicular lymphomas are under investigation, such as use of the antibody protein (or nucleic acid vaccine encoding the idiotype-portion of the antibody protein) coupled to an immunogenic carrier.
Cellular vaccines involving the leukemia cell that is modified to enhance its capacity to induce an immune response or dendritic cells pulsed with putative leukemia-associated antigens are under investigation. One strategy is to modify the leukemia cell through transduction of an adenovirus encoding the ligand for CD40 (CD154). Ligation of CD40 can induce CLL cells to express immune costimulatory molecules that are required for stimulation of allogeneic or autologous T cells. Because CLL cells stimulated in this fashion can induce generation of autologous cytotoxic T cells,53 methods for ligating CD40 have been incorporated into strategies for treating this disease. There are different approaches in animal models using either intratumor injection of viral vectors encoding CD15454 or cell-based vaccines composed of either dendritic cells or tumor cells that had been modified to express CD154.55–,58 Transduction of primary follicle center lymphoma B cells59 or CLL B cells53 with adenovirus encoding CD154 can enhance autologous cellular immune recognition of neoplastic cells. Moreover, Ad-CD154 transduced CLL B cells showed promising results in a Phase I clinical trial study.60 A Phase II study currently is evaluating the effects of multiple injections of autologous Ad-CD154-transduced CLL B cells.
Activated T-cell Therapy
T cells activated via CD3/CD28 coligation display functional characteristics of effector cells that are able to induce cytolysis of autologous leukemia cells via the extrinsic cell-death pathway. This effect appears to be mediated in part by CD154 (CD40-ligand), Fas-L, and/or TRAIL, which are expressed by such activated T cells.61 Because of their noted expression of CD154, infusions of activated, autologous T cells potentially could have effects similar to that noted in CD154-gene therapy.61 Processing of CLL patients’ blood mononuclear cells ex vivo with anti-CD3/CD28 microspheres leads to the rapid activation and 100- to 1000-fold expansion of T cells over 11 to 14 days, even in patients who have received prior treatment with chemotherapy (e.g., fludara). The cultured cells consist of T cells that exhibit no apparent functional defects and few, if any, B cells. The anti-CD3/CD28 bead-activated T cells express high levels of interferon (IFN)-γ, TNF-α, CD154, CD137 (4-1BBL), and other key effector molecules, such as CD134 (OX-40), CD54 (ICAM-1), and CD25 (IL-2 receptor). Owing to their expression of CD154 and TNF-α, CD3/CD28-activated T cells can induce neoplastic B cells to express CD80, CD86, and ICAM-1 (CD54).62,63 These molecules play important roles in the immune costimulation of T cells responding to peptides presented by major histocompatibility complex (MHC) antigens of antigen-presenting cells.64 As such, some of the immune suppressive features of the leukemia cell are reversed, at least transiently.
Because of these potential benefits, a Phase I trial testing the safety and biologic activity of activated T-cell therapy has been initiated at University of California, San Diego with Xcyte Therapies, Inc (Seattle, Wash). For this, the patients undergo leukapheresis to remove mononuclear cells that subsequently are cultured ex vivo with anti-CD3/CD28 microspheres. The cultivated autologous T cells subsequently are reinfused as a single intravenous infusion. A dose-escalation study currently is under way in patients who have failed standard chemotherapy or who elect to receive such treatment prior to chemotherapy. Conceivably, this approach could be combined with other treatment regimens that ordinarily cause T-cell depletion, potentially allowing us to mitigate the problems associated with the immune dysfunction caused by most other standard treatments for this disease.
III. Do We Have the Tools to Cure CLL?
Michael J. Keating, MB, BS*
University of Texas MD Anderson Cancer Center, Department of Leukemia, 1400 Holcombe Boulevard, Box 428, Houston TX 77030
The traditional wisdom of management of CLL is based on the principle to first “do no harm.” This approach was based on two “pillars of wisdom.” First, CLL patients are considered to be an elderly population who are more likely to die of causes other than CLL. The second pillar is that treatment was ineffective in prolonging survival that was associated with a very low frequency of complete remissions.1 From the earliest time it was apparent that CLL was a disease with very heterogeneous outcome.2 Many patients could be followed for decades without any evidence of progressive disease development whereas some other patients developed early symptoms of bone marrow failure, repeated infections, and transformation into more aggressive forms of the disease. The earlier attempts to categorize these patients into prognostic subcategories led to the hallmark classifications of Rai and Binet separating patients into various stages.3,4 While this approach was generally effective, it provided limited information on prognosis for individual patients.
The development of newer prognostic factors has allowed for further discrimination of patients into risk categories. A number of traditional prognostic factors such as age, sex, and tumor burden were useful, and serologic staging parameters such as beta-2-microglobulin (β2M), thymidine kinase (TK), and soluble CD23 emerged as being independently discriminatory after accounting for the stage of disease.5–,8 More recently, seminal breakthroughs in the understanding of the variability in CLL were obtained by appreciation of the importance of mutation status of immunoglobulin genes. In 1999, two groups of investigators demonstrated that patients with a memory cell immunophenotype with mutated immunoglobulin genes had a very favorable outcome and a low probability of developing progressive disease, whereas those with unmutated immunoglobulin genes were much more likely to develop progressive disease and be associated with a shorter survival.9,10 Damle and colleagues also demonstrated that patients with low expression of CD38 on the CLL cells were associated with a mutated phenotype and had a very favorable prognostic outcome.9 Further studies have confirmed that mutation status is a powerful predictor of prognosis whereas CD38, in some hands, is less powerfully predictive.11 The reason for the unmutated status may be associated with the observation that unmutated patients have spliced variants of the enzyme activation induced cytidine deaminase (AID) which is emerging as a major focus for research and understanding of the biology of CLL.12,13 More recently, analysis of gene microarray data has highlighted the association of ZAP70 (a tyrosine kinase) with the unmutated phenotype.14,15 ZAP70 has independent prognostic importance and it is obvious that within a few years clarification of the relative impacts of the immunoglobulin gene mutation status, CD38, and ZAP70 will allow categorization of patients into those with minimal risk of dying of CLL and a separate subset type of patients more likely to develop progression of the disease.
A misconception, which has been perpetuated over many years, is that patients seldom die of CLL. It is clear now from observations in the modern era that patients who do have progression of their CLL predominantly die of complications of the disease, especially from infections.16 As the disease progresses, immune dysfunction and myelosuppression become more and more severe leading to an increased susceptibility to a variety of infections. With the increasing awareness that patients with progressive CLL die of their disease, a strictly palliative approach does not appear to be viable and attempts at curative strategies for subsets of patients with progressive CLL should be undertaken.
Curability of Lymphoid Malignancies
Formerly Hodgkin’s disease, acute lymphocytic leukemia, and diffuse large B-cell lymphoma were considered to be incurable diseases. The development of effective therapies has transformed these conditions into reliably curable diseases in subsets of patients. It is also clear that increasing quality of response is associated with the prospect of improved survival and prospect for cure. The question persists as to whether this concept applies to CLL or whether CLL is more like follicular lymphoma with a propensity to multiple responses with relapse. A major weakness of CLL research has been the lax response criteria that have been applied. A major contribution of the National Cancer Institute (NCI) Working Group and the International Working Group on CLL (IWCLL) was the development of new criteria for response with provided comparability between studies.17,18 The disappearance of clinical evidence of disease on examination, return of hematopoiesis to normal, and normalization of the bone marrow were important elements in defining complete remission (CR). Even as late as 1986, persistent nodules in the bone marrow biopsy were allowed for patients to be classified as complete remissions.18,19 It is now obvious that the persistence of these nodules is associated with persistence of CLL with a higher propensity for relapse.20
Even in patients who achieved a bone marrow biopsy remission along with the other criteria for CR, detection of residual disease was comparatively easy once flow cytometry with monoclonal CD5 + 19 coexpressing cells (the hallmark of CLL) was possible.20 The CR patients who had more than 10% residual CLL cells on flow cytometry had shorter remission duration. However, there was no significant impact on survival.
Up until recently, rarely were patients described to have achieved PCR negativity for the immunoglobulin heavy chain (IgVH) sequence and were classified as molecular remissions.21–,23 The PCR technique has been useful in predicting the long-term survival of patients with low-grade lymphoma.24 While there is evidence that some patients who are in long-term remission remain PCR positive and those who become PCR negative consistently have longer remissions than those who have residual disease detected by the PCR technique,25,26 other investigators have suggested that sophisticated flow analysis can be as sensitive as PCR for detection of residual disease.27 A question that can be posed in CLL is whether flow cytometry negativity or PCR negativity are reasonable surrogate endpoints for prolonged survival and prospects for cure and whether these endpoints are consistently achievable with modern approaches to therapy. I propose that these are reasonable endpoints and modern clinical trials should try to achieve this state in a significant proportion of patients.
Tool 1—Chemotherapy
Historically, the approach to the treatment of CLL was based on the alkylating agents, predominantly chlorambucil but also cyclophosphamide. Corticosteroids were also reported to be useful but were associated with a high incidence of infection; combinations of chlorambucil with prednisone became the benchmark of therapy in CLL.28 A major breakthrough that occurred in chemotherapy management was the discovery of the activity of purine analogs fludarabine monophosphate (Fludara), 2-chlorodeoxyadenosine (2-CDA), and pentostatin (DCF, deoxycoformycin).29–,31 The activity of fludarabine in previously treated patients was rapidly confirmed in previously untreated patients29 and 2-CDA was also confirmed to be very active in both subsets of patients. The addition of corticosteroids did not appear to be helpful. Once the activity of fludarabine and 2-CDA was confirmed, randomized comparative trials were conducted. Fludara has been shown to be superior in terms of complete remission rate and remission duration when compared with the CAP regimen (cyclophosphamide, doxorubicin, and prednisone) and chlorambucil.31,32 In the American Intergroup study of fludarabine versus chlorambucil, there was a very significantly higher CR rate, overall response rate, and remission duration in the Fludara-treated patients.32 Comparisons of 2-CDA + prednisone to chlorambucil and prednisone have also confirmed the greater activity of the purine analogs compared to alkylating agents.33 None of these studies, however, has led to a survival advantage as patients have largely relapsed and gone on to subsequent therapy. The conclusion reached from these comparative studies has been that the purine analogs are the most active single group of agents in CLL and should form the building block of subsequent therapies.
The purine analogs are potent inhibitors of DNA repair. Thus, combinations of fludarabine and cyclophosphamide have been developed34,35 and more recently fludarabine, cyclophosphamide, and mitoxantrone.36 These agents had high response rates in single institution studies and appear to have higher CR rates than single agent purine analogs. Randomized comparative trials are now being conducted to evaluate the benefit of combinations compared to single agent approaches. A small number of patients treated with these combinations have been able to achieve PCR negativity.36 The results of chemotherapy protocols with or without rituximab are shown in Table 2 .
Tool 2—Monoclonal Antibodies
The development of monoclonal antibodies with activity against surface antigens on CLL cells has led to a flurry of research in this arena (Table 3 ). The first monoclonal antibody approved for use in lymphoid malignancies was rituximab. The pivotal clinical trial, however, demonstrated a low level of responsiveness in patients’ small lymphocytic lymphoma, the counterpart of CLL.44 This low response rate was attributed to both the lower antigen density of CD20, the target for rituximab on CLL and SLL cells compared to follicular lymphoma, and a more rapid clearance of the antibody from plasma of patients with SLL compared to follicular lymphoma. This postulated “antigen sink” was largely unexplained but recent data suggest that there is a substantial amount of soluble CD20, which, as a measure of turnover of CLL cells, may allow immune complex formation with more rapid clearance of antibodies from plasma.45 Other studies have confirmed a low response rate with conventional doses of rituximab of 375 mg/m2 per week for 4 weeks.39
Two approaches have been undertaken to try to improve the response rate to rituximab in CLL. The first was the increase in dose intensity on the once a week schedule;41 the second, a tripling of dose intensity by giving the conventional dose 3 times a week.40 Both approaches have increased the response rate to approximately 40%. Newer clinical trials have demonstrated that administration of rituximab to previously untreated patients is associated with a substantially higher overall response rate and CR rate.46,47
Another antibody, Campath-1H (alemtuzumab), targets CD52, an antigen that is ubiquitous in CLL with heterogeneity of antigen density. Early studies demonstrated clear activity in CLL but were associated with a significant incidence of opportunistic infections presumably associated with the T-cell immunodeficiency, which occurs subsequent to alemtuzumab administration. CD52 is present on both B- and T-lymphocytes and monocytes. In a pivotal study, Campath-1H was evaluated in fludarabine-refractory patients who had previously been exposed to alkylating agents and who demonstrated a 33% response rate with excellent activity in clearing peripheral blood and bone marrow.42 When Campath-1H was moved into initial therapy administered as a subcutaneous injection, the response rate was comparable to that achieved with fludarabine.43 Other studies have taken the approach that monoclonal antibodies will be more effective in the management of minimal residual disease (MRD) and have demonstrated that low amounts of residual tumor can be cleared by the administration of Campath-1H.48,49 Many of these Campath-1H-treated patients become PCR negative when treated for MRD. Another approach to MRD has been to use the monoclonal antibody zevalin,50 which is associated with significant myelosuppression and has been less effective in our experience. Other monoclonal antibodies such as epratuzumab and anti-HU1D10 antibodies are being explored in CLL.
Tool 3—Chemo-immunotherapy
In vitro studies have demonstrated that rituximab sensitizes cell lines to the cytotoxicity of a number of agents including fludarabine and cyclophosphamide.51 There is also evidence that rituximab will downregulate bcl2 perhaps by inhibition of IL10.52 Fludarabine downregulates CD55 and CD59 which can be considered as complement defense proteins.53 Based on the in vitro evidence of additive or synergistic activity, studies combining rituximab with fludarabine have been designed and implemented. Cancer and Leukemia Group B (CALGB) has compared simultaneous administration of rituximab with fludarabine versus fludarabine followed by rituximab.37 A higher complete response rate was seen in the simultaneous arm of the study compared to the sequential. There was no additional toxicity apart from an increase in neutropenia but not of infections, either traditional or opportunistic. The study will continue to mature and evaluate long-term benefit.
At the MD Anderson Cancer Center (MDACC) fludarabine and cyclophosphamide have been combined with rituximab in the FCR regimen with fludarabine being given at 25 mg/m2/day for 3 days, days 1 to 3, and cyclophosphamide 250 mg/m2 3 times a day. Rituximab is given at a dose of 375–500 mg/m2 on day 1.38 There were 204 patients with previously untreated CLL with either advanced stage disease or progressive earlier stage disease that were treated. The overall response rate is 95% with 69% complete remission rate. Of interest, 82% of the CR patients have a CD5 + 19 percentage in the bone marrow of < 1%. In addition, approximately half of the patients are able to become PCR negative for the IgVH gene.38 A number of the PR patients have complete marrow clearance but have persistent cytopenia, which prevents them from being classified as having a CR. This regimen is associated with significant tumor lysis requiring the administration of allopurinol. Neutropenia of grade 3 and 4 is common but not associated with a high incidence of infection with only 2% of courses being associated with major infection. Failure-free survival and overall survival are illustrated in Figure 4 and remission duration by responses in Figure 5. CR rate and response duration are significantly superior to FC.
Less information is available on the combination of fludarabine with Campath-1H. In a small study from the United Kingdom, Hellman and colleagues demonstrated that patients refractory to fludarabine have a very good response to the addition of Campath-1H.54 A German study illustrated that fludarabine and Campath-1H can be administered to previously treated patients with CLL with a high overall and complete response rate.55 Other combinations are under development.
Tool 4—Stem Cell Transplantation
Stem cell transplantation (SCT) was not widely used in the management of CLL for a number of reasons. Prior to the development of the purine analogs, adequate clearing of blood and bone marrow to collect uncontaminated stem cells for autologous transplantation was not possible. In addition, the advanced age of the patient population with CLL limited the allogeneic SCT approach. In 1996, Michallet et al published a study of 54 patients with an allogeneic transplant.56 Twenty-four patients were still in remission with a median follow-up of 27 months. Most other studies have had smaller numbers of patients in the range of 12–25. The impression from these studies is that a number of patients (30%–40%) appear to stay in a long-term disease-free state.
A greater number of patients have been transplanted with autologous stem cells. The largest study was from the Dana Farber Cancer Institute with 154 patients.57 The treatment related deaths were 6 (4%) out of 154; 132 patients were in ongoing complete remission. Five-year event-free survival for these 154 patients is 65%. An update of this study is needed. In a study of 14 autologous stem cell transplants, 9 patients became MRD negative; of these 9, 2 relapsed, and 4 became MRD positive.58 Of the 5 patients who remained MRD positive, 4 relapsed. In the 12 allogeneic transplant patients on the same study, 3 have died and 8 of the remaining 9 became MRD negative on a long-term basis.
There is clear evidence in a number of studies that a graft-versus-leukemia (GVL) effect is associated with allogeneic SCT. Nonablative allogeneic transplants have increasingly been performed to explore the graft versus leukemia effect. Of 30 patients from the German Cooperative Group, 12 achieved complete remissions and 8 were MRD negative.59 The follow-up is too short to draw any conclusions. Seventy-seven patients have had nonablative transplants from the European Bone Marrow Transplant Registry with a projected event-free survival of 3 to 4 years of 50%.60 The development of nonablative transplants has extended the range of patients eligible for allogeneic SCT to 75 years of age. In a comparison of ablative versus nonablative transplants at MDACC, the long-term survival appears to be similar (~40%) despite the higher age range of the nonablative SCT.61,62 Generally, patients who are MRD negative have longer disease-free intervals with overall survival than patients who remain MRD positive.
Strategic Application of Present Modalities
With the development of new chemo-immunotherapy programs, antibody treatment for MRD, and autologous and allogeneic stem cell transplants, the options for therapy of patients have become increasingly complex. The ability to achieve MRD negativity in other diseases has translated into longer event-free survival and association with long-term survival. A feasible strategy, therefore, is for patients who are undergoing initial therapy to have the most effective remission induction regimen. If they become and stay MRD negative, then no further therapy should be offered to them. On the other hand, if they remain MRD positive and if the level of positivity increases with time intensification or consolidation with antibody therapy, SCT is reasonable. At the present time, it appears that autologous SCT is not curative but prolongs remission duration. On the other hand, the GVL effect of ablative and nonablative allogeneic SCT can be associated with long-term control. The ability to achieve MRD negativity with all of these modalities gives rise to the prospect of long-term survival in a substantial number of patients with CLL.
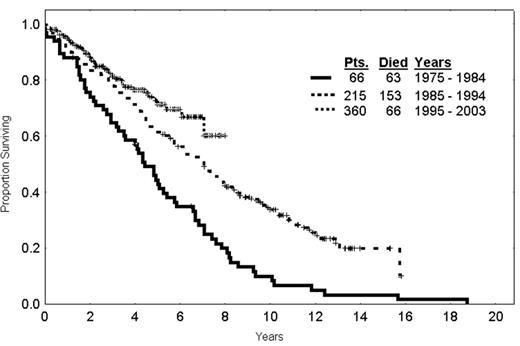
The question arises as to whether therapy has modified the survival of patients over time. An analysis of patients receiving initial therapy for CLL from 1970–1985, 1985–1995, and 1995 on, illustrates that there is a steady increase in the survival fraction of patients over time (Figure 6 ). Obviously, covariate adjustment needs to be made for the patients going on to the various studies but at least the question has arisen as to whether patients with CLL are surviving for longer periods of time with modern therapy. The conclusion is that the tools are available for curative strategies to be developed in CLL challenging further developments to make treatments more tolerable. Immune therapy using vaccines and gene therapy approaches are promising and will probably have a major application in situations of MRD.
Molecular, phenotypic, and clinical characteristics of the Ig V gene-defined B-cell chronic lymphocytic leukemia (B-CLL) subgroups.*
| . | Subgroup I . | Subgroup II . |
|---|---|---|
| * Subgroup I is defined by the expression of unmutated (< 2% difference from germline counterpart) BCRs, and Subgroup II by the expression of mutated (≥ 2% difference from germ line) BCRs. Patients in Subgroup I experience a much more aggressive clinical course. | ||
| Abbreviations: Ig, immunoglobulin; BCR, B-cell receptor. | ||
| Stage at presentation | Often Rai intermediate/high risk | Often Rai low risk |
| Age at presentation | No difference | No difference |
| Gender | More males | M = F |
| Lymphocyte doubling time | Frequently < 12 months | Frequently > 12 months |
| V gene mutations | Few or none | Significant numbers |
| Antigen selection | Yes | Yes |
| ZAP70 expression | High | Low |
| CD38 expression | High | Low |
| Activation markers | 38+/69+ | 71+/62L+ |
| Telomere length | Uniformly short | Diverse lengths |
| Telomerase activity | High | Low |
| Retention of BCR signaling | Yes | No |
| Ongoing Ig V gene mutations | Yes | Yes |
| Intermittent expression of AID | Yes | Yes |
| Chromosomal abnormalities | Poor prognosis | Better prognosis |
| Treatment requirement | Usual | Unusual |
| . | Subgroup I . | Subgroup II . |
|---|---|---|
| * Subgroup I is defined by the expression of unmutated (< 2% difference from germline counterpart) BCRs, and Subgroup II by the expression of mutated (≥ 2% difference from germ line) BCRs. Patients in Subgroup I experience a much more aggressive clinical course. | ||
| Abbreviations: Ig, immunoglobulin; BCR, B-cell receptor. | ||
| Stage at presentation | Often Rai intermediate/high risk | Often Rai low risk |
| Age at presentation | No difference | No difference |
| Gender | More males | M = F |
| Lymphocyte doubling time | Frequently < 12 months | Frequently > 12 months |
| V gene mutations | Few or none | Significant numbers |
| Antigen selection | Yes | Yes |
| ZAP70 expression | High | Low |
| CD38 expression | High | Low |
| Activation markers | 38+/69+ | 71+/62L+ |
| Telomere length | Uniformly short | Diverse lengths |
| Telomerase activity | High | Low |
| Retention of BCR signaling | Yes | No |
| Ongoing Ig V gene mutations | Yes | Yes |
| Intermittent expression of AID | Yes | Yes |
| Chromosomal abnormalities | Poor prognosis | Better prognosis |
| Treatment requirement | Usual | Unusual |
Reported complete and overall response rates (CR and OR) in various clinical trials for chronic lymphocytic leukemia (CLL).
| Regimen . | Patients . | %CR . | %OR . | Ref. . |
|---|---|---|---|---|
| Chlorambucil | 181 | 3 | 37 | 32 |
| Fludarabine (Flu) | 170 | 20 | 70 | 32 |
| Flu+Cyclophosphamide (FC) | 34 | 35 | 88 | 34 |
| Flu+Rituximab (Concurrent) | 51 | 77 | 90 | 37 |
| (Sequential) | 53 | 28 | 77 | 37 |
| FC+Rituximab | 202 | 69 | 95 | 38 |
Reported results with monoclonal antibody therapy in chronic lymphocytic leukemia (CLL).
| Regimen . | Previously Untreated . | Prior Rx . | Dose (4-week schedule) . | %CR + PR . | Ref. . |
|---|---|---|---|---|---|
| Abbreviations: CR, complete response; PR, partial response | |||||
| Rituximab | 43 | — | 375 mg/m2 qw × 4 | 9 + 49 | 46 |
| 6 | 27 | 375 mg/m2 tiw × 4 | 3 + 42 | 40 | |
| — | 50 | 500–2250 mg/m2 qw × 4 | 0 + 36 | 41 | |
| Campath | 0 | 93 | 30 mg tiw × 12 wks IV | 2 + 31 | 42 |
| 38 | — | 30 mg tiw × 18 wks SC | 19 + 68 | 43 | |
| Regimen . | Previously Untreated . | Prior Rx . | Dose (4-week schedule) . | %CR + PR . | Ref. . |
|---|---|---|---|---|---|
| Abbreviations: CR, complete response; PR, partial response | |||||
| Rituximab | 43 | — | 375 mg/m2 qw × 4 | 9 + 49 | 46 |
| 6 | 27 | 375 mg/m2 tiw × 4 | 3 + 42 | 40 | |
| — | 50 | 500–2250 mg/m2 qw × 4 | 0 + 36 | 41 | |
| Campath | 0 | 93 | 30 mg tiw × 12 wks IV | 2 + 31 | 42 |
| 38 | — | 30 mg tiw × 18 wks SC | 19 + 68 | 43 | |
Characteristics of the most common and the most unique B-cell chronic lymphocytic leukemia (B-CLL) B-cell receptors (BCRs).
The prototypic unmutated BCR is represented by the rearrangement that uses the VH1-69 gene, whereas the prototypic mutated BCR is represented by the VH3-07 rearrangement. The VH4-34 rearrangements have characteristics of both the prototypic unmutated and mutated BCRs. The arrangements involving these 3 VH genes are the most common in B-CLL. Less common but remarkably unique BCRs are represented by the VH4-39 and VH3-21 rearrangements that pair with defined D, JH, VL, and JL genes. These latter BCRs are the most striking examples of antigen selection of a specific antigen-binding site. Adapted, with permission, from the Annual Review of Immunology, Volume 21, 20032 (www.annualreviews.org).
Abbreviations: BCR, B-cell receptor; B-CLL, B-cell chronic lymphocytic leukemia; CDR, complementarity determining region.
Characteristics of the most common and the most unique B-cell chronic lymphocytic leukemia (B-CLL) B-cell receptors (BCRs).
The prototypic unmutated BCR is represented by the rearrangement that uses the VH1-69 gene, whereas the prototypic mutated BCR is represented by the VH3-07 rearrangement. The VH4-34 rearrangements have characteristics of both the prototypic unmutated and mutated BCRs. The arrangements involving these 3 VH genes are the most common in B-CLL. Less common but remarkably unique BCRs are represented by the VH4-39 and VH3-21 rearrangements that pair with defined D, JH, VL, and JL genes. These latter BCRs are the most striking examples of antigen selection of a specific antigen-binding site. Adapted, with permission, from the Annual Review of Immunology, Volume 21, 20032 (www.annualreviews.org).
Abbreviations: BCR, B-cell receptor; B-CLL, B-cell chronic lymphocytic leukemia; CDR, complementarity determining region.
Time-to-treatment failure and survival of 202 chronic lymphocytic leukemia (CLL) patients treated with fludarabine, cyclophosphamide, and rituximab (FCR) as initial therapy.
Time-to-treatment failure and survival of 202 chronic lymphocytic leukemia (CLL) patients treated with fludarabine, cyclophosphamide, and rituximab (FCR) as initial therapy.
Time-to-treatment failure of 202 chronic lymphocytic leukemia (CLL) patients treated with fludarabine, cyclophosphamide, and rituximab (FCR) as initial therapy according to response (NCIWG criteria).
Abbreviations: CR, complete response; PR, partial response; NR, no response; NCIWG, National Cancer Institute Working Group.
Time-to-treatment failure of 202 chronic lymphocytic leukemia (CLL) patients treated with fludarabine, cyclophosphamide, and rituximab (FCR) as initial therapy according to response (NCIWG criteria).
Abbreviations: CR, complete response; PR, partial response; NR, no response; NCIWG, National Cancer Institute Working Group.
Survival of chronic lymphocytic leukemia (CLL) patients requiring therapy by era (dated from the start of initial therapy).
Survival of chronic lymphocytic leukemia (CLL) patients requiring therapy by era (dated from the start of initial therapy).
Acknowledgments: These studies were supported in part by grants from the NCI (CA 81554 and CA 87956), the Jean Walton Fund for Lymphoma & Myeloma Research, the Joseph Eletto Leukemia Research Fund, and AIRC.