Abstract
The treatment recommendations for chronic myelogenous leukemia (CML) are evolving rapidly. In the past year, pegylated interferon and STI571 (Gleevec, imatinib mesylate), a Bcr-Abl tyrosine kinase inhibitor, have become commercially available and non-myeloablative stem cell transplants continue to be refined. Clinicians and patients face a bewildering array of treatment options for CML. In this article Dr. Sawyer reviews the clinical results with STI571 and ongoing investigations into mechanisms of resistance to STI571. Given the newness of STI571, a practical overview on the administration of STI571 is presented by Drs. Druker and Ford, focusing on aspects such as optimal dose, management of common side effects, and potential drug interactions. The most recent data on interferon-based regimens are reviewed by Dr. Baccarani in the third section. In the last section Dr. Goldman presents recent results of allogeneic stem cell transplants, including the reduced intensity conditioning regimens. Lastly, the proposed place of each of these treatments in the management of CML patients is addressed to assist in deciding amongst treatment options for CML patients.
I. Clinical Development of STI571 in Chronic Myelogenous Leukemia
Charles L. Sawyers, MD,*
UCLA, 10833 Le Conte Avenue, 11-934 Factor Building, Los Angeles CA 90024
Introduced in the treatment of chronic myelogenous leukemia (CML) 20 years ago, interferon (IFN)-based regimens are now considered as the standard therapy for newly diagnosed patients in chronic phase CML who have no matched bone marrow donor.1 IFN is the only drug that has consistently been shown to prolong survival as compared to chemotherapy. IFN monotherapy of newly diagnosed patients is usually associated with a rate of major cytogenetic response of 10-38% in comparison with only 0-5% with chemotherapy. Despite these results, there is at present no evidence of cure with IFN therapy. Minimal residual disease remains almost invariably detectable by sensitive polymerase chain reaction (PCR) methods. The vast majority of patients ultimately develop resistance to IFN and die of their disease.
Patients who become resistant or intolerant to IFN are usually treated with either hydroxyurea or busulfan. Although this second-line treatment is associated with a good level of hematologic control (the rate of complete hematologic response is approximately 50%), the achievement of a major cytogenetic response remains rare. Accelerated phase (AP) is usually considered the first manifestation of resistance to therapy. The diagnosis of AP dictates therapeutic changes, either an increase in the dose of the drugs already used to control the chronic phase or the introduction of new treatments. However, no standard therapy exists in this setting. Some degree of hematologic response or a return to a second chronic phase can be achieved in approximately 50% of patients, but these responses are usually short-lived. Complete responses are uncommon while cytogenetic responses are anecdotal.
Blast crisis is the terminal event in the clinical course of CML, defined usually as the presence of ≥ 30% marrow blasts.2 Patients are treated with a multiagent chemotherapy regimen commonly used to treat acute lymphoblastic or myeloid leukemia, as appropriate. A hematologic response can be achieved in approximately 20% to 40% of patients, but it is complete in only 5% to 30% of patients and generally short-lived. Over the past 30 years, the prognosis of patients with CML in blast crisis has remained extremely poor, with a median survival of only 3 to 6 months.
Phase I Clinical Trial Results with STI571
Based on the above, the efficacy and safety of STI571 (also called imatinib or Gleevec) has been investigated in patients with chronic phase CML failing prior IFN therapy, in accelerated phase CML or in blast crisis.
Chronic phase
In a phase I dose-escalation study, 83 patients with chronic phase CML were enrolled and treated at doses ranging from 25 mg to 1000 mg daily. The data indicates a clear dose-response relationship with a rate of complete hematologic response (CHR) of 38% (11/29) in patients treated with doses below 300 mg and 98% (53/54) in patients receiving 300 mg or higher.3 The plasma levels of STI571 in the 300 mg dose cohort correlate with the levels needed to achieve Bcr-Abl target enzyme inhibition and growth suppression in CML cell lines. This dose-response relationship was further evaluated using an Emax model. When relative response (% fall in WBC after 1 month of treatment) was related to exposure (expressed as daily dose, AUC, Cmin or Cmax, or time above the 1μM plasma level leading to apoptosis in vitro), the best fit was obtained with dose, suggesting that outcome is essentially dose-dependent.4
Blast crisis
In 58 patients with blast crisis or acute Ph+ leukemias treated at doses ranging from 300 mg to 1000 mg, hematologic responses were also seen in 55% of patients with myeloid blast crisis (21/38) and 70% of patients with lymphoid blast crisis or Ph+ ALL (14/20).5 In some cases, complete cytogenetic remissions were observed. Unfortunately, nearly all patients with lymphoid disease relapsed within 2-3 months. Relapse was also common in myeloid blast crisis, occurring in 60% of responders within 6 months. As longer follow up data is obtained, it appears that the remissions observed in the remaining 40% may not be durable beyond one year.
In these phase I studies, STI571 was generally well tolerated, and almost all non-hematologic adverse events (AEs) were of grade 1/2 severity. The most frequent non-hematological AEs that were considered drug-related included nausea, musculoskeletal symptoms (most commonly muscle cramps, arthralgia and myalgia), edema (most commonly periorbital), and skin rash. Based on non-hematologic toxicity, a maximum tolerated dose (MTD) was not reached, and dose escalation was stopped at 1000 mg. In chronic phase patients, even though these events were not dose limiting, there was a trend for a higher frequency of grade 3/4 AEs and grade 3/4 cytopenias at doses of 750 mg daily or higher. No clear dose relationship was apparent in patients with blast crisis or other Ph+ acute leukemias.
Taking into account an observed inter-patient variability in PK parameters, a dose of 400 mg offered a favorable safety versus efficacy margin in terms of achieving therapeutic drug levels in chronic phase CML (i.e. trough plasma levels above the concentrations required for in vitro cell growth inhibition). Therefore, this dose was selected for the subsequent phase II study in patients with chronic phase CML. A dose of 400 mg was also initially selected for the studies in AP and blast crisis (BC). However, preclinical in vitro data suggested that an important mechanism of drug resistance is the amplification of the bcr-abl gene, leading to increased amounts of the target BCR-ABL protein.6- 8 It was hypothesized that high doses could be more effective in inhibiting the target kinase in patients with overall poor prognosis. Thus, when more safety data became available from the phase I study, the protocols were amended to make 600 mg the starting dose, which was the highest dose known to be safe and effective at that time.
Phase I Clinical Trial Results with STI571
Chronic phase trial
Patients in chronic phase CML were eligible for this study if they met one of the following criteria for IFN failure: hematologic failure (failure to achieve a CHR after ≥ 6 months of IFN or relapse with a rising WBC to ≥ 20 x109/L), cytogenetic failure (failure to achieve a major cytogenetic response after ≥ 12 months of IFN or relapse with a ≥ 30% increase in the percentage of Ph+ marrow metaphases to ≥ 65%), or intolerance to IFN, defined as a ≥ grade 3 non-hematological IFN-related toxicity persisting for ≥ 1 month. Five hundred thirty-two patients were enrolled, of whom 454 (85%) had a confirmed diagnosis of CML in chronic phase. Out of these 454 patients, IFN failure was documented as either hematologic failure (133 patients), cytogenetic failure (160 patients), or intolerance to IFN (161 patients). Patients were late in the course of the disease with a median time from diagnosis of 34 months. These patients had received prior IFN (given alone or in combination with other drugs) at a dose ≥ 25 MIU/week for a median of 14 months. The patient baseline characteristics were typical of pretreated chronic phase CML: 40% of the patients were ≥ 60 years (10% older than 70 years).
Accelerated phase trial
A total of 235 CML patients were enrolled in this study, of whom 181 had a confirmed diagnosis of CML in accelerated phase on the basis of criteria developed by the Houston group.9 The primary endpoint of the study was the rate of hematologic response, described as either CHR, no evidence of leukemia, or return to chronic phase. Among the 181 patients with a confirmed diagnosis of AP, 62 were started at 400 mg and 119 patients were started at 600 mg. AP was newly diagnosed in 62 patients (34%), while 119 patients (66%) had received prior therapy for CML in AP, most often with hydroxyurea (101 patients), IFN (33 patients), or cytarabine (23 patients). Approximately 12% of enrolled patients were aged > 70 years.
Myeloid blast crisis trial
In this study, patients with previously untreated or treated myeloid BC were enrolled. The primary endpoint was the rate of hematologic response, defined as in the AP study. A total of 260 CML patients were enrolled, of whom 229 had a confirmed diagnosis of CML in blast crisis. The first 37 patients were treated at a dose of 400 mg and the subsequent 223 patients at 600 mg.
Aggregate results of all three phase II trials
As shown in Table 1 , responses were observed at all stages of the disease. In patients with chronic phase, the rate of complete hematologic response was 91%, and 55% of the patients achieved a major cytogenetic response. In patients with AP or BC, the rate of sustained hematologic response was 69% and 29%, respectively (i.e. responses lasting 4 weeks or more). Unexpectedly, a high rate of major cytogenetic response was also observed in these advanced stages of the disease (24% and 16%, respectively).10- 12
Current Clinical Trials
Phase III comparison to IFN in upfront CML treatment
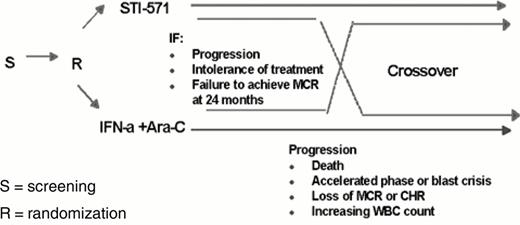
The efficacy results summarized above and the favorable safety profile have established STI571 as effective therapy for patients with advanced phase CML and patients in chronic phase failing first line IFN therapy. The drug was approved by the FDA on May 10, 2001 for these indications. However, a number of questions remain to be elucidated to better define the use of this potent new agent in the treatment of CML (Table 2 ). The first question relates to the activity of single-agent STI571 in patients with newly diagnosed CML. To address this question, a randomized phase III study comparing STI571 400 mg/day versus a combination of IFN and low dose Ara-C 13 was established that accrued more than 1106 patients in less than 5 months. The design is shown in Figure 1 , and the first results are expected in 2002.
STI571 combination clinical trials
In advanced phase CML, particularly in BC, resistance develops in a significant fraction of patients after an initial response to therapy. Importantly, several in vitro studies have shown that the combination of STI571 with IFN, Ara-C, or daunorubicin may have synergistic antiproliferative activity.14,15 On the basis of these findings, several clinical studies are currently underway to assess the feasibility of combining STI571 with various chemotherapeutic regimens or with IFN or Pegylated-IFN. Additional studies are also evaluating the feasibility of administering STI571 before or after bone-marrow transplantation, or to treat relapses after bone marrow transplantation.
Drug Resistance
Several mechanisms of resistance to STI571 have been identified from in vitro studies of CML cell lines. Mechanisms include amplification or increased expression of the Bcr-Abl gene or overexpression of the Pgp protein.6- 8 For clinical purposes, resistance to STI571 needs to be classified into distinct clinical scenarios, for which the mechanisms of resistance are likely to be quite different. In this review, we are distinguishing between upfront resistance to STI571 (i.e., the patient fails to respond) and resistance due to relapsed disease while on STI571 after an initial response. In addition, hematologic and cytogenetic resistance need to be considered separately, at least until a greater mechanistic understanding is obtained.
Upfront STI571 resistance— hematologic and cytogenetic
The frequency of upfront resistance varies dramatically, depending on the stage of disease (chronic, accelerated or blast crisis) as well as the definition of response. In chronic phase patients who fail IFN, less than 10% of patients have upfront hematologic resistance. However, about 45-50% of such patients have cytogenetic resistance, meaning that a major cytogenic response is not observed with the first 9 months of treatment. The fact that these cytogenetic non-responders do have good hematologic disease control indicates that STI571 is clearly exerting a therapeutic effect. The reason for the failure to convert from a hematologic to a cytogenetic response in these patients is unknown. One possibility is that higher doses of STI571 might be required in these patients, perhaps because progenitor cells in bone marrow, which are measured in karyotype assays, are less sensitive to STI571 than mature blood cells. The potential benefit of dose escalation is being tested in the phase II chronic phase trial by escalation from 400 mg/day to 800 mg/day in patients who fail to obtain a major cytogenetic response at one year. An alternative explanation is that Bcr-Abl independent signals are responsible for the continued survival of these cells despite effective targeting of Bcr-Abl by STI571. Laboratory measurements of Bcr-Abl signal transduction in these cells, and examination of other pathways should distinguish between these models.
Upfront hematologic resistance in AP and BC is more common than in chronic phase, occurring in 18% and 48% of patients, respectively. These percentages are based on a definition of response (reduction in bone marrow blasts) that does not require that the response be sustained for one month, since many patients respond briefly then relapse. As with chronic phase, it will be important to know if upfront resistance in advanced stage CML is caused by failure to inhibit Bcr-Abl effectively or by the presence of specific secondary mutations that allow the leukemia cells to proliferate independent of Bcr-Abl.
Mechanisms of resistance in patients with advanced stage CML who relapse
More information about STI571 resistance is available in patients who initially respond to treatment but subsequently relapse while on remaining on drug. In a study of 11 such patients with BC CML or Ph+ ALL, 3 had amplification of the Bcr-Abl gene and 6 had a point mutation in Bcr-Abl that prevented STI571 from inhibiting its kinase activity.16 Larger numbers of patients need to be examined to characterize the full range of potential mechanisms of relapse (Figure 2 ). Nevertheless, these findings are remarkable because they suggest that the Bcr-Abl oncogene remains a critical drug target even in late stage CML, when numerous secondary oncogenic mutations are present. This result also raises the possibility that additional drugs targeted against Bcr-Abl, designed to work by a different mechanism, may have utility in patients with advanced disease. To date, relapse on STI571 has not been a significant issue in patients with chronic phase disease, suggesting that prolonged single agent treatment with STI571 may be adequate treatment if given early.
Summary
Phase I and phase II clinical trials of STI571 in patients with CML in chronic phase refractory to IFN, AP and BC have established its utility in CML treatment and led to its fast track approval by the FDA in May, 2001. Current trials will define its role as a single agent in newly diagnosed CML as compared to combination therapy with IFN and Ara-C. Additional trials are evaluating the safety and efficacy of STI571 given in combination with chemotherapy. The lower response rate and high relapse rates observed with single agent STI571 in advanced stage CML make it imperative that effective combination treatment be found. Insights into the mechanisms of STI571 resistance, either at the time of treatment initiation (upfront resistance) or at relapse, should provide additional clues about how to optimize the treatment of CML with targeted therapeutics.
For patients with chronic phase disease, the primary determinant of whether single agent therapy is appropriate will be the durability of hematologic and cytogenetic responses. At the time of this writing, these responses are durable at one year, but longer follow-up is needed to make fully informed decisions. In the interim, we can only make suggestions for how to advise patients based on our own experience. In patients with no cytogenetic response, we are examining higher dose STI571 (800 mg per day) or the addition of IFN or low dose cytarabine to STI571, all in the context of clinical trials. For patients who have a cytogenetic response, we are continuing single agent STI571 until evidence of disease progression. Bone marrow cytogenetics and FISH are monitored every six months. Since many patients have no detectable disease with these measures, it is likely that PCR tests which quantify the level of BCR-ABL transcript in peripheral blood will become an important tools for monitoring response to STI571.
II. Practical Aspects of STI571 Administration
Brian J. Druker, MD,1
Oregon Health and Science University, 3181 SW Sam Jackson Park Road, L592, Portland OR 97201-3098
Novartis Pharma AG, Basel, Switzerland
Dr. Druker is a consultant for Novartis.
Although therapy with STI571 is generally well tolerated, it is not devoid of side effects. Particularly common side effects include myelosuppression, nausea, vomiting, edema, muscle cramps, arthralgias, diarrhea, and skin rashes (Tables 3 and 4 ). Elevated transaminases are observed less frequently but occasionally necessitate the discontinuance of therapy. The purpose of this article is to provide information about these side effects and possible management strategies. Decisions for an individual patient need to be made on a case-by-case basis, taking into account the patient's specific circumstances.
Myelosuppression
Myelosuppression is particularly common in CML patients treated with STI571 (Table 4). In a phase II trial in chronic phase patients who had failed IFN, it was mandated by the protocol to interrupt therapy with STI571 for grade 3 myelosuppression. Using these guidelines, 25% of patients experienced grade 3 neutropenia (ANC < 1000/mm3) and 16% developed grade 3 thrombocytopenia (platelets < 50,000/mm3). In addition, 8% of patients developed grade 4 neutropenia (ANC < 500/mm3) (Kantarjian HM et al, submitted). In advanced phase patients (AP and BC), due to the more life-threatening nature of the disease, treatment was not interrupted except for prolonged myelosuppression, and accordingly a higher percentage of patients developed grade 3 and 4 neutropenia and thrombocytopenia (Talpaz M et al, submitted; Sawyers CL et al, submitted, respectively). In CML patients in blast crisis, almost 50% of patients developed grade 4 neutropenia. A number of deaths have been attributed to STI571-induced myelosuppression, almost all of which were seen in advanced phase disease.
Although myelosuppression can occur at any time during STI571 therapy, it generally occurs within the first 2-4 weeks in blast phase patients, though slightly later in chronic and AP patients. Factors associated with myelosuppression besides advanced disease may include prior myelosuppression from IFN therapy or prior therapy with busulfan. The risk of developing myelosuppression may depend on the amount of residual normal hematopoiesis, and this may be negatively correlated with the above-mentioned factors.
The management of myelosuppression requires an understanding of the mechanism of action of STI571, the observed dose-response relationship, and an exploration of the ability of STI571 to inhibit normal hematopoiesis.
Mechanism of Action and the Therapeutic Dose of STI571
The mechanism of action of STI571 is to inhibit the Bcr-Abl tyrosine kinase. To achieve maximum therapeutic benefit, it seems likely that one needs to use a dose that maximally inhibits Bcr-Abl kinase activity or alternatively that inhibits sufficient Bcr-Abl kinase activity to induce apoptosis. At present, this optimal dose is not known; however, 1 μM levels appear optimal for cell killing in vitro and 1 μM trough levels are achieved in patients using STI571 at a daily dose of 300 mg.1,2
Dose-Response Relationships
When examining the dose-response curve in chronic phase patients, it appears that the therapeutic benefit plateaus at or slightly above a dose of 300 mg.2 These data were obtained from a relatively small number of patients during the phase I dose escalation study and are summarized in Table 5 . In this study, at a dose of 200 mg, 3 of 9 (33%) patients achieved a complete hematologic response, but only 1 of these patients (11%) had bone marrow revert to a normal morphology. This was the only patient in this dose cohort to have a cytogenetic response, and this was a complete cytogenetic response. At 250 mg, a complete hematologic response occurred in 4 of 7 patients (57%). In this cohort, 2 of 7 patients (28%) had their marrow morphology revert to normal, but only 1 patient (14%) had a cytogenetic response. In contrast, at 300 mg or greater, 53 of 54 (98%) patients achieved complete hematologic responses. The majority of these patients had bone marrows that appeared normal, and the cytogenetic response rate was 54% (29/54) with 31% major cytogenetic responders.3 These data were confirmed in a phase II study using 400 mg where the complete hematologic response (CHR) rate was 91% and the major cytogenetic response rate was 55%, including 36% of patients with complete cytogenetic responses (Kantarjian HM et al, submitted).
Inhibition of Normal Hematopoiesis by STI571
As the Bcr-Abl positive clone is responsible for the majority of hematopoiesis in CML patients, myelosuppression is an expected therapeutic effect. Myelosuppression might also be due to inhibition of c-kit by STI571 given the expression of c-kit on hematopoietic progenitor cells. When addressing dose modifications of STI571, it is necessary to consider whether any dose of STI571 is able to inhibit normal hematopoiesis. For example, if STI571 does not inhibit normal hematopoiesis and myelosuppression is simply an indication of therapeutic benefit, then dose modification should be unnecessary. However, if STI571 inhibits hematopoiesis, lowering the dose might allow the recovery of normal hematopoietic elements.
A number of findings suggest that the inhibition of normal hematopoiesis by STI571 is minimal. Studies treating gastrointestinal stromal tumor (GIST) patients with doses of 400 and 600 mg of STI571 showed that myelosuppression was much less frequent than in CML patients (though not completely absent) and was seen principally in patients who had received significant prior chemotherapy.4 In colony forming assays using normal hematopoietic progenitors, doses of 1 μM of STI571 resulted in a 10-20% inhibition of colony formation.5,6 In advanced phase CML patients, recovery of normal counts has been observed in patients receiving continuous therapy with STI571. Taken together, these observations suggest that the inhibition of normal hematopoiesis by STI571 does occur but is not pronounced.
The foregoing data suggest that 300 mg is a threshold dose for achieving an optimal therapeutic response and that STI571 has only marginal effects on normal hematopoiesis. Based on these considerations, it is recommended that STI571 should rarely, if ever, be used at doses of less than 300 mg.
Maximum Tolerated Dose of STI571
During the phase I study, a maximum dose of 1000 mg was reached and there was no convincing dose limiting toxicity; however, doses of 750, 800 and 1000 mg were less well tolerated than doses of 400 and 600 mg with a higher frequency of nausea, vomiting, edema, fatigue and diarrhea.3,7 In an EORTC study in GIST patients, 1000 mg was the maximally tolerated dose, with nausea and vomiting as the dose limiting toxicities.8 In a phase II study in accelerated phase patients, 77 patients were treated with 400 mg of STI571 and 158 patients with 600 mg. The rates of hematologic response in these two groups were similar; however, there was a statistically significantly longer time to progression and superior survival in patients receiving 600 mg (Talpaz M et al, submitted). The majority of blast phase patients have been treated at 600 mg. Based on these data, 600 mg is the recommended dose for patients in accelerated phase and blast crisis. For patients in chronic phase, all studies have been conducted at 400 mg. In the absence of comparative data in this patient population and given the high response rates noted above, 400 mg is the recommended dose in chronic phase patients. In patients not responding to 400 or 600 mg, dose escalation to 800 mg is warranted with caution, but dose escalation beyond this is not advised.
Is flat dosing with 400 and 600 mg appropriate, regardless of patient size?
From population-based pharmacokinetic studies, there is no evidence that the size of the patient has an impact on the plasma levels of STI571.9 In our institution, we have treated 17 chronic phase patients weighing over 250 pounds (113 kg) who had failed prior therapy with IFN. Fifteen of these 17 patients (88%) achieved a CHR and 10/17 (58%) achieved a major cytogenetic response. Although based on only a small number of patients, these response rates are similar to those seen in phase II studies. Thus, our experience confirms the pharmacokinetic data that flat dosing is appropriate.
Practical Dose Considerations and Dose Modifications for Myelosuppression
Dose and hematologic monitoring
In CML patients in chronic phase, the recommended starting dose of STI571 is 400 mg. CBCs should be monitored weekly for the first month. Thereafter, the frequency of blood count monitoring depends on the stability and the level of the blood count. Thus, if a patient's ANC falls to less than 1500/mm3 and/or the platelet count to less than 100,000/mm3, blood counts should be monitored weekly. If blood counts are higher than these levels, monitoring can be reduced to every 2 weeks until 12 weeks of treatment is reached, and depending on the stability of the counts, the frequency of monitoring can be lengthened to monthly. For patients with advanced phase CML, a starting dose of 600 mg of STI571 is recommended. Blood counts should be monitored at least weekly, if not more often, depending on the clinical situation.
Institution of STI571 therapy
STI571 can be started when white blood counts (WBCs) are normal or at any level above normal. For patients with a WBC over 20,000/mm3, concomitant therapy with allopurinol is recommended until the WBC is consistently less than 20,000/mm3. Tumor lysis syndrome has been rare, even in advanced phase patients, but maintaining adequate hydration is essential, and advanced phase patients should be monitored for this complication. After initiating therapy with STI571, the WBC should begin to fall within the first two weeks and usually normalizes within four to six weeks. The decline in platelet counts is commonly delayed by a week or two. In patients receiving therapy with hydroxyurea and who have normal blood counts, the hydroxyurea can be tapered and discontinued within the first week of STI571 therapy. For patients with elevated WBCs, the hydroxyurea may need be continued for one to three weeks while closely monitoring the WBC. Similar guidelines apply for patients with elevated platelet counts on therapy with anagrelide, but with a week or two added to the time course. For patients with a WBC or platelet count below the lower limits of normal due to recent CML therapy (typically, an IFN-treated patient), all therapy should be discontinued and the blood counts allowed to recover to at least an ANC of 1500/mm3 and a platelet count of at least 100,000/mm3 before starting STI571. If blood counts are below normal due to advanced phase disease, STI571 can be started regardless of the blood counts.
Management of myelosuppression
The primary consideration guiding recommendations for the management of myelosuppression is to match the intensity of treatment to the acuity of the underlying illness. STI571 has the potential to induce severe and prolonged myelosuppression, and caution must be exercised in its administration, particularly in patients with minimal residual normal hematopoiesis. Thus, in otherwise healthy chronic phase patients, it does not seem reasonable to put patients at risk of developing febrile neutropenic episodes or for patients to become platelet transfusion dependent. This is particularly true knowing that, over time, some patients can achieve recovery of normal hematopoiesis and a major cytogenetic response despite having experienced recurrent grade 3 neutropenia and thrombocytopenia, and frequent dose interruptions,.
In chronic phase patients, it is recommended that STI571 be held if the ANC falls below 1000/mm3 or platelets to less than 50,000/mm3. Treatment should be restarted when the ANC recovers to 1500/mm3 and the platelets to 100,000/mm3. This recommendation may be modified slightly in patients with higher risk features, such as higher blast or basophil percentage or chromosomal evolution. For patients with prolonged times to recover their peripheral counts (e.g., greater than 2-4 weeks), consideration should be given to reducing the dose to 300 mg and to re-escalating to 400 mg after several months only if myelosuppression does not recur. As noted above, dose reductions below 300 mg are not generally recommended since these doses are subtherapeutic. Rather, dose interruptions for recurrent myelosuppression have so far been the preferred course of action.
In patients with more immediately life-threatening advanced phase disease, the suggested course of action is less clear. One approach has been to allow no dose modifications based on thrombocytopenia and to support patients with platelet transfusions with a platelet count under 10,000/mm3 or under 50,000/mm3 with clinically evident bleeding. Another approach has been to hold STI571 in these situations. Obviously, if clinically significant bleeding occurs, STI571 should be held until the bleeding is controlled. For an ANC less than 500/mm3, it is suggested that STI571 be continued and that marrows be examined for cellularity and residual leukemia. In patients whose marrows remain hypercellular or with blasts greater than 30%, it is recommended that STI571 be continued. If the marrow is hypocellular and the ANC is less than 500/mm3 for 2-4 weeks, than either the dose of STI571 should be reduced, STI571 should be held or consideration should be given to growth factor treatment while continuing the STI571. All of these approaches have been used and there are not enough data to know which, if any, of these approaches is the most appropriate. For blast crisis patients, we have tended towards a more aggressive approach to therapy knowing the seriousness of the illness and that standard chemotherapy would frequently result in bone marrow aplasia lasting 4-6 weeks.
Nausea
The most common side effect of STI571 is nausea (Table 3). Nausea is usually grade 1, is dose-related and is likely due to the local irritant properties of the compound. Nausea is much more common when STI571 is taken on an empty stomach and can be avoided in most patients if STI571 is taken with food. As the pharmacokinetics of STI571 are not altered whether STI571 is taken with food or fasting,10 it is recommended that STI571 be taken with the largest meal of the day. Due to its local irritant properties, it is also recommended that STI571 be taken at least two hours before bedtime, especially in patients with a history of esophagitis or hiatal hernia. In patients who continue to have difficulties with nausea despite following these recommendations, the total daily dose can by split in half and taken with two separate meals. In particular, patients treated with 800 mg of STI571 are best treated with 400 mg b.i.d. If nausea is a recurrent problem, anti-nausea medications such as prochlorperazine (compazine) or ondansetron (zofran) can effectively control this side effect.
Muscle cramps
Muscle cramps are relatively common, and the most common sites of occurrence are the hands, feet, calves and thighs. Some patients describe the cramps with features reminiscent of tetanic contractions. The pattern, frequency, and severity of muscle cramps does not seem to change over time. There are also no clear precipitating factors, although some patients have noted a nocturnal pattern or a relationship to exertion. Ionized calcium and magnesium levels are not clearly abnormal in STI571-treated patients, and the precise pathogenesis of this side effect is unknown. Despite this, calcium supplements can alleviate this symptom, and on occasion magnesium supplements may also be of some help. Quinine supplementation has also been reported to reduce symptoms in some patients.
Bone pain and arthralgias
Bone pain and arthralgias have been reported by 20-40% of patients. Their onset tends to be in the first month of therapy and they frequently abate after a month or two. The pain most frequently occurs in the long bones, typically femurs or tibias, as well as hips or knees. On occasion, the pain can be quite severe and disabling. The etiology of this symptom is unclear, but in some patients it has correlated with clearance of cells from the marrow. If the patient's platelet count is over 100,000/mm3, this symptom can be successfully treated with nonsteroidal anti-inflammatory drugs (NSAIDs). Due to the gastric irritant properties of STI571 and the occurrence of rare gastrointestinal (GI) bleeding in patients treated with STI571, an NSAID with a lower incidence of GI bleeding should be selected. NSAIDs should either be avoided in patients with a history of GI bleeding or administered concomitantly with an H-2 blocker or proton pump inhibitor. If the platelet count is under 100,000/mm3 or the patient has other contraindications to the use of NSAIDs, acetaminophen could be tried cautiously (see below) or mild narcotic pain medications should be used. Regardless, patients should be reassured that this side effect is usually self-limiting.
Skin rashes
There are several types of STI571-induced skin rashes. The most common rash associated with STI571 is a maculopapular rash that is most prominent over the forearms and trunk and occasionally occurs on the face. In most cases, the rash is mild and easily manageable with antihistamines and/or topical steroids. In more severe cases, a short course of oral steroids may be required. A few patients develop a severe, desquamative rash that mandates immediate discontinuation of STI571 and institution of steroid therapy. Depending on the clinical situation, it has been possible to restart STI571 after the rash has resolved with a gradual dose escalation of STI571. In these cases, prednisone has typically been given at 1 mg/kg, tapering to 20 mg over several weeks. STI571 has been restarted at 100 mg per day and the dose increased by 100 mg per week while tapering the steroids, assuming that the rash has not recurred. This approach should only be considered in patients for whom no other treatment option exists other than STI571. Lastly, we have observed rare patients with extremely high basophil counts (> 30%) develop urticarial eruptions after taking STI571, presumably due to histamine release from basophils. This rash can be managed by premedication with an antihistamine and will usually resolve as the basophil counts normalizes.
Diarrhea
Loose stools have been noted by patients treated with STI571 and the condition is dose-related. It is possible that this side effect is due to inhibition of c-kit, which is highly expressed by the interstitial cells of Cajal, the pacemaker cells of the intestine, responsible for intestinal motility. It may also be due to the local irritant effects of the compound since a sizable fraction of unchanged drug is excreted in the feces following biliary elimination. This side effect is easily managed with antidiarrheal medications in symptomatic patients.
Edema and fluid retention
Edema is one of the most common side effects of STI571, occurring in 50% or more of patients, and is clearly dose-related. The most common manifestation of this side effect is periorbital edema that is typically worse in the morning. Peripheral edema, most commonly lower extremity, is also seen. In a much lower number of patients (around 1-2%), manifestations of more generalized fluid retention develop with combinations of one or more of the following: pulmonary edema, pleural or pericardial effusions, ascites or anasarca. Fluid retention can potentially be life threatening, and at least one death has been reported in a blast crisis patient due to pulmonary edema. Edema and fluid retention tend to be more common in older patients and in patients with a prior history of cardiac disease. This symptom may respond poorly to diuretic therapy. Although the precise cause of the edema and fluid retention is unknown, PDGF receptor null and Abl/Arg (Abl-related gene) double knockout animals have edema,11,12 suggesting that the fluid retention is due to pharmacological inhibition of these targets.
No specific therapy is required for most cases of periorbital edema. Some patients have found that limiting salt intake is helpful in ameliorating the symptoms. Diuretics may be indicated in more severe cases, and topical therapy with 1% hydrocortisone may also be of some benefit.
Because of an increased likelihood of fluid retention, STI571 should be used with caution in older patients and in patients with cardiac or renal impairment. In these patients, it is advisable to initiate therapy with 300 mg of STI571, with dose increases to 400 or 600 mg as tolerated. Patients should be monitored closely for evidence of peripheral edema or rapid weight gain and diuretic therapy should be initiated or the dose of diuretics increased as soon as possible. In patients with severe fluid retention, STI571 should be discontinued, the edema controlled with diuretics and STI571 restarted, possibly at a reduced dose, while maintaining or increasing diuretic therapy.
Hepatotoxicity
The typical pattern of liver function abnormalities has been a transaminitis (Table 4). The median time of onset is around 100 days, though some occur as early as the first week and others only after many months of therapy. The etiology of the hepatoxicity is unclear though it appears to be a typical drug induced hypersensitivity on liver biopsy. It is recommended the liver function tests (LFTs) be monitored every other week for the first month of therapy and monthly thereafter, with more frequent evaluations in patients with elevated transaminases. Our current approach is to hold STI571 if patients develop grade 3 elevations in ALT or AST (> 5 times the upper limit of normal). When the LFTs fall to grade 1 or less (< 2.5 times the upper limit of normal for transaminases or < 1.5 times for bilirubin), STI571 is reintroduced at a reduced dose. If the liver toxicity does not recur within 6-12 weeks, re-escalation to the initial dose can be performed, while closely monitoring the LFTs. If grade 3 toxicity recurs, a more thorough hepatic evaluation is indicated as described below. With recurrent grade 3 toxicity, STI571 should normally be permanently discontinued. Fortunately, less than 1% of patients treated with STI571 have had to discontinue therapy due to persistent elevations in LFTs.
In patients with grade 2 elevations of transaminases (2.5-5 times the upper limit of normal), we carefully review the patient's intake of potential hepatoxins. For example, patients are cautioned to avoid acetaminophen and other non-essential hepatotoxic medications, as well as alcoholic beverages. Essential medications that have potential hepatoxicity should be carefully evaluated to determine whether a less hepatotoxic substitute can be safely administered. If grade 2 toxicity persists, an evaluation to include a viral hepatitis panel, ferritin level, alpha-1 antitrypsin level and possibly an ultrasound or liver biopsy should be considered. The decision to continue STI571 with ongoing grade 2 transaminitis needs to be made in light of the clinical situation and at a minimum, a dose reduction of STI571 may be warranted.
There has been controversy regarding the safety of acetaminophen in patients treated with STI571. A death due to hepatic failure occurred in an AP patient who had daily fevers and was taking high doses (more than 3 grams daily) of acetaminophen together with STI571 (Talpaz M et al, submitted). The causal relationship of this drug combination to the death is undetermined. Numerous other patients have safely taken these two medications in combination. Nevertheless, caution is recommended and patients should be advised to use acetaminophen in moderation and only on an intermittent basis and certainly not to exceed 2 grams per day.
Other side effects
Other side effects of STI571 include weight gain and fatigue. The weight gain may be in part related to fluid retention. However, it is clear that fluid retention cannot account for the progressive increases in weight seen in some patients. An increased appetite has been reported by some patients while taking STI571, which abates during treatment breaks. Another aspect of weight gain has been the return of a normal appetite following the discontinuation of IFN. Patients prone to weight problems need to be cautioned about the association between STI571 and weight gain. Measures such as decreased caloric intake and increased exercise are recommended to prevent or treat this problem. The etiology of fatigue may be partially due to a mild anemia that can be observed during initial STI571 therapy. A decrease in hemoglobin of 1-2 gm/dl is frequently observed in the first month of therapy with an increase to baseline over the next months. Other patients have developed a macrocytosis with or without anemia that may be attributable to c-kit inhibition.
Drug interactions
STI571 is predominantly metabolized in the liver by the CYP3A4/5 p450 enzyme system. STI571 does not induce increased levels of this enzyme since plasma levels of STI571 remain stable over time. However, reduced plasma STI571 levels may occur in patients treated concomitantly with inducers of this enzyme, leading to decreased therapeutic efficacy of STI571. Major inducers of this enzyme include phenytoin, carbamazepine, and phenobarbital, among others (Table 6 ). As an example, a patient treated with phenytoin was found to have fourfold lower plasma levels of STI571. The indications for treatment with medications inducing CYP3A4/5 should be carefully reviewed and if indicated, an appropriate substitution may be warranted.
Conversely, drugs that inhibit the CYP3A4/5 enzyme might be expected to result in increased levels of STI571. Major inhibitors include erythromycin, ketoconazole, ritonavir, and saquinavir, among others (Table 6). Grapefruit juice is also an inhibitor of this enzyme, and patients should be cautioned against excessive intake. Fortunately, the therapeutic window of STI571 is relatively broad. Caution needs to be exercised in patients treated with drugs affecting this enzyme, particularly in patients on higher doses of STI571.
There are numerous drugs that are also metabolized by this enzyme system that may compete with STI571 for metabolism, potentially leading to increased levels of both drugs. For example, increased levels of cyclosporin have been observed in posttransplant patients treated with STI571, and cyclosporin levels should be monitored closely in these patients and doses adjusted accordingly.
STI571 is also an inhibitor of CYP2D6 and CYP2C9, and drugs metabolized by these enzymes should also be used with caution. Patients on warfarin have demonstrated both increases and decreases in INR. Because warfarin is metabolized by CYP2C9, it is considered advisable to use low-molecular weight or standard heparin for patients who require anticoagulation while taking STI571. Due to concerns about bleeding, the combination of STI571 and anticoagulants should be used with great caution in patients with platelet counts below 100,000/mm3, and the risks and benefits of using the medications in combination should be carefully considered.
Summary
STI571 therapy is generally well tolerated. Most common sides effects are mild and can be easily managed. However, therapy requires frequent and careful monitoring, particularly for myelosuppression, fluid retention, and hepatotoxicity which occasionally are severe.
III. Non-Transplant Treatment Options for Patients with Newly Diagnosed Chronic Myeloid Leukemia
Michele Baccarani, MD*
Istituto di Ematologia e Oncologia Medica “H L. eA. Seràgnoli”, Università di Bologna, Bologna, Italy
Dr. Baccarani was a consultant for Roche (1994) and Novartis (2001). He was a principal investigator of several studies of the treatment of CML sponsored by Roche, Novartis, and Schering Plough.
Acknowledgments: the contribution of the members, the scientific board and the technical staff of the Italian Cooperative Study Group on CML is gratefully acknowledged, with special reference to Domenico Russo MD, Eliana Zuffa MD, Gianantonio Rosti MD, Antonio de Vivo MD, Francesca Bonifazi MD, Katia Vecchi and Sandra Cescutti.
Planning the treatment of CML was simple for almost one century during which the aim of the treatment was to control and contain the leukemic cell mass. This type of treatment has been called conventional.1-,3 It was based on ionizing radiation during the first half of the century and on selected cytotoxic agents, mainly busulfan and hydroxyurea (HU), during the second half. It provided a good quality of life but was not able to prevent or even delay substantially the progression from chronic phase (CP) to AP and BC or prolong survival. The recognition of the limits of conventional treatment led to some attempts at treatment intensification,4-,9 but before these attempts could be exploited in depth they were bypassed and obscured by the rapid development of allogeneic bone marrow transplantation (alloBMT).10-,12 CML was the main indication for alloBMT, and the main questions became how to submit more patients to alloBMT by increasing the age limits and resorting to unrelated marrow donors, how to control transplant related morbidity and mortality, and how to exploit the therapeutic potential of the allogeneic immune system.13-,15 To this point the story was clear and the opinion was univocal. CML was identified as a fatal disease with a median survival of 4 years and a 10-year survival of 5%. Conventional treatment was recognized to be useful but palliative. AlloBMT was the only option whenever it was possible. The improvement of alloBMT was always based on the principle that “more is better,” because it was felt that the conditioning regimen that was given for transplantation was necessary for the eradication of leukemia and that transplanting the marrow was necessary to survive the treatment. Later on it was shown that this was not the case and that the allogeneic immune system played a crucial role.16-,19 In the mean time, the principle that “more is better” was tested also in several other ways.4-,9,20-,23 These attempts, including non conventional polychemotherapy, splenectomy and autologous BMT (autoBMT) had and still have some rationale, but in 30 years we have not been able to provide evidence that any of these attempts was consistently and reproducibly better than single agent conventional chemotherapy. In this area the only evidence-based conclusions were that busulphan was better than ionizing radiations,24 that splenectomy was not useful,5,6 and that HU was better than busulphan.25 The acute leukemia like regimens4-,9 were never tested in a randomized study, and all the procedures of so-called autoBMT have been so far as fascinating as elusive, and it has been very difficult to plan a randomized study of autoBMT.8,9,20- 23 On the other hand when it became clear that the success of allo BMT depended more on graft versus leukemia reaction, the principle that more is better became less appealing and more difficult to pursue.
However, the introduction of α-interferon (IFN-α) in the mid-1980s definitively displaced conventional chemotherapy and began to limit the indications for alloBMT. The therapeutic effect of IFN-α was investigated in several noncontrolled studies,26-,33 which have provided valuable information and generated the prospective randomized studies that were required to establish the role of IFN-α in the treatment of CML.34-,38 The results of these studies (listed in Table 7 ) show the superiority of IFN-α over conventional single-agent chemotherapy in 4 of 5 studies.34-,37 Two studies, the German one35 and the Benelux one,38 failed to show a superiority of IFN-α over HU in term of survival, but when the German study was revisited using the criteria of the Italian study, the difference between IFN-α and HU became significant.39 A meta-analysis of all randomized studies provided conclusive evidence that IFN-α significantly prolonged survival in comparison to conventional single-agent chemotherapy.40
The major problem with IFN-α is how to increase the response rate, how to improve and to prolong the response and how to increase survival. Other important issues are the dose, the duration of the treatment, the side effects, the relationship with alloBMT, the possibility of a cure, the relationship with the prognostic risk score, and the cost. All these issues are important to identify properly the patients who are candidates for an IFN-α-based regimen as first-line treatment.
Improving Response and Survival
To improve the rate, the quality and the duration of response and to prolong survival, IFN-α has been combined with conventional cytotoxic agents, homoharringtonine, non-conventional chemotherapy and autoBMT,7-,9,22,23,41-,47 but only the combination with cytosine arabinoside (Ara-C) has been tested in controlled randomized studies. The rationale for the combination with Ara-C was provided by a study that was published in 1987, reporting a preferential inhibition by Ara-C of Ph+ colony-forming-units granulocyte macrophage (CFU-GM) by comparison with normal CFU-GM.48 The concentration of Ara-C that was required for the inhibition of Ph+ CFU-GM was so low (4.0 ± 0.9 ng/ml) that it could be obtained with a continuous infusion or a subcutaneous administration of a low dose of the drug.49 Therefore, a low dose of Ara-C (LDAC; 20mg/m2 daily) was combined with IFN-α, and the association was shown to be effective even in patients who were resistant to IFN-α or were in late CP or even in more advanced phases of the disease.41-,43,46,47 A national French study has tested IFN-α versus IFN-α + LDAC and has shown that the combination is better both for cytogenetic response and survival.45 A national Italian study, testing the same treatment with a very similar protocol, has confirmed that the combination is better for cytogenetic response, but it failed to confirm a difference in survival.50 The main results of the two studies are summarized in Table 8 . These results will be reviewed, because the differences may depend on differences in treatment protocol design, and will be meta-analysed to account for statistical variability. This meta-analysis is required to assess more precisely the benefit that is expected from the addition of LDAC to IFN-α.
IFN-α Dose
The dose of IFN-α that was scheduled in the main randomized and non-randomized studies of IFN-α is reported in Table 9 , ranging between 3 MIU (total dose) 3 times a week and 5 MIU/m2 daily. It should not be overlooked that in several studies the administered dose was lower and sometimes was not reported. In the two Italian studies34,50 and in the French study45 the ratio between the scheduled dose and the administered dose ranged between 0.70 and 0.80 during the first 2 years and declined thereafter. In other studies, this ratio was clearly lower. Based on available data it is impossible to show a dose-response relationship; it is expected that if such a relationship actually exists, it would be difficult to detect. It will be even more difficult to establish whether the dose can influence the duration of the response and the survival. A prospective, randomized, multinational (UK and the Netherlands) study of low dose versus standard dose is in progress, and an interim analysis is expected soon. In the Italian study of IFN-α versus IFN-α + LDAC,50 the patients who are in major cytogenetic remission after 3 years were randomized to continue IFN-α at maximum tolerated dose versus 3 MIU 3 times a week, but the results of this randomization will not be available for 3 years.
IFN-α Treatment Duration
The problem of treatment duration has two faces: 1) should treatment be discontinued in case of a poor response or loss of the response; and 2) should treatment be continued in case of a stable complete response, and for how long? Neither question has an evidence-based answer. If the response is poor or incomplete continuing the treatment can be of some benefit,34,36 but this small benefit must be weighed not only against the cost and the toxicity but also against the benefits that may be offered by other treatments. The current policy of the Italian group is to discontinue an IFN-α-based regimen if the hematologic response is not complete after 6 months and if the cytogenetic response is not clearly detectable (Ph negative metaphases > 35%) after 1 year and is not major (Ph negative metaphases > 65%) after 2 years. Treatment discontinuation is recommended when the cytogenetic response is lost. If the cytogenetic response is stable, we suggest that treatment should be continued in case of a partial response (Ph negative metaphases 66-99%) while it can be discontinued after 2 years if the cytogenetic response is complete and stable. A quantitative molecular assessment of residual disease may provide a better guide to treatment discontinuation.51- 54
IFN-α Side Effects: Tolerance
Tolerance is poor in the elderly, especially with reference to neurologic and psychiatric side effects, such as depression. Although there is no evidence supporting this conclusion, caution is recommended. While no drugs have been identified to limit the side effects of IFN-α, an effort has been made to modify IFN-α itself to make its administration easier and to minimize side effects. The pegylation of IFN-α provides a pharmacokinetic profile that allows for weekly injections and is expected to limit toxicity, efficacy being equal. A pegylated form of human recombinant IFN-α2b is currently tested in an international randomized study of CML, and the first analysis of this study is expected soon.
IFN-α Relationship with alloBMT
The possibility that prior treatment with IFN-α may affect negatively the outcome of alloBMT was raised by Beelen et al.55 The reason is not clear, but it is possible that IFN-α triggers or enhances GVHD by enhancing the expression of class I and II HLA molecules. However, other studies have not confirmed an adverse effect of prior IFN-α treatment. The data (listed in Table 10 ) suggest that only the administration of IFN-α immediately prior to alloBMT may have an adverse effect on transplant related mortality.60- 61 This problem will become less important when the treatment program of a patient is designed from the very beginning according to the risk of the disease, the probability of responding to treatment (for IFN-α) and the risk of dying from treatment (for alloBMT). Once the treatment is selected in such a way, the possible disadvantages of prescribing or proscribing IFN-α will be minimized.
IFN-α, Response and Prognostic Risk Score
IFN-α is superior to conventional single-agent chemotherapy irrespective of the risk,40 but the relationship between the risk and the response is important (Table 11 ) and has important therapeutic implications. Not only do more low risk patients respond to IFN-α but, in case of response, and response being equal, the absolute benefit is much higher for a low risk patient than for a high risk patient (Table 11). Therefore two selections are required for rational use of IFN-α. The first selection occurs at diagnosis and is based on the risk. The risk can be calculated either using the old Sokal's formulation63 or the new, IFN-α-adapted, Euro formulation64 (Table 12 ). Low risk cases, who account for about 50% of all CML cases, are good candidates for IFN-α. The second selection is made during treatment; a complete hematologic response at 6 months, a partial cytogenetic response at 12 months and a complete cytogenetic response at 2 years identify the patients who can obtain the maximum benefit from IFN-α treatment and can become long-term survivors.50,65
IFN-α and Cure of CML
This issue has been the object of a recent debate.51-,54 Treatment with IFN-α can induce a complete molecular remission only occasionally, and it is not known if such a complete remission is stable. However, IFN-α can induce a complete cytogenetic remission in 10 to 30% of cases, and the patients who achieve such a remission can become long-term survivors. In a European collaborative study of 317 patients who became complete cytogenetic responders, the projected 10-year survival is 72% and about 75% of the patients who are alive are still in continuous complete remission.66
IFN-α and the Cost of Treatment
Two studies have concluded that IFN-α is a cost-effective initial therapy for patients with CP CML67 and is superior to conventional single-agent chemotherapy in terms of quality-adjusted survival.68 However the estimated marginal cost-effectiveness of IFN-α was quite high, ranging from US $34,800 in one study67 to more than $50,000 in the other study,68 per quality-adjusted year of life saved. These estimates were influenced significantly by the cost of the drug and by the degree of tolerance and were based on prior clinical studies where the dose of IFN-α was set at 5 MIU/m2 daily and IFN-α was given to all the patients, irrespective of risk and response, until progression or death. Today the dose and the cost of IFN-α can be lower and selecting the right candidates decreases significantly the cost for those who are treated and avoids using the drug for those who do not benefit.
In summary, there is evidence that HU is better than busulfan;25 there is no evidence as yet in favor of non-conventional cytotoxic treatment, including autoBMT; and there is evidence in favor of IFN-α. Soon there will be more evidence concerning the correct use of IFN-α, including the dose, the pegylated preparation and the combination with LDAC. However, the major problem is that we have not yet been able to understand the molecular basis of the therapeutic activity of IFN-α. Lacking this basic information may make further progress difficult. Understanding the mechanisms of action of IFN-α and the mechanisms of resistance to IFN-α is likely to be more important than embarking on other clinical studies. We are approaching the end of the use of IFN-α as an empiric treatment of CML, but we are at the beginning of a era of molecularly targeted treatment in which IFN-α may have a useful role, provided that we know the molecular targets of IFN-α and the rationale for the combination of IFN-α with the new investigational agents.
The new wave of investigational agents (Table 13 ) is expected to grow quickly based on the rapid progress in the field of molecular genomics. Up to now the chief actor is a protein tyrosine kinase inhibitor that prevents phosphorylation from the BCR/ABL proteins and turns off BCR/ABL positive leukemic cells.69 The compound was synthesized in the Ciba-Geigy labs as CGP57148B, was developed by Novartis as STI571 and is now available as Gleevec (or Glivec). STI571 is felt to be the best first-line treatment of CML because of its molecular specificity, its in vitro activity and preliminary clinical data. Although expectations are high and have a sound biologic basis, the clinical data are not yet mature, although in less than 3 years STI571 has completed successfully phase 1 and 2 studies and is currently being tested in a phase 3 study versus IFN-α + LDAC. The efficacy of the drug is expected to be higher than that of IFN-α, because STI571 is active also in accelerated and sometimes in blastic phases of CML and also in cases that are not sensitive to IFN-α.70,71 The first interim analysis of the prospective study of STI571 versus IFN-α + LDAC will provide a comparative evaluation of the rapidity and the quality of hematologic response and of early cytogenetic response. Both responses are good short-term surrogates for survival in patients treated with IFN-α, but we do not know as yet if they will also be a good surrogate for STI571.
CML is born from the BCR/ABL gene but undergoes other genetic alterations that are only partially known and are responsible for progression. We know that any treatment of CML, including not only IFN-α but also alloBMT, is more effective in early CP than in late CP and loses efficacy after progression to AP and BC. STI571 is likely to perform better than other agents but may face the same problems. STI571 is targeted specifically against P210 and other BCR/ABL proteins. From the recent clinical experience it is already clear that blocking BCR/ABL proteins is not sufficient to turn off leukemia once leukemia has progressed.70,71 In other words, once leukemic cells have acquired other abnormalities they tend to become independent of the original oncogenic protein. That is why STI571 is less effective in AP and even less effective in BC. Administering STI571 in a early clinical phase may turn off leukemia so effectively that there would be no chance for the development of additional abnormalities. However, since the development of additional abnormalities may spread over a long period of time, it is reasonable to expect that a yet unpredictable number of cases will escape STI571, providing a rationale for the combination of tyrosine kinase inhibitors with other agents. The first agents to be combined with STI571 are likely to be those with an already proven efficacy and a different mechanism of action, like IFN-α and Ara-C. Phase 2 studies of Gleevec and pegylated IFN-α and of Gleevec and LDAC are ongoing in the UK, Italy, and the US to assess feasibility and tolerance. Moreover other experimental agents are coming (Table 13) that offer a promise of neutralizing or bypassing the sequence of the leukemogenic events initiated by BCR/ABL. One example is provided by the family of the farnesyl and geranyl-geranyl transferase inhibitors that block the activation of the Ras pathway downstream to BCR/ABL proteins by preventing Ras prenylation.72- 74
For clinicians and patients it is important to understand that the concept of gold-standard treatment is misleading. Treating CML will be easier than in the past, but treatment should not yet be regarded as a simple prescription of few well-tolerated pills. Treatment options are developing so quickly that treatment must remain experimental and must allow the rapid accumulation of better knowledge of the therapeutic and side effects of the agents that have been already approved, of those that are investigational, and of their combinations. CML is a relatively rare disease. If patients with CML are not enrolled in controlled prospective treatment protocols, any further progress will be slow and difficult.
IV. Stem Cell Transplantation for CML: Its Place in Treatment Algorithms—2001
John M. Goldman, DM, FRCP, FRCPath*
Department of Haematology, Imperial College School of Medicine, Hammersmith Hospital, Ducane Road, W12 0NN, London, United Kingdom
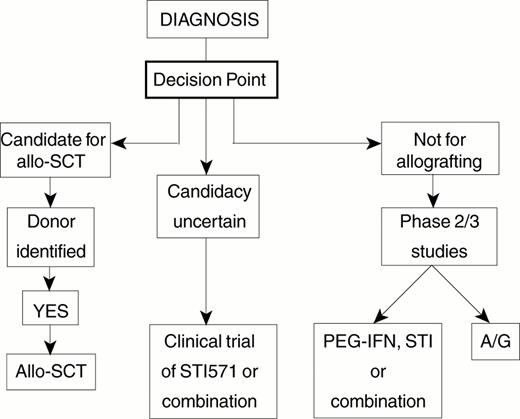
There is today general agreement that allogeneic stem cell transplantation (allo-SCT) can cure selected patients with CML1 and that cure depends on the contribution of a poorly defined ‘graft-versus-leukemia' effect.2 However, there is still great uncertainty concerning the optimal indications for allografting and there are differences of opinion about many of the details of the transplant procedure itself. Moreover the recent introduction of STI571, which is highly effective in the management of CML in the short term3 and may prove to prolong life in comparison with IFN-α, has greatly complicated the choice of primary therapy for newly diagnosed patients. This choice must be governed by consideration of the prognostic factors for survival for patients treated by allo-SCT and by consideration of what is known of prognostic factors for survival for patients treated by non-transplant techniques. These issues are reviewed below. I present a simplified algorithm that may help to guide decision-making for the individual patient; it may need to be adjusted quite soon in the light of experience with STI571 used alone and in combination with other agents.
Allogeneic Stem Cell Transplantation
Prognostic factors for survival
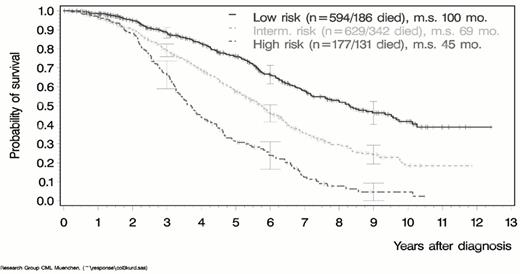
The decision whether to offer an individual patient the option of treatment by allo-SCT must be based in part on an assessment of the probability of success using the best available stem cell donor. Gratwohl et al4 have defined five principal prognostic factors for survival after allo-SCT on the basis of data submitted to the European Group for Blood and Marrow Transplantation (Table 14 ). They allocated a score of 0, 1 or 2 to each of the five factors in accordance with the degree to which the influence of that factor was favorable or unfavorable for a particular patient. Thus for each factor in a given transplant procedure a patient's total score could be 0 (most favorable) or 2 (least favorable). The aggregate prognostic score calculated in this way correlated well with actual survival (Figure 3 ). This approach is extremely useful for helping a clinician to make recommendations and the patient to decide whether or not to undergo allo-SCT. It must be conceded, however, that many of the factors to be considered in such decision-making are ‘empirical.' There is a complete absence of prospective studies addressing the results of allo-SCT in CML—a point stressed in the report from the American Society of Hematology Committee on Practice Guidelines.5
Defining alternative donors
If allo-SCT with HLA-identical sibling donors can cure selected patients with CML, it is logical to consider the role of alternative donors for allografting patients who lack suitable siblings. Such alternative donors include phenotypically HLA-matched or near-matched family members, phenotypically matched unrelated volunteers and phenotypically matched donors of cord blood stem cells. The definition of HLA-matching is more complicated for alternative donors than for siblings. It depends usually on the use of DNA-based techniques to characterize genes of both class I and class II categories (i.e. HLA A, B, C, DR, DQ and DP). Van Rood et al have suggested a system for categorizing the degree of match between patient and donor.6 When more than one donor appears suitable for an individual patient the assay of alloreactive cytotoxic T-lymphocyte precursors in the blood of the prospective donor can aid donor selection.7 In general the results of allografting with stem cells from alternative donors are not as good as results of allografting comparable patients with stem cells from HLA-identical siblings.8 This may be due in part to the frequency with which CMV is reactivated and causes clinical disease in seropositive patients, more often severe or fatal than after sibling transplant recipients.9 Relatively good results were reported in 1998 from the Seattle group.10 They showed that actuarial survival at 5 years was 57%. However, the incidence of GVHD appeared to be higher than what might have been expected following sibling transplants. A small number of patients, both children and adults, have been treated successfully by allo-SCT using umbilical cord blood cells from unrelated donors,11 but this approach is not yet widely established.
Standard or reduced intensity conditioning regimens?
It has been conventional since the 1980s to ‘condition' the CML patient with high dose chemoradiotherapy (usually cyclophosphamide and total body irradiation) or chemotherapy alone (usually busulfan and cyclophosphamide) before stem cell transfusion to order to provide adequate immune suppression and to maximize leukemia cell kill. The recognition that eradication of leukemia after allo-SCT depends to a large degree on a lymphocyte-mediated graft-versus-leukemia effect1 has led in recent years to the concept that low dose conditioning designed predominantly to tolerize the patient to lymphoid tissues of the donor may be an attractive approach to minimizing the toxicity of the transplant procedure and perhaps also reducing the severity of GVHD.12,13 Persisting leukemia can then be eliminated by transfusion of donor lymphocytes at a later date. The range of different ‘reduced intensity conditioning' regimens (also known as ‘nonmyeloablative transplants' or ‘mini-allografts') is extensive (Table 15 ). A number of CML patients treated by reduced intensity conditioning regimens have indeed achieved complete donor chimerism and are negative when studied by RT-PCR techniques. (See the Section III.)
Blood or marrow as source of stem cells?
The introduction into clinical practice of G-CSF in the late 1980s made it possible to collect sufficient numbers of pluripotential stem cells (or more precisely CD34+ cells) from the peripheral blood of selected patients to allow engraftment in the autologous setting. Subsequently it became clear that allografts could also be performed with stem cells or CD34+ cells mobilized into the peripheral blood of normal donors. In general, collections of peripheral blood contain substantially more CD34+ cells and perhaps 10 times more lymphocytes than comparable collections of bone marrow. Recovery of neutrophil and platelet numbers is more rapid in recipients of blood-derived allogeneic stem cells than in those receiving marrow-derived stem cells.14 The relapse rate after allografting for CML seems to be lower in patients who receive blood-derived stem cells. Conversely the incidence and severity of chronic GVHD seem to be greater in recipients of blood-derived stem cells.15 A number of prospective studies comparing clinical results of using blood or marrow stem cells are still in progress. For the present it seems reasonable to use marrow stem cells for the patient undergoing an allograft in chronic phase.14 One might speculate that for patients undergoing an allograft for CML in advanced phase, the use of peripheral blood stem cells would be preferable on account of the presumed increased potential of these cells for mediating a graft-versus-leukemia effect.
Prevention of graft-versus-host disease
Depletion of T-lymphocytes from the allogeneic marrow or blood is a highly effective method of abrogating GVHD. Such depletion may be achieved by incubating blood or marrow cells with an anti-T or antilymphocyte monoclonal antibody or by intravenous administration of the antibody to the patient at the time of the transplant. T-cell depletion has, however, a number of disadvantages. It increases the risk of non-engraftment, it delays immune reconstitution, and most importantly, increases the risk of relapse.16 This last complication can be counteracted by prophylactic or pre-emptive administration of donor-derived T-lymphocytes starting soon after the transplant procedure. Clinical results of one published study suggest that this approach may indeed be valuable in the management of CML.17
Recognition and management of relapse
The incidence of relapse within the first 5 years after allo-SCT for CML in chronic phase ranges between 0 and 30% and depends in part on the details of the transplant procedure and the method employed for preventing GVHD. As mentioned above, T-cell depletion is associated with a greatly increased risk of relapse. Similarly patients who receive cyclosporine plus methotrexate post-transplant may have a higher incidence of relapse than those who receive cyclosporine alone but this does not impact on survival. Patients allografted in advanced phase CML have a higher incidence of relapse than those transplanted in chronic phase. The majority of relapses occur within the first 4 years post-allo-SCT, but patients remain at risk of relapse for much longer, possibly indefinitely.
When it occurs, relapse usually proceeds in an orderly manner, starting with molecular evidence of disease and progressing thereafter to cytogenetic relapse and eventually to hematological relapse. The whole progression may take months or years. Occasionally molecular or cytogenetic relapses reverse spontaneously. On rare occasions patients apparently in complete remission relapse directly to blastic phase disease.18
For these reasons it seems logical to monitor patients indefinitely by molecular methods (e.g. RT-PCR for BCR-ABL transcripts present in the blood) or possibly by fluorescence in situ hybridization of peripheral blood neutrophils. Patients who satisfy current criteria for relapse should then be considered for further therapy. Currently the choice of treatments for relapse includes administration of STI571 or IFN-α, use of donor lymphocyte infusions (DLI) or a second allo-SCT using the same donor. Until further experience is gained with the use of STI571 in this setting, the best approach is probably to administer T-lymphocytes collected from the original transplant donor. Whereas originally such DLI were given as a single bulk dose, this method was associated with a substantial risk of inducing GVHD or marrow aplasia.19,20 The modified approached introduced by the group in Sloan-Kettering Cancer Center (New York) whereby DLI were administered on an escalating dose schedule induces complete remission with equal reliability and with much reduced risks of GVHD and graft failure.21,22 This therefore is probably the optimal approach to the management of relapse after allo-SCT, until the role of STI571 is more fully evaluated.
Overall contribution of allografting to cure of CML
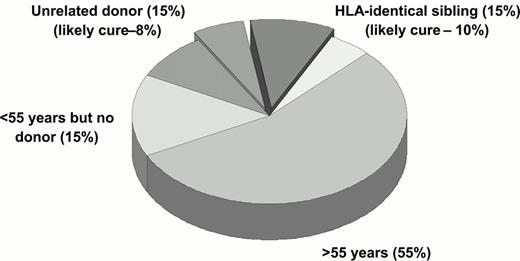
If one considers a typical cohort of newly diagnosed patients with CML in the western world, only 40-50% at most will be young enough to be considered for a standard allo-SCT. Of these, about 30% will prove to have HLA-identical sibling donors, although this figure depends of course of average family size in the population under study. If all patients with sibling donor proceed to transplant, one may anticipate a cure rate of the order of 65%. If then one searches for an unrelated donor for ‘younger' patients lacking matched siblings, one may find an acceptable donor for at most half of these; this figure will of course depend of the patient's ethnic background and the criteria used to define an acceptable donor. Of those who proceed to allo-SCT with unrelated donors one may at present estimate a cure rate of 50%. These rough calculations means that one may be able to offer a transplant to 22% of the original patient cohort, and perhaps 18% may expect to be cured (Figure 4 ).
Conventional (Non-Transplant) Treatment of CML
Because the transformation of a patient's disease from chronic phase to an advanced phase appears to be spontaneous and the precise timing is therefore difficult to predict, it has in the past been assumed that the major determinant of transformation is a series of chance events that cannot be quantitated. This view is almost certainly erroneous since it is intuitively more likely that the disease is intrinsically different in different patients—a view gaining support from recent molecular and clinical observations.
Molecular heterogeneity at diagnosis
The mean length of telomeres derived from the chromosomes of leukemia cells is significantly shorter than that of telomeres from corresponding normal cells and that the rate of telomere shortening is correlated with the duration of chronic phase.23,24 About 70% of patients express a (reciprocal) ABL-BCR gene present on the derivative 9q+ that results from the Philadelphia translocation25 and chromosomal material in the region of the ABL-BCR gene is sometimes deleted.26 It was reported recently that about 20% of patients have such deletions involving the ABL-BCR gene and variable quantities on DNA from the upstream ABL and downstream BCR sequences, whereas no such deletions can be detected in the majority of patients.27,28 The patients with such deletions had a very significantly shorter survival than those without deletions.28,29 These two observations suggest that molecular features that can be characterized at the time of diagnosis may help to predict the duration of survival in individual patients. It is likely that such ‘molecular staging' will be further refined in the near future.
Clinical heterogeneity at diagnosis
The first clinically useful attempt to assess duration of survival at the time of diagnosis was made by Sokal et al, who devised a mathematical equation incorporating the patient's age, spleen size, percentage of blast cells in the blood and platelet count to produce a risk index for an individual patient.30 A given cohort of patients could thereby be divided into three categories with relatively short, intermediate and long median survivals. The Sokal scoring system was based on study of patients treated predominantly with busulfan. Recently Hasford et al. collected data on a large series of patients treated with IFN-α and used a similar approach to devise a scoring system (the EURO scoring system) that takes into account the same factors that were used by Sokal and in addition the basophil and eosinophil counts at diagnosis31 (see details in Table 12). This scoring system appears to give a better separation of sub-groups than the Sokal system (Figure 5 ). It is likely that these clinical scoring systems will need to be updated when survival data for patients treated with STI571 become available.
Clinical heterogeneity in response to treatment
The rate of leukocyte count doubling and the quantity of busulfan necessary to control the leukocyte count differ among patients.32 The amount of busulfan administered to individual patients in the first year after diagnosis is inversely related to the duration of chronic phase.33 It is now known that the speed with which the leukocyte count is restored to normal by treatment with IFN-α is associated with the probability of achieving a cytogenetic response34 and the attainment of cytogenetic response itself predicts for long survival. The various observations could be due to differences in drug metabolism or differences in other aspects of host response to treatment, but more likely reflect an intrinsic heterogeneity of the leukemia in the patients under study. Such hematologic or cytogenetic responses to treatment can therefore be exploited for deciding whether or not a given patient should be offered treatment by allo-SCT.
Decision-making for the Newly Diagnosed Patient
The decision how best to treat the patient newly diagnosed with CML in chronic phase now poses a considerable challenge for patient and hematologist. Lee and colleagues have used the technology of decision analysis to compare the value to the patient of transplant and non-transplant therapies where a well-matched unrelated donor35 or an HLA-identical sibling is available.36 These studies were however completed before the advent of STI571. They concluded that for a newly diagnosed patient with an unrelated donor transplantation performed within the first year of diagnosis provided the greatest quality-adjusted ‘expected survival,' although the benefit decreased with increasing age. For example for a 35 year old patient with intermediate prognosis (Sokal) CML, transplantation within the first year resulted in 5.3 more discounted quality-adjusted years of life expectancy than no transplant. For a patient with a sibling donor or for a patient with high or intermediate risk CML with a sibling donor or a well-matched unrelated donor, they recommended transplant up to the age of 50 years. The calculations are based on a number of assumptions that seem reasonable but are not necessarily always valid.
An alternative way of addressing the issue is to consider and to discuss with the patient two contrasting approaches to his or her initial management:37
Option 1.
One approach is to recommend that every newly diagnosed patient should receive initial treatment with STI571, IFN-α or a suitable combination, ideally in the context of a clinical trial. Patients in the appropriate age range who ‘fail' this trial of therapy and who have HLA-identical siblings or HLA-matched alternative donors would then be offered an allo-SCT. The problem with this approach relates in part to the difficulty in defining a meaningful ‘response' or ‘failure' to STI571 (or the combination) and in part to the risk that the delay of SCT might permit the disease to progress. Although it seems unlikely that prior treatment with STI571 would adversely influence the results of a subsequent allograft, it is known that a longer interval from diagnosis to transplant increases the risk of transplant-related mortality and this ‘delay to transplant' might apply independently of any particular prior therapy.
Option 2.
The second approach would be to try to decide within a few weeks of diagnosis about the advisability of transplant for a given patient (Figure 6 ). For this purpose one must set an arbitrary level of risk of transplant-related mortality (TRM) above which an ‘early' transplant would not automatically be recommended and another level of risk of TRM above which a transplant would not be undertaken in any circumstance. Newly diagnosed patients would likely fit into one of three categories: (a) patients deemed eligible for an early transplant; (b) patients for whom a transplant is thought to carry a somewhat higher risk; and (c) patients for whom a transplant could not reasonably be considered.
On this scheme patients in category (a) would proceed to transplant soon after diagnosis. Taking into account the various factors that impact on transplant-related mortality, one might assume that a patient under the age of 45 with an HLA-identical sibling donor or a patient under the age of 35 years with a molecularly matched alternative donor might be a good candidate for an early allograft. One could speculate that these upper age limits for transplant might be reduced by 10 years for patients in the Hasford good risk category. For patients in category (b) it would be reasonable to offer a trial of therapy with STI571 or IFN-α and then to assess the response after 6 or 12 months. Patients who failed to achieve and maintain a reasonable degree of cytogenetic improvement would then be offered an allograft. Patients in category (c) might be offered primary treatment with STI571 or a combination of STI571 with IFN-α or cytarabine.
At the time of writing we believe the best advice for the newly diagnosed patient is Option 2. Relatively young patients with newly diagnosed CML who have HLA-identical sibling donors or molecularly HLA-matched unrelated donors should receive a conventional transplant within the first year of diagnosis (Table 16 ). The role of reduced intensity conditioning allo-SCT cannot yet be reliably assessed.
Although at present the curative potential of STI571 is unknown, it is entirely possible that STI571 alone, in combination with other agents or in conjunction with a novel approach to immunotherapy, could eradicate CML. As clinical trials that test the various combinations are now in progress, patients not wanting to undergo allo-SCT should be encouraged to enroll in one of these studies. If one or two years from now it becomes clear that few if any of the patients responding to STI571 progress to advanced phase disease and that the cytogenetic responses achieved are durable, then Option 1 involving initial treatment with STI571 (or an STI571-containing combination) will become the treatment of choice. This view will gain additional support if some of the patients who achieve complete cytogenetic responses also achieve durable molecular remissions.
Hematologic and cytogenetic response in phase II studies.
| (patients with confirmed diagnosis) . | Study 0110 Chronic phase, Interferon failure (n=454) . | Study 0109 Accelerated phase (n=181) . | Study 0102 Myeloid blast crisis (n=229) . |
|---|---|---|---|
| Hematologic response | 415 (91%) | 125 (69%) | 66 (29%) |
| Complete hematologic response | 415 (91%) | 61 (34%) | 16 (7%) |
| No evidence of leukemia | -22 (12%) | 7 (3%) | |
| Return to chronic phase | -42 (23%) | 43 (19%) | |
| Major cytogenetic response | 248 (55%) | 43 (24%) | 36 (16%) |
| Complete | 164 (36%) | 30 (17%) | 15 (7%) |
| Partial | 84 (19%) | 13 (7%) | 21 (9%) |
| (patients with confirmed diagnosis) . | Study 0110 Chronic phase, Interferon failure (n=454) . | Study 0109 Accelerated phase (n=181) . | Study 0102 Myeloid blast crisis (n=229) . |
|---|---|---|---|
| Hematologic response | 415 (91%) | 125 (69%) | 66 (29%) |
| Complete hematologic response | 415 (91%) | 61 (34%) | 16 (7%) |
| No evidence of leukemia | -22 (12%) | 7 (3%) | |
| Return to chronic phase | -42 (23%) | 43 (19%) | |
| Major cytogenetic response | 248 (55%) | 43 (24%) | 36 (16%) |
| Complete | 164 (36%) | 30 (17%) | 15 (7%) |
| Partial | 84 (19%) | 13 (7%) | 21 (9%) |
Clinical questions to be addressed in clinical trials.
| Abbreviations: BMT, bone marrow transplant; IFN, interferon; ABMT, allogeneic BMT; DLI, donor lymphocye infusions; Ara-C, cytarabine | |
| Frontline treatment | Phase III study ongoing |
| Combination | Feasibility of combination with IFN or Peg-IFN |
| Feasibility of combination with low-dose Ara-C | |
| Feasibility of combination with chemotherapy | |
| BMT | Safety of BMT after STI571 therapy |
| Feasibility of prophylactic treatment after BMT | |
| Treatment of relapses post-BMT | |
| Feasibility of combination with DLI | |
| ABMT | In vivo purging |
| Maintenance therapy after ABMT | |
| Abbreviations: BMT, bone marrow transplant; IFN, interferon; ABMT, allogeneic BMT; DLI, donor lymphocye infusions; Ara-C, cytarabine | |
| Frontline treatment | Phase III study ongoing |
| Combination | Feasibility of combination with IFN or Peg-IFN |
| Feasibility of combination with low-dose Ara-C | |
| Feasibility of combination with chemotherapy | |
| BMT | Safety of BMT after STI571 therapy |
| Feasibility of prophylactic treatment after BMT | |
| Treatment of relapses post-BMT | |
| Feasibility of combination with DLI | |
| ABMT | In vivo purging |
| Maintenance therapy after ABMT | |
Adverse experiences reported in clinical trials of STI571 (≥ 10% of all patients in any trial).(1)
| . | Myeloid blast crisis . | Accelerated phase . | Chronic phase, IFN failure . | |||
|---|---|---|---|---|---|---|
| . | n= 260 . | n = 235 . | n = 532 . | |||
| . | 600 mg n=223 . | 600 mg n = 158 . | . | |||
| . | 400 mg n=37 . | 400 mg n = 77 . | 400 mg . | |||
| . | (%) . | (%) . | (%) . | |||
| Preferred term . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . |
| (1) All adverse events occurring in ≥ 10% of patients are listed regardless of suspected relationship to treatment. | ||||||
| (2) Other fluid retention events include pleural effusion, ascites, pulmonary edema, pericardial effusion, anasarca, edema aggravated, and fluid retention not otherwise specified. | ||||||
| Abbreviations: CNS, central nervous system | ||||||
| Nausea | 69 | 3 | 71 | 5 | 58 | 2 |
| Fluid retention | 69 | 10 | 71 | 6 | 56 | 2 |
| - Superficial edemas | 65 | 5 | 69 | 3 | 55 | 1 |
| - Other fluid retention events(2) | 16 | 6 | 9 | 3 | 3 | 0.6 |
| Muscle cramps | 26 | 0.4 | 37 | 0.4 | 50 | 1 |
| Diarrhea | 41 | 3 | 53 | 4 | 37 | 1 |
| Vomiting | 52 | 4 | 55 | 3 | 30 | 0.9 |
| Hemorrhage | 48 | 17 | 39 | 8 | 16 | 0.8 |
| - CNS hemorrhage | 5 | 3 | 1 | 0.4 | 0.6 | 0.6 |
| - Gastrointestinal hemorrhage | 5 | 3 | 3 | 1 | 0.4 | 0 |
| Musculoskeletal pain | 42 | 9 | 43 | 9 | 32 | 1 |
| Skin rash | 34 | 4 | 43 | 5 | 39 | 3 |
| Headache | 26 | 4 | 29 | 2 | 30 | 0.2 |
| Fatigue | 28 | 3 | 36 | 3 | 31 | 0.6 |
| Arthralgia | 24 | 4 | 29 | 6 | 30 | 0.6 |
| Dyspepsia | 10 | 0 | 20 | 0 | 21 | 0 |
| Myalgia | 8 | 0 | 20 | 2 | 21 | 0.2 |
| Weight increased | 5 | 0.8 | 11 | 2 | 24 | 4 |
| Pyrexia | 40 | 7 | 37 | 8 | 15 | 1 |
| Abdominal pain | 26 | 5 | 28 | 2 | 23 | 0.6 |
| Cough | 13 | 0.8 | 23 | 0.9 | 12 | 0 |
| Dyspnea | 13 | 4 | 18 | 6 | 6 | 0.2 |
| Anorexia | 12 | 2 | 14 | 2 | 4 | 0 |
| Constipation | 15 | 2 | 14 | 0.9 | 5 | 0.2 |
| Nasopharingitis | 8 | 0 | 14 | 0 | 14 | 0.2 |
| Night sweats | 12 | 0.8 | 13 | 1 | 8 | 0.2 |
| Pruritus | 8 | 1 | 11 | 0.9 | 10 | 0.8 |
| Epistaxis | 13 | 3 | 11 | 0 | 4 | 0 |
| Hypokalemia | 12 | 3 | 8 | 2 | 3 | 0 |
| Petechiae | 10 | 2 | 4 | 0.9 | 0.9 | 0 |
| Pneumonia | 10 | 6 | 8 | 6 | 2 | 0.4 |
| Weakness | 12 | 3 | 9 | 3 | 5 | 0.2 |
| Upper respiratory tract infection | 3 | 0 | 8 | 0.4 | 12 | 0 |
| Dizziness | 10 | 0.4 | 11 | 0 | 10 | 0 |
| Insomnia | 10 | 0 | 11 | 0 | 11 | 0.2 |
| Sore throat | 8 | 0 | 11 | 0 | 10 | 0 |
| Ecchymosis | 10 | 0.4 | 6 | 0.9 | 1 | 0 |
| Rigors | 8 | 0 | 10 | 0.4 | 7 | 0 |
| . | Myeloid blast crisis . | Accelerated phase . | Chronic phase, IFN failure . | |||
|---|---|---|---|---|---|---|
| . | n= 260 . | n = 235 . | n = 532 . | |||
| . | 600 mg n=223 . | 600 mg n = 158 . | . | |||
| . | 400 mg n=37 . | 400 mg n = 77 . | 400 mg . | |||
| . | (%) . | (%) . | (%) . | |||
| Preferred term . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . | All grades . | Grade 3/4 . |
| (1) All adverse events occurring in ≥ 10% of patients are listed regardless of suspected relationship to treatment. | ||||||
| (2) Other fluid retention events include pleural effusion, ascites, pulmonary edema, pericardial effusion, anasarca, edema aggravated, and fluid retention not otherwise specified. | ||||||
| Abbreviations: CNS, central nervous system | ||||||
| Nausea | 69 | 3 | 71 | 5 | 58 | 2 |
| Fluid retention | 69 | 10 | 71 | 6 | 56 | 2 |
| - Superficial edemas | 65 | 5 | 69 | 3 | 55 | 1 |
| - Other fluid retention events(2) | 16 | 6 | 9 | 3 | 3 | 0.6 |
| Muscle cramps | 26 | 0.4 | 37 | 0.4 | 50 | 1 |
| Diarrhea | 41 | 3 | 53 | 4 | 37 | 1 |
| Vomiting | 52 | 4 | 55 | 3 | 30 | 0.9 |
| Hemorrhage | 48 | 17 | 39 | 8 | 16 | 0.8 |
| - CNS hemorrhage | 5 | 3 | 1 | 0.4 | 0.6 | 0.6 |
| - Gastrointestinal hemorrhage | 5 | 3 | 3 | 1 | 0.4 | 0 |
| Musculoskeletal pain | 42 | 9 | 43 | 9 | 32 | 1 |
| Skin rash | 34 | 4 | 43 | 5 | 39 | 3 |
| Headache | 26 | 4 | 29 | 2 | 30 | 0.2 |
| Fatigue | 28 | 3 | 36 | 3 | 31 | 0.6 |
| Arthralgia | 24 | 4 | 29 | 6 | 30 | 0.6 |
| Dyspepsia | 10 | 0 | 20 | 0 | 21 | 0 |
| Myalgia | 8 | 0 | 20 | 2 | 21 | 0.2 |
| Weight increased | 5 | 0.8 | 11 | 2 | 24 | 4 |
| Pyrexia | 40 | 7 | 37 | 8 | 15 | 1 |
| Abdominal pain | 26 | 5 | 28 | 2 | 23 | 0.6 |
| Cough | 13 | 0.8 | 23 | 0.9 | 12 | 0 |
| Dyspnea | 13 | 4 | 18 | 6 | 6 | 0.2 |
| Anorexia | 12 | 2 | 14 | 2 | 4 | 0 |
| Constipation | 15 | 2 | 14 | 0.9 | 5 | 0.2 |
| Nasopharingitis | 8 | 0 | 14 | 0 | 14 | 0.2 |
| Night sweats | 12 | 0.8 | 13 | 1 | 8 | 0.2 |
| Pruritus | 8 | 1 | 11 | 0.9 | 10 | 0.8 |
| Epistaxis | 13 | 3 | 11 | 0 | 4 | 0 |
| Hypokalemia | 12 | 3 | 8 | 2 | 3 | 0 |
| Petechiae | 10 | 2 | 4 | 0.9 | 0.9 | 0 |
| Pneumonia | 10 | 6 | 8 | 6 | 2 | 0.4 |
| Weakness | 12 | 3 | 9 | 3 | 5 | 0.2 |
| Upper respiratory tract infection | 3 | 0 | 8 | 0.4 | 12 | 0 |
| Dizziness | 10 | 0.4 | 11 | 0 | 10 | 0 |
| Insomnia | 10 | 0 | 11 | 0 | 11 | 0.2 |
| Sore throat | 8 | 0 | 11 | 0 | 10 | 0 |
| Ecchymosis | 10 | 0.4 | 6 | 0.9 | 1 | 0 |
| Rigors | 8 | 0 | 10 | 0.4 | 7 | 0 |
Lab abnormalities.
| . | Myeloid blast crisis . | Accelerated phase . | Chronic phase, interferon failure . | |||
|---|---|---|---|---|---|---|
| . | n = 260 . | n = 235 . | n = 532 . | |||
| . | (%) . | (%) . | (%) . | |||
| . | Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . |
| CTC grades: neutropenia (grade 3 ≥ 0.5 – 1.0 x 109/L, grade 4 < 0.5 x 109/L), thrombocytopenia (grade 3 ≥ 10 – 50 x 109/L, grade 4 < 10 x 109/L), anemia (hemoglobin ≥ 65 – 80 g/L, grade 4 < 65 g/L), elevated creatinine (grade 3 > 3-6 x upper limit normal range (ULN), grade 4 > 6 x ULN), elevated bilirubin (grade 3 > 3-10 x ULN, grade 4 > 10 x ULN), elevated alkaline phosphatase (grade 3 > 5-20 x ULN, grade 4 > 20 x ULN), elevated SGOT or SGPT (grade 3 > 5-20 x ULN, grade 4 > 20 x ULN). According to protocol, treatment was interrupted for > Grade 3 or neutropenia or thrombocytopenia in chronic phase patients. Guidelines for accelerated and blast patients were as noted in the text. | ||||||
| Hematology parameters | ||||||
| • Neutropenia | 16 | 47 | 23 | 35 | 26 | 8 |
| • Thrombocytopenia | 27 | 33 | 31 | 12 | 17 | <1 |
| • Anemia | 40 | 11 | 33 | 6 | 5 | <1 |
| Biochemistry parameters | ||||||
| • Elevated creatinine | 1.2 | 0 | 1.3 | 0 | 0.2 | 0 |
| • Elevated bilirubin | 3.5 | 0 | 1.7 | 0 | 0.6 | 0 |
| • Elevated alkaline phosphatase | 4.6 | 0 | 5.1 | 0.4 | 0.2 | 0 |
| • Elevated SGOT (AST) | 1.9 | 0 | 2.1 | 0 | 1.7 | 0 |
| • Elevated SGPT (ALT) | 2.3 | 0.4 | 3.0 | 0 | 1.7 | 0 |
| . | Myeloid blast crisis . | Accelerated phase . | Chronic phase, interferon failure . | |||
|---|---|---|---|---|---|---|
| . | n = 260 . | n = 235 . | n = 532 . | |||
| . | (%) . | (%) . | (%) . | |||
| . | Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . |
| CTC grades: neutropenia (grade 3 ≥ 0.5 – 1.0 x 109/L, grade 4 < 0.5 x 109/L), thrombocytopenia (grade 3 ≥ 10 – 50 x 109/L, grade 4 < 10 x 109/L), anemia (hemoglobin ≥ 65 – 80 g/L, grade 4 < 65 g/L), elevated creatinine (grade 3 > 3-6 x upper limit normal range (ULN), grade 4 > 6 x ULN), elevated bilirubin (grade 3 > 3-10 x ULN, grade 4 > 10 x ULN), elevated alkaline phosphatase (grade 3 > 5-20 x ULN, grade 4 > 20 x ULN), elevated SGOT or SGPT (grade 3 > 5-20 x ULN, grade 4 > 20 x ULN). According to protocol, treatment was interrupted for > Grade 3 or neutropenia or thrombocytopenia in chronic phase patients. Guidelines for accelerated and blast patients were as noted in the text. | ||||||
| Hematology parameters | ||||||
| • Neutropenia | 16 | 47 | 23 | 35 | 26 | 8 |
| • Thrombocytopenia | 27 | 33 | 31 | 12 | 17 | <1 |
| • Anemia | 40 | 11 | 33 | 6 | 5 | <1 |
| Biochemistry parameters | ||||||
| • Elevated creatinine | 1.2 | 0 | 1.3 | 0 | 0.2 | 0 |
| • Elevated bilirubin | 3.5 | 0 | 1.7 | 0 | 0.6 | 0 |
| • Elevated alkaline phosphatase | 4.6 | 0 | 5.1 | 0.4 | 0.2 | 0 |
| • Elevated SGOT (AST) | 1.9 | 0 | 2.1 | 0 | 1.7 | 0 |
| • Elevated SGPT (ALT) | 2.3 | 0.4 | 3.0 | 0 | 1.7 | 0 |
Dose-response to STI571.
| Dose of STI571 . | Complete Hematologic Response . | Marrow Response . | Cytogenetic Response . |
|---|---|---|---|
| Data are from the phase I clinical trial of STI571 in chronic phase CML patients who failed therapy with interferon.3 Marrow response means return of marrow morphology to normal. Cytogenetic response requires less than 65% Ph positive metaphases. | |||
| 200 mg | 3/9 (33%) | 1/9 (11%) | 1/9 (11%) |
| 250 mg | 4/7 (57%) | 2/7 (28%) | 1/7 (14%) |
| > 300 mg | 53/54 (98%) | > 80% | 29/54 (53%) |
| Dose of STI571 . | Complete Hematologic Response . | Marrow Response . | Cytogenetic Response . |
|---|---|---|---|
| Data are from the phase I clinical trial of STI571 in chronic phase CML patients who failed therapy with interferon.3 Marrow response means return of marrow morphology to normal. Cytogenetic response requires less than 65% Ph positive metaphases. | |||
| 200 mg | 3/9 (33%) | 1/9 (11%) | 1/9 (11%) |
| 250 mg | 4/7 (57%) | 2/7 (28%) | 1/7 (14%) |
| > 300 mg | 53/54 (98%) | > 80% | 29/54 (53%) |
CYP3A4/5 inducers and inhibitors.
| CYP3A3/4 Inducers . | CYP3A3/4 Inhibitors . | . |
|---|---|---|
| Adapted from Cytochrome P-450 Enzymes and Drug metabolism. 13 | ||
| Carbamazepine | Amiodarone | Metronidazole |
| Dexamethasone | Anastrozole | Mibefradil |
| Ethosuximide | Azithromycin | Miconazole (moderate) |
| Glucocorticoids | Cannabinoids | Nefazodone |
| Griseofulvin | Cimetidine | Nelfinavir |
| Nafcillin | Clarithromycin | Nevirapine |
| Nelfinavir | Clotrimazole | Norfloxacin |
| Nevirapine | Cyclosporine | Norfluoxetine |
| Oxcarbazepine | Danazol | Omeprazole (weak) |
| Phenobarbital | Delavirdine | Oxiconazole |
| Phenylbutazone | Dexamethasone | Paroxetine (weak) |
| Phenytoin | Diethyldithiocarbamate | Propoxyphene |
| Primidone | Diltiazem | Quinidine |
| Progesterone | Dirithromycin | Quinine |
| Rifabutin | Disulfiram | Quinupristin and |
| Rifampin | Entacapone (high dose) | dalfopristin |
| Rofecoxib (mild) | Erythromycin | Ranitidine |
| St John's wort | Ethinyl estradiol | Ritonavir |
| Sulfadimidine | Fluconazole (weak) | Saquinavir |
| Sulfinpyrazone | Fluoxetine | Sertindole |
| Troglitazone | Fluvoxamine | Sertraline |
| Gestodene | Troglitazone | |
| Grapefruit juice | Troleandomycin | |
| Indinavir | Valproic acid (weak) | |
| Isoniazid | Verapamil | |
| Itraconazole | Zafirlukast | |
| Ketoconazole | Zileuton | |
| CYP3A3/4 Inducers . | CYP3A3/4 Inhibitors . | . |
|---|---|---|
| Adapted from Cytochrome P-450 Enzymes and Drug metabolism. 13 | ||
| Carbamazepine | Amiodarone | Metronidazole |
| Dexamethasone | Anastrozole | Mibefradil |
| Ethosuximide | Azithromycin | Miconazole (moderate) |
| Glucocorticoids | Cannabinoids | Nefazodone |
| Griseofulvin | Cimetidine | Nelfinavir |
| Nafcillin | Clarithromycin | Nevirapine |
| Nelfinavir | Clotrimazole | Norfloxacin |
| Nevirapine | Cyclosporine | Norfluoxetine |
| Oxcarbazepine | Danazol | Omeprazole (weak) |
| Phenobarbital | Delavirdine | Oxiconazole |
| Phenylbutazone | Dexamethasone | Paroxetine (weak) |
| Phenytoin | Diethyldithiocarbamate | Propoxyphene |
| Primidone | Diltiazem | Quinidine |
| Progesterone | Dirithromycin | Quinine |
| Rifabutin | Disulfiram | Quinupristin and |
| Rifampin | Entacapone (high dose) | dalfopristin |
| Rofecoxib (mild) | Erythromycin | Ranitidine |
| St John's wort | Ethinyl estradiol | Ritonavir |
| Sulfadimidine | Fluconazole (weak) | Saquinavir |
| Sulfinpyrazone | Fluoxetine | Sertindole |
| Troglitazone | Fluvoxamine | Sertraline |
| Gestodene | Troglitazone | |
| Grapefruit juice | Troleandomycin | |
| Indinavir | Valproic acid (weak) | |
| Isoniazid | Verapamil | |
| Itraconazole | Zafirlukast | |
| Ketoconazole | Zileuton | |
Results of the five randomized studies of α-interferon (IFN-α) versus single-agent conventional chemotherapy and of their meta-analysis. For cytogenetic response all the differences are significant. For survival the differences are significant with the exception of the German study (IFN-α vs hydroxyurea [HU]) (Hehlmann et al, 1994) and of the Benelux study (IFN-α vs. HU). In the Benelux study the scheduled dose of IFN-α was 3 MIU five times a week.
| . | No. of cases . | . | Major cytogenetic response . | 5-year survival . | |||
|---|---|---|---|---|---|---|---|
| . | IFN-α . | HU/BUS . | IFN-α MIU/day . | IFN-α . | HU/BUS . | IFN-α . | HU/BUS . |
| Abbreviations: ICSG, Italian Cooperative Study Group; CML, chronic myelogenous leukemia; HU, hydroxyurea; BUS, busulfan | |||||||
| ICSG on CML, 1994 | 218 | 94/ 10 | 5/m2 | 19% | 1% | 60% | 45% |
| Hehlmann et al, 1994 | 133 | 194/186 | 5/m2 | 6% | 1%/1% | 58% | 48%/33% |
| Allan et al, 1995 | 293 | 142/152 | 5/m2 | 10% | 2% | 50% | 32% |
| Ohnishi et al, 1995 | 85 | 0/ 85 | 5/m2 | 15% | 5% | 63% | 37% |
| Benelux Study Group, 1998 | 100 | 95/ 0 | 3 total | 16% | 2% | 54% | 54% |
| CML Trialists' Collaborative Group (Meta-analysis), 1997 | 640 | 286/345 | NR | NR | 57% | 46%/34% | |
| . | No. of cases . | . | Major cytogenetic response . | 5-year survival . | |||
|---|---|---|---|---|---|---|---|
| . | IFN-α . | HU/BUS . | IFN-α MIU/day . | IFN-α . | HU/BUS . | IFN-α . | HU/BUS . |
| Abbreviations: ICSG, Italian Cooperative Study Group; CML, chronic myelogenous leukemia; HU, hydroxyurea; BUS, busulfan | |||||||
| ICSG on CML, 1994 | 218 | 94/ 10 | 5/m2 | 19% | 1% | 60% | 45% |
| Hehlmann et al, 1994 | 133 | 194/186 | 5/m2 | 6% | 1%/1% | 58% | 48%/33% |
| Allan et al, 1995 | 293 | 142/152 | 5/m2 | 10% | 2% | 50% | 32% |
| Ohnishi et al, 1995 | 85 | 0/ 85 | 5/m2 | 15% | 5% | 63% | 37% |
| Benelux Study Group, 1998 | 100 | 95/ 0 | 3 total | 16% | 2% | 54% | 54% |
| CML Trialists' Collaborative Group (Meta-analysis), 1997 | 640 | 286/345 | NR | NR | 57% | 46%/34% | |
Comparison of the main results of the French45 and the Italian50 studies of α-interferon (IFN-α) + low dose cytosine arabinoside (LDAC) versus IFN-α alone. A difference in favor of IFN-α + LDAC was confirmed for major cytogenetic response (Ph neg 66-100%), but not for overall survival.
| . | French study (n = 721) . | Italian study (n = 538) . | ||||
|---|---|---|---|---|---|---|
| . | IFN-α + LDAC . | IFN-α . | P . | IFN-α + LDAC . | IFN-α . | P . |
| Abbreviations: NR, not reported | ||||||
| Complete hematologic response at 6 months | 66% | 55% | 0.003 | 62% | 55% | 0.11 |
| Major cytogenetic response (Ph neg 66-100%) at 12 months | 35% | 21% | 0.001 | 21% | 13% | 0.012 |
| Major cytogenetic response (Ph neg 66-100%) at 24 months | NR | NR | 28% | 18% | 0.003 | |
| 5-year survival | 70% | 62% | 0.02 | 68% | 65% | 0.77 |
| . | French study (n = 721) . | Italian study (n = 538) . | ||||
|---|---|---|---|---|---|---|
| . | IFN-α + LDAC . | IFN-α . | P . | IFN-α + LDAC . | IFN-α . | P . |
| Abbreviations: NR, not reported | ||||||
| Complete hematologic response at 6 months | 66% | 55% | 0.003 | 62% | 55% | 0.11 |
| Major cytogenetic response (Ph neg 66-100%) at 12 months | 35% | 21% | 0.001 | 21% | 13% | 0.012 |
| Major cytogenetic response (Ph neg 66-100%) at 24 months | NR | NR | 28% | 18% | 0.003 | |
| 5-year survival | 70% | 62% | 0.02 | 68% | 65% | 0.77 |
Results of the main studies of interferon-α (IFN-α) alone or in combination with low dose cytosine arabinoside (LDAC).The studies are ordered by year of publication. Notice that the dose of IFN-α is the dose that was scheduled. The dose that was actually given ranged from less than 50% to 80% of scheduled. In the study of Schofield et al30 IFN-α was scheduled daily for the first month and three times a week thereafter. In the Benelux study38 IFN-α was scheduled five times a week.
| . | . | IFN-α dose (MIU/day) . | No. of cases . | Major cytogenetic response* . | 5-year survival . |
|---|---|---|---|---|---|
| * Major cytogenetic response = Ph neg metaphases 66-100%. | |||||
| 1 Multicenter non-randomized study | |||||
| 2 Single center randomized study of IFN-α versus conventional chemotherapy. | |||||
| 3 Multicenter non-randomized study | |||||
| 4 Multicenter randomized study of IFN-α versus IFN-α + LDAC. | |||||
| Abbreviations: ICSG, Italian Cooperative Study Group | |||||
| Ozer et al 1993 | IFN-α 1 | 5/m2 | 128 | 24% | 52% |
| ICSG on CML 1994 | IFN-α 2 | 5/m2 | 218 | 19% | 60% |
| Schofield et al 1994 | IFN-α 3 | 2/m2 | 41 | 17% | 62% |
| Hehlmann et al 1994 | IFN-α 2 | 5/m2 | 133 | 6% | 58% |
| Kantarjian et al 1995 | IFN-α 3 | 5/m2 | 274 | 38% | 62% |
| Allan et al 1995 | IFN-α 2 | 5/m2 | 293 | 10% | 50% |
| Ohnishi et al 1995 | IFN-α 2 | 5/m2 | 85 | 15% | 63% |
| Thaler et al 1996 | IFN-α 1 | 3.5 total | 80 | 12% | 50% |
| Guilhot et al 1997 | IFN-α 4 | 5/m2 | 361 | 21% | 62% |
| Guilhot et al 1997 | IFN-α+LDAC4 | 5/m2 | 360 | 35% | 70% |
| Benelux 1998 | IFN-α 2 | 3 total | 100 | 16% | 54% |
| Mahon et al 1998 | IFN-α 3 | 5/m2 | 116 | 43% | 68% |
| Kantarjian et al 1999 | IFN-α+LDAC3 | 5/m2 | 186 | 45% | 68% |
| ICSG on CML 1999 | IFN-α1 | 5/m2 | 272 | 22% | 63% |
| Lindauer et al 1999 | IFN-α+LDAC1 | 5 total | 65 | 11% | 56% |
| Kloke et al 2000 | IFN-α3 | 4/m2 | 71 | 30% | 60% |
| ICSG on CML 2001 | IFN-α4 | 5/m2 | 263 | 18% | 65% |
| ICSG on CML 2001 | IFN-α+LDAC4 | 5/m2 | 275 | 28% | 68% |
| . | . | IFN-α dose (MIU/day) . | No. of cases . | Major cytogenetic response* . | 5-year survival . |
|---|---|---|---|---|---|
| * Major cytogenetic response = Ph neg metaphases 66-100%. | |||||
| 1 Multicenter non-randomized study | |||||
| 2 Single center randomized study of IFN-α versus conventional chemotherapy. | |||||
| 3 Multicenter non-randomized study | |||||
| 4 Multicenter randomized study of IFN-α versus IFN-α + LDAC. | |||||
| Abbreviations: ICSG, Italian Cooperative Study Group | |||||
| Ozer et al 1993 | IFN-α 1 | 5/m2 | 128 | 24% | 52% |
| ICSG on CML 1994 | IFN-α 2 | 5/m2 | 218 | 19% | 60% |
| Schofield et al 1994 | IFN-α 3 | 2/m2 | 41 | 17% | 62% |
| Hehlmann et al 1994 | IFN-α 2 | 5/m2 | 133 | 6% | 58% |
| Kantarjian et al 1995 | IFN-α 3 | 5/m2 | 274 | 38% | 62% |
| Allan et al 1995 | IFN-α 2 | 5/m2 | 293 | 10% | 50% |
| Ohnishi et al 1995 | IFN-α 2 | 5/m2 | 85 | 15% | 63% |
| Thaler et al 1996 | IFN-α 1 | 3.5 total | 80 | 12% | 50% |
| Guilhot et al 1997 | IFN-α 4 | 5/m2 | 361 | 21% | 62% |
| Guilhot et al 1997 | IFN-α+LDAC4 | 5/m2 | 360 | 35% | 70% |
| Benelux 1998 | IFN-α 2 | 3 total | 100 | 16% | 54% |
| Mahon et al 1998 | IFN-α 3 | 5/m2 | 116 | 43% | 68% |
| Kantarjian et al 1999 | IFN-α+LDAC3 | 5/m2 | 186 | 45% | 68% |
| ICSG on CML 1999 | IFN-α1 | 5/m2 | 272 | 22% | 63% |
| Lindauer et al 1999 | IFN-α+LDAC1 | 5 total | 65 | 11% | 56% |
| Kloke et al 2000 | IFN-α3 | 4/m2 | 71 | 30% | 60% |
| ICSG on CML 2001 | IFN-α4 | 5/m2 | 263 | 18% | 65% |
| ICSG on CML 2001 | IFN-α+LDAC4 | 5/m2 | 275 | 28% | 68% |
Summary of the data concerning the possible adverse effects of prior IFN-α treatment on the outcome of alloBMT.In the IBMTR study of Giralt et al62 it was found that the patients who were pretreated with IFN-α had a slightly higher risk of non-engraftment (2% vs 0.2%, p = 0.01) and a slightly lower risk of relapse (1% vs 8%, p = 0.01).
| . | No. of Cases . | Marrow Donors . | Detectable Effects . |
|---|---|---|---|
| Abbreviations: Sib, sibling; VU, volunteer unrelated marrow donor; BMT, bone marrow transplant. | |||
| Giralt et al 1993 | 23 | SIB | None |
| Beelen et al 1995 | 50 | SIB/VU | Adverse, if treatment for more than 1 year |
| Shepherd et al 1995 | 53 | SIB/VU | None |
| Zuffa et al 1998 | 16 | SIB | None |
| Tomàs et al 1998 | 30 | SIB | None |
| Beelen et al 1999 | 94 | SIB/VU | Adverse, if treatment for more than 1 year and discontinued less than 3 months before alloBMT |
| Hehlmann et al 1999 | 86 | SIB/VU | Adverse if treatment discontinued less than 3 months before alloBMT |
| Giralt et al (IBMTR) 2000 | 209 | SIB | None |
| . | No. of Cases . | Marrow Donors . | Detectable Effects . |
|---|---|---|---|
| Abbreviations: Sib, sibling; VU, volunteer unrelated marrow donor; BMT, bone marrow transplant. | |||
| Giralt et al 1993 | 23 | SIB | None |
| Beelen et al 1995 | 50 | SIB/VU | Adverse, if treatment for more than 1 year |
| Shepherd et al 1995 | 53 | SIB/VU | None |
| Zuffa et al 1998 | 16 | SIB | None |
| Tomàs et al 1998 | 30 | SIB | None |
| Beelen et al 1999 | 94 | SIB/VU | Adverse, if treatment for more than 1 year and discontinued less than 3 months before alloBMT |
| Hehlmann et al 1999 | 86 | SIB/VU | Adverse if treatment discontinued less than 3 months before alloBMT |
| Giralt et al (IBMTR) 2000 | 209 | SIB | None |
Low risk patients benefit from α-IFN treatment more than high risk patients. The benefit for intermediate risk patients is less clear. The data are based on the prospective studies of the Italian Coop. Study Group on CML.65
| . | Low Risk . | High Risk . |
|---|---|---|
| Hematologic response | ≥ 90% | 40 - 60% |
| Cytogenetic response | ≥ 50% | 10 - 20% |
| Survival of responders | ≥ 10 years | 5 - 8 years |
| Relative benefit | yes | yes |
| Absolute benefit | greater | smaller |
| . | Low Risk . | High Risk . |
|---|---|---|
| Hematologic response | ≥ 90% | 40 - 60% |
| Cytogenetic response | ≥ 50% | 10 - 20% |
| Survival of responders | ≥ 10 years | 5 - 8 years |
| Relative benefit | yes | yes |
| Absolute benefit | greater | smaller |
Sokal's63 and Euro64 formulation for the definition of the risk profile at diagnosis. Sokal's formulation was developed from conventionally treated cases (single-agent chemotherapy).The Euro formulation was developed from αIFN treated cases. Low risk patients have a relative risk (RR) < 0.8 with Sokal's and ≤ 780 with Euro. Intermediate risk patients have a RR between 0.8 and 1.2 with Sokal's and between 781 and 1479 with Euro. High risk patients have a RR > 1.2 with Sokal's and ≥ 1480 with Euro.
| . | SOKAL . | EURO . |
|---|---|---|
| (1) Maximum distance from costal margin; (2) Percent in peripheral blood | ||
| Age, years | 0.0116 (age - 43.4) | 0.6666 when age ≥ 50 |
| Spleen (1), cm | 0.0345 (spleen - 7.51) | 0.042 x spleen |
| Platelet, x 109/L | 0.188 [( \(\frac{Platelet}{700}\) )2 - 0.563 ] | 1.0956 when ≥ 1500 |
| Myeloblasts (2), % | 0.0887 (myeloblasts - 2.10) | 0.0584 x myeloblasts |
| Eosinophils (2), % | — | 0.0413 x eosinophils |
| Basophils (2), % | — | 0.2039 when basophils ≥ 3% |
| Relative risk (RR) | Exponential of the total | Total x 1000 |
| . | SOKAL . | EURO . |
|---|---|---|
| (1) Maximum distance from costal margin; (2) Percent in peripheral blood | ||
| Age, years | 0.0116 (age - 43.4) | 0.6666 when age ≥ 50 |
| Spleen (1), cm | 0.0345 (spleen - 7.51) | 0.042 x spleen |
| Platelet, x 109/L | 0.188 [( \(\frac{Platelet}{700}\) )2 - 0.563 ] | 1.0956 when ≥ 1500 |
| Myeloblasts (2), % | 0.0887 (myeloblasts - 2.10) | 0.0584 x myeloblasts |
| Eosinophils (2), % | — | 0.0413 x eosinophils |
| Basophils (2), % | — | 0.2039 when basophils ≥ 3% |
| Relative risk (RR) | Exponential of the total | Total x 1000 |
Investigational agents. STI571 has already been approved for the treatment of chronic myelogenous leukemia (CML). Other agents are in a preclinical or in a early clinical phase.
| - Proteine tyrosine kinase inhibitors | - STI571 (Gleevec) |
| - Other | |
| - Ras inhibitors | - Farnesyl transferase inhibitors |
| - Other | |
| - Other signal transduction inhibitors | |
| - Proteasome inhibitors | |
| - Histone deacetylase inhibitors | |
| - Vaccines (e.g. protein, pulsed dendritic cells) | |
| - Proteine tyrosine kinase inhibitors | - STI571 (Gleevec) |
| - Other | |
| - Ras inhibitors | - Farnesyl transferase inhibitors |
| - Other | |
| - Other signal transduction inhibitors | |
| - Proteasome inhibitors | |
| - Histone deacetylase inhibitors | |
| - Vaccines (e.g. protein, pulsed dendritic cells) | |
Risk score for individual transplant procedures as established by the European Group for Blood and Marrow Transplantation.32
| Feature . | Score . |
|---|---|
| A. Donor type | |
| HLA-identical sibling | 0 |
| Unrelated/non-identical | 1 |
| B. Stage of disease: | |
| Chronic phase | 0 |
| Accelerated phase | 1 |
| Blast crisis | 2 |
| C. Age: | |
| < 20 years | 0 |
| 20-40 years | 1 |
| >40 years | 2 |
| D. Donor/recipient sex combination: | |
| Other | 0 |
| Female donor for male recipient | 1 |
| E. Interval from diagnosis to transplant: | |
| < 12 months | 0 |
| > 12 months | 1 |
| Feature . | Score . |
|---|---|
| A. Donor type | |
| HLA-identical sibling | 0 |
| Unrelated/non-identical | 1 |
| B. Stage of disease: | |
| Chronic phase | 0 |
| Accelerated phase | 1 |
| Blast crisis | 2 |
| C. Age: | |
| < 20 years | 0 |
| 20-40 years | 1 |
| >40 years | 2 |
| D. Donor/recipient sex combination: | |
| Other | 0 |
| Female donor for male recipient | 1 |
| E. Interval from diagnosis to transplant: | |
| < 12 months | 0 |
| > 12 months | 1 |
Approaches to conditioning used in for reduced intensity allogeneic stem cell transplants.
| Preparative regimen . | GVHD prophylaxis . |
|---|---|
| Table modified from Barrett & Childs12 | |
| Abbreviations: GVHD, graft-versus-host disease; ATG, antithymocyte globulin; MTX, methotrexate: MMF, mycophenolate mofetil | |
| Cyclophosphamide, fludarabine | Cyclosporine |
| Cyclophosphamide, fludarabine, ATG | Cyclosporine, ATG |
| Fludarabine, busulfan, ATG | Cyclosporine |
| Fludarabine, melphalan, | MTX, FK506 |
| Total body irradiation (200 cGy) | Cyclosporine, MMF |
| Cyclophosphamide, ATG, thymic irradiation | Cyclosporine |
| Preparative regimen . | GVHD prophylaxis . |
|---|---|
| Table modified from Barrett & Childs12 | |
| Abbreviations: GVHD, graft-versus-host disease; ATG, antithymocyte globulin; MTX, methotrexate: MMF, mycophenolate mofetil | |
| Cyclophosphamide, fludarabine | Cyclosporine |
| Cyclophosphamide, fludarabine, ATG | Cyclosporine, ATG |
| Fludarabine, busulfan, ATG | Cyclosporine |
| Fludarabine, melphalan, | MTX, FK506 |
| Total body irradiation (200 cGy) | Cyclosporine, MMF |
| Cyclophosphamide, ATG, thymic irradiation | Cyclosporine |
Suggested indications for early allogeneic stem cell transplantation for a newly diagnosed patient with chronic myeloid leukemia (CML).
| • Intermediate or high risk (European score) |
| HLA-identical sibling donor |
| Age less than 45 years |
| • Low risk (European score) |
| HLA-identical sibling donor |
| Age less than 35 years |
| • Intermediate or high risk (European score) |
| Molecularly matched unrelated (or family) donor |
| Age less than 35 years |
| • Low risk (European score) |
| Molecularly matched unrelated (or family) donor |
| Age less than 25 years |
| • Intermediate or high risk (European score) |
| HLA-identical sibling donor |
| Age less than 45 years |
| • Low risk (European score) |
| HLA-identical sibling donor |
| Age less than 35 years |
| • Intermediate or high risk (European score) |
| Molecularly matched unrelated (or family) donor |
| Age less than 35 years |
| • Low risk (European score) |
| Molecularly matched unrelated (or family) donor |
| Age less than 25 years |
Design of randomized clinical trial of STI571 versus interferon/cytarabine arabinoside (IFN/Ara-C).
Abbreviations: S, screening; R, randomization; MCR, major cytogenetic response; CHR, complete hematologic response
Design of randomized clinical trial of STI571 versus interferon/cytarabine arabinoside (IFN/Ara-C).
Abbreviations: S, screening; R, randomization; MCR, major cytogenetic response; CHR, complete hematologic response
Potential mechanisms of STI571 resistance.
Abbreviations: MDR, multidrug resistance; AGP, α1 acid glycoprotein
Potential mechanisms of STI571 resistance.
Abbreviations: MDR, multidrug resistance; AGP, α1 acid glycoprotein
Survival and transplant related mortality for patients with chronic myelogenous leukemia (CML) whose clinical data were reported to the European Group for Blood and Marrow Transplantation according to risk score calculated on the basis of factors listed in Table 14. Reproduced from Gratwohl et al4with permission of the authors and publishers.
Survival and transplant related mortality for patients with chronic myelogenous leukemia (CML) whose clinical data were reported to the European Group for Blood and Marrow Transplantation according to risk score calculated on the basis of factors listed in Table 14. Reproduced from Gratwohl et al4with permission of the authors and publishers.
Pie chart showing that only about 18% of patients with chronic myelogenous leukemia (CML) can in fact be cured by allogeneic stem cell transploant (allo-SCT).
Pie chart showing that only about 18% of patients with chronic myelogenous leukemia (CML) can in fact be cured by allogeneic stem cell transploant (allo-SCT).
Probability of survival for 1300 CML patients categorized at diagnosis in accordance with the European Prognostic Scoring system devised by Hasford et al.31
Probability of survival for 1300 CML patients categorized at diagnosis in accordance with the European Prognostic Scoring system devised by Hasford et al.31
Schematic representation of Option 2 by which some patients are offered treatment by allogeneic stem cell transplant (allo-SCT) soon after diagnosis if they are young and have a suitable donor. Others are deemed ineligible for allo-SCT. The intermediate group is offered initial treatment with STI571 or an STI-containing combination) for a period of months and may proceed to allo-SCT if they are deemed to have failed the ‘trial' of STI571.
Abbreviations: PEG-IFN, pegylated interferon; A/G, autografting
Schematic representation of Option 2 by which some patients are offered treatment by allogeneic stem cell transplant (allo-SCT) soon after diagnosis if they are young and have a suitable donor. Others are deemed ineligible for allo-SCT. The intermediate group is offered initial treatment with STI571 or an STI-containing combination) for a period of months and may proceed to allo-SCT if they are deemed to have failed the ‘trial' of STI571.
Abbreviations: PEG-IFN, pegylated interferon; A/G, autografting