Abstract
Infection in the neutropenic patient has remained a major clinical challenge for over three decades. While diagnostic and therapeutic interventions have improved greatly during this period, increases in the number of patients with neutropenia, changes in the etiologic agents involved, and growing antibiotic resistance have continued to be problematic.
The evolving etiology of infections in this patient population is reviewed by Dr. Donowitz. Presently accepted antibiotic regimens and practices are discussed, along with ongoing controversies.
In Section II, Drs. Maki and Crnich discuss line-related infection, which is a major infectious source in the neutropenic. Defining true line-related bloodstream infection remains a challenge despite the fact that various methods to do so exist. Means of prevention of line related infection, diagnosis, and therapy are reviewed.
Fungal infection continues to perplex the infectious disease clinician and hematologist/oncologist. Diagnosis is difficult, and many fungal infections will lead to increased mortality even with rapid diagnosis and therapy. In Section III, Dr. Pappas reviews the major fungal etiologies of infection in the neutropenic patient and the new anti-fungals that are available to treat them.
Finally, Dr. Rolston reviews the possibility of outpatient management of neutropenic fever. Recognizing that neutropenics represent a heterogeneous group of patients, identification of who can be treated as an outpatient and with what antibiotics are discussed.
I. Infections in the Neutropenic Patient: An Overview
Gerald R. Donowitz, MD*
University of Virginia Health System, P.O. Box 801343, Charlottesville VA 22908-1343
Infection in the compromised host has been a topic of clinical concern, research and discussion for over three decades. Our understanding of risk factors for infection, means of diagnosis, and therapeutic options has increased greatly during this period of time. However, the increasing numbers of patients who are immunocompromised, the changing epidemiology of infection, and the growing resistance of bacteria to commonly used antimicrobial agents has made this problem one of the most persistent in infectious disease, hematology-oncology and general internal medicine.
Neutropenia and the Immunocompromised State
The compromised state occurs when any of the host's major defense systems are undermined in a manner that increases the chance of infection.1 Host defenses can be undermined by the underlying disease (myeloma, lymphoma, chronic lymphocytic leukemia), specific therapy of the underlying disease (steroids, cytotoxic chemotherapy) or an array of iatrogenic manipulations that occur when a patient is hospitalized (exposure to broad-spectrum antibiotics as prophylaxis or therapy, use of long-dwelling right-atrial catheters, exposure to hospital pathogens). As a rule, a variety of host defense defects will occur in patients with malignancy undergoing chemotherapy that will predispose them to infection. Neutropenia remains the major defect for many patients and therefore continues to serve as a model system for dealing with infections in patients who are immunocompromised.
The role of neutropenia as a major host defense defect was defined by Bodey in 1966 when he demonstrated that as the absolute neutrophil count dropped below 500-1000/mm3, the incidence of severe infection, the number of days spent on antibiotics, and the number of days of fever increased.2 The incidence of infection was 14% if the neutrophil count fell below 500-1000/mm3, and 24-60% if the neutrophil count fell to < 100/mm3. The longer the duration of neutropenia and the more rapid the decline in white cells, the greater the incidence of infection.2,3 If the granulocytopenia was prolonged for more than 5 weeks, then the incidence of infection was 100%. Neutrophil counts less than 500 cells/mm3 for greater than 10 days is now viewed as a general threshold for more frequent and severe infections.2,4
While neutropenia remains of critical importance in establishing the risk of infection, it is only one of the risks. It is now clear that patients with neutropenia represent a heterogeneous population with varying rates of infection-related morbidity and mortality.5 As discussed in Section IV, additional parameters help define the true risk of infection, as well as the mortality, and therefore help determine the possibility for inpatient versus outpatient therapy with oral versus parenteral antibiotics.
The relationship of host defense defects and infection risk is in continual flux (Figure 1 ). As new chemotherapy regimens are developed, and as new antibiotics are introduced for prophylaxis or therapy, new infection risks have been defined. Use of the newer purine analogs such as fludarabine in patients with chronic lymphocytic leukemia resulted in increased infection with Listeria monocytogenes, Pneumocystis carinii, and other organisms associated with T-cell dysfunction.6,7 Prophylaxis with agents such as trimethoprim-sulfamethoxazole and ciprofloxacin in the setting of severe mucositis has been associated with bacteremia with streptococcus viridans species.8,9 Thus each new chemotherapeutic regimen or new antibiotic must be evaluated and monitored for its long-term effect on infection.
Etiology of Infection in the Neutropenic: A Changing Landscape
In the late 1960s, 1970s and into the 1980s, aerobic gram-negative bacilli were the predominant organisms causing infection in the neutropenic patient. In Schimpff's landmark study of the utility of empiric antibiotic usage in neutropenia, it was shown that 64% of fevers were associated with a documented infection. Of those infections that were microbiologically proven, aerobic gram-negative bacilli were involved approximately 60-80% of the time, with Pseudomonas aeruginosa being a leading isolate.10 Of the gram-positive organisms isolated, Staphylococcus aureus was the most important. Aerobic gram-negative rods predominated in all centers. Consequently as empiric antibiotic regimens were developed, coverage of aerobic gram-negative bacilli, especially P. aeruginosa, was mandatory.
In the mid 1980s, the spectrum of bacteria causing infection began to change. A steady increase in gram-positive infections occurred until presently 60-70% of bacteremias with a single organism identified will be caused by gram-positive cocci.11,12 Coagulase-negative staphylococci and S. aureus are the predominant organisms. Why this change from gram-negative to gram-positive organisms occurred is not absolutely clear, and is probably multifactorial. Important considerations include aggressive chemotherapeutic regimens that cause more severe mucositis, longer durations of neutropenia, almost uniform use of long-dwelling right-atrial catheters, use of H2 antagonists, and use of prophylactic antibacterial agents with relatively weak coverage of gram-positive organisms.13
In addition to the change from gram-negative infections to those caused by gram-positive organisms, “new” gram-positive organisms have become important etiologies of infection (Table 1 ).11,14 Several organisms deserve special emphasis.
One of the most important gram-positive organisms infecting the neutropenic is viridans streptococci. Streptococcus mitis, S. oralis, S. salivarius and S. millerei have been the organisms most commonly involved.8,9,13,14 The majority of patients will present with fever and bacteremia that rapidly respond to antibiotics. However, 10% will develop a toxic shock-like syndrome with fever, hypotension, diffuse rash with subsequent desquamation, and development of acute respiratory distress syndrome (ARDS). Mortality rates of 6-30% have been observed. A major predisposing factor appears to be the use of certain prophylactic antibiotics in the setting of severe mucositis. Other associations include use of high dose cytosine arabinoside and use of H2-receptor antagonists.15 Of note, increasing resistance of the viridans streptococci to penicillin and some second- and third-generation cephalosporins has been documented, impacting the choice of empiric therapy.16,17
The enterococcus is becoming a more common agent colonizing and infecting neutropenic patients, mirroring its emerging role as a nosocomial pathogen in general. Of these organisms, E. faecium is overtaking E. faecealis as the predominant organism. As has occurred in other hospital settings, vancomycin-resistant enterococci have been involved in outbreaks.18,19 Even in neutropenics, colonization is a more frequent occurrence than true infection. However, in the setting of neutropenia, bacteremia with vancomycin-resistant enterococci may be associated with a mortality rate of over 70%.
The other organisms listed in Table 1 account for ≈5% of all isolates in febrile neutropenics. Their potential for causing severe infection and their variable susceptibility to commonly used antibiotics needs to be recognized. One of the more challenging areas in the febrile neutropenic is the question of diagnosis and therapy of line-related infection. While gram-positive organisms predominate, an array of bacteria and fungi may be involved. This topic will be reviewed in Section II.
A variety of previously unappreciated gram-negative organisms have also been identified as causes of infection in the neutropenic patient. Among these isolates include Stenotrophomonas maltophilia, Legionella species, Alcaligenes xylosoxidans, and Burkholderia cepacia.11
Also important is the increasing resistance of more common aerobic gram-negative bacilli to the antibiotic “standards” that have been utilized over the last decade.20 Resistance of Pseudomonas aeruginosa to third-generation cephalosporins is 9-12%, to imipenem is 8.3%, and to ciprofloxacin is 13.3%. Enterobacter species have resistance rates of 10-21% to ceftazidime and piperacillin/tazobactam.20 Resistance of gram-negative bacilli to ciprofloxacin has increased at some centers to over 25%, especially where the agent has been used for prophylaxis.20
Empiric Therapy of Fever in the Neutropenic Patient: Suggestions, Not Rules
In the setting of changing flora and susceptibility patterns to antibiotics, guidelines as to “best therapy” of infection in the neutropenic patient must be evaluated on the basis of local patterns of infection and local and regional resistance patterns.
The approach to therapy of the febrile neutropenic patient has evolved slowly over the last thirty years in several distinct stages. The first stage stemmed from the work of Schimpff in 1971 determining that empiric antibiotic therapy was required and that combinations of antibiotics lead to reasonable outcomes.10 The next stage began with the introduction of third-generation cephalosporins with potent activity against aerobic gram-negative bacilli including P. aeruginosa and carbapenems, which allowed monotherapy to be considered. The present stage involves the consideration of antibacterial resistance patterns prior to the use of any empiric therapy. Since these stages overlap, each needs to be considered.
Aminoglycosides and anti-pseudomonas penicillins became established therapy for neutropenic fever in 1971, with overall response rates between 60-70%. For the next decade a variety of studies utilized various aminoglycosides (gentamicin, amikacin, tobramycin, netilmicin) as well as various anti-pseudomonas penicillins (ticarcillin, piperacillin, mezlocillin) and somewhat later, β-lactam/β-lactamase inhibitor combinations (ticarcillin-clavulanic acid, piperacillin-tazobactam). Despite differences in in vitro susceptibility testing, there has never been a clear or consistent predominance of one combination versus any other. In general, the potential advantages of combination chemotherapy over monotherapy include potential synergy against strains of aerobic gram-negative bacilli, activity against anaerobes especially when β-lactam/β-lactamase inhibitor combinations are used, and a possible decrease in the emergence of resistant strains. While none of these regimens are “first-line” therapy against gram-positive cocci, they may be adequate to stabilize the patient until specific gram-positive etiologies are identified. The major drawback of combination therapy is the oto- and nephrotoxicity of aminoglycosides, which require monitoring of serum levels and careful dose adjustment. Single daily dosing of aminoglycoside has been utilized in the neutropenic population, but it remains unclear whether this means of dosing is as effective and less toxic than the more standard dosing interval. Recently, ciprofloxacin has been shown to be equivalent to tobramycin as part of combination therapy with piperacillin, with fewer clear episodes of drug-related nephrotoxicity noted in the ciprofloxacin group.21 With the development of aztreonam, a monobactam with potent coverage against aerobic gram-negative bacilli but no coverage of gram-positive cocci, therapy with azeotronam plus clindamycin or vancomycin was added to the possible combination regimens for use in the febrile neutropenic with particular usefulness in the penicillin-allergic patient.
In the 1980s as third-generation cephalosporins and carbapenems became available, renewed interest in monotherapy, or at least modified monotherapy, developed. The anti-pseudomonas third-generation cephalosporins (ceftazidime, cefepime) and carbapenems (imipenem, meropenem) all have potent activity against aerobic gram-negative-bacilli including P. aeruginosa, and had at least some activity against many strains of gram-positive cocci. Ceftazidime received earliest attention both as monotherapy as well as in combination with short- or long-term aminoglycoside. While overall efficacy of ceftazidime monotherapy was comparable to combination therapy, patients with documented infection with gram-negative bacilli did better when an aminoglycoside was used along with it.23,24 The concept of modified monotherapy or “front-loaded” therapy was therefore established using an aminoglycoside for the first 72 hours of therapy, then discontinuing it if initial cultures were negative for aerobic gram-negative bacilli. This was thought to provide broad initial coverage but would not expose patients to aminoglycosides unless it was really needed. While the initial work was done with ceftazidime, cefepime has been used in the same way. Both agents have been used as pure monotherapy as well.
The carbapenems presently available, imipenem and meropenem, have been clearly demonstrated to be excellent agents used as monotherapy. Unlike ceftazidime, where aminoglycosides improved outcomes in the setting of documented gram-negative infection, no such effect was noted with imipenem.24 With similar activity and penetration, meropenem also has been used as monotherapy. Ciprofloxacin, as well as other quinolones, have recently been studied as potential monotherapeutic agents.25,26 The data for quinolones remains somewhat limited and the data should be viewed as suggestive only.
Large, well-designed, blinded, randomized, controlled trials have not been carried out to compare third-generation cephalosporins to each other or to compare carbapenems to each other. Studies carefully examining whether anything is to be gained by adding a second antibiotic to a monotherapeutic regimen are rare. In general, each of the agents mentioned is probably adequate as initial empiric therapy. Should microbiologic confirmation of infection occur, adjustment of the regimen can be done safely without risk to the patient as long as broad-spectrum coverage is maintained. Which antibiotic or antibiotic combination to use should be determined by local susceptibility patterns and local frequencies of various pathogens.
Ongoing controversies persist concerning various aspects of antibiotic therapy of the compromised host. Some of these include:
1. Should vancomycin be utilized as part of the initial regimen?
Clearly, the predominance of gram-positive cocci as the etiologic agents of microbiologically proven infection in this population would suggest the use of vancomycin especially in an era of methicillin-resistant S. aureus (MRSA), coagulase-negative staphylococci, enterococci and viridans streptococci. Definitive data, however, is lacking. Some studies have shown that vancomycin when used initially may be associated with fewer break-through bacteremias and local infections with S. aureus.27,28 Subsequent studies suggested that there was no increase in morbidity or mortality overall if vancomycin was held until it was needed, that is, until a gram-positive organism was identified and the patient was not responding to the initial regimen.29,30 A significant exception is bacteremia with viridans streptococci, which may have a higher mortality if not initially treated with vancomycin.8
Overall, the general consensus concerning vancomycin is that there is no clear indication for its use as initial empiric therapy unless the patient is known to be colonized with MRSA, is at an institution where fulminant gram-positive infections are frequent, or is at an institution where infection with viridans streptococci are frequent or suspected. If vancomycin is used but no gram-positive infections are identified after appropriate culturing at 48-72 hours, vancomycin should be discontinued.4 If cultures are positive for gram-positive organisms from initial cultures and the patient is not doing well on the initial antibiotic regimen, vancomycin could be added until the final antibiotic susceptibilities are established.4
2. How long does it take for antibiotics to work?
In reviewing results in over 480 episodes of febrile neutropenia, Elting et al observed that the median time to clinical response was 5-7 days.31 However, the time to response differed with the specific antibiotics being used, with some agents leading to a consistent response within 3 days. It would seem reasonable that antibiotic changes should not be carried out during the initial 3-7 days of therapy unless the patient's clinical status deteriorates. The “juggling” of antibiotics during this time otherwise does not appear helpful or supported by any literature.
3. If the patient responds, how long should therapy be continued?
The majority of patients with febrile neutropenia will not have a microbiologically documented infection. Therefore, duration of therapy will not be guided by monitoring sterilization of cultures or by the presence of a specific organism. Based on data by Pizzo et al, some would argue that therapy until neutropenia resolved (neutrophil count ≈ 300-500/mm3) is warranted.32 The relatively small number of patients examined in the study does not allow a definitive answer to be derived. Others would argue that either 10-14 days of therapy, therapy for a minimum of 7 days, or until cultures are cleared and the signs and symptom of infection resolve is adequate independent of the circulating neutrophil count.4 Discontinuation of antibiotics prior to resolution of neutropenia has been suggested only if patients are stable, have intact mucous membranes and skin, and are not scheduled for further chemotherapy or invasive procedures.4 Arguments for defined periods of therapy in the setting of neutropenia include: the association of prolonged antibiotic therapy with development of fungal infection, development of antimicrobial resistance, and drug-related toxicities.
A further point of debate is whether parenteral therapy should be utilized for the total duration of therapy; whether an early switch to oral therapy is reasonable; or whether total oral therapy is possible. The utility of outpatient antibiotic therapy is discussed in Section IV. For those patients whose neutropenia is expected to be prolonged (>14 days) and profound (neutrophil count < 100 cells/mm3) parenteral therapy seems reasonable. Though strong data are lacking, it has been suggested that if there is not clear infection noted, no positive cultures, and the patient is stable, parenteral therapy can be changed to oral therapy after 2 or more days for completion of a course. Antibiotic therapy with ciprofloxacin and amoxicillin/clavulanic or cefixime alone has been suggested as “reasonable” oral agents for “follow-up” therapy.33– 36 That most of the studies using oral agents were done in low risk patients suggests a note of caution when intravenous therapy is switched to oral therapy.
4 . What happens when the patient does not respond to empiric antibiotics after 3-5 days?
This probably represents the most worrisome of scenarios. The lack of response may be due to an array of possibilities including (i) a non-bacterial pathogen, (ii) an organism that is resistant at least in part to the antibiotic regimen being used, (iii) a new superinfection, (iv) an infection at a difficult to treat site (an abscess or a catheter infection) or (v) lower than expected serum or tissue levels of the antibiotic.4 Drug fever, and a number of other non-infectious possibilities such as atelectasis, pulmonary embolism, and phlebitis are also possible. Repeat history and physical examination as well as use of computerized tomography (CT) and magnetic resonance imaging (MRI) imaging are important considerations. If a detailed review of the patient reveals no new findings, then one or two interventions are usually used. The first is the addition of vancomycin if it was not part of the initial regimen. The thought is that gram-positive organisms are the most likely possibility and vancomycin is probably the best agent. If vancomycin is added but there is no clear response or no isolation of a gram-positive organism, vancomycin should then be discontinued.
The second choice is the addition of amphotericin or a comparable anti-fungal. As will be discussed In Section III, since we do poorly at diagnosing fungal infections and since neutropenia and exposure to broad-spectrum antibiotics are major predisposing factors for fungal infection, this is a reasonable intervention. Historically, approximately 66% of patients will respond to amphotericin in this setting.37 If a fungal infection is found, the duration of antifungal therapy will depend on the organism. More likely, however, the patient will respond without a documented fungal infection detected. The duration of therapy in this case is not clearly defined. Treatment until neutropenia resolves or at least 2 weeks of therapy are commonly followed procedures.
Use of Adjunctive Therapy in Febrile Neutropenia: Logic, but no Definitive Data
Use of hematopoietic growth factors in neutropenic patients with fever would seem like a logical adjunct to antibiotics. However, in the randomized controlled trials using either G-CSF or GM-CSF, no clear or definitive reduction in morbidity and mortality has occurred.38– 40 Patients with profound, prolonged neutropenia with documented infection have been identified as a subgroup of patients that may benefit from use of growth factors. With even fewer supportive data, some experts would also use growth factors for patients who are not improving from severe infections despite appropriate antibiotic therapy.
Similarly, data concerning transfusions of white blood cells in febrile neutropenic patients has not yielded definitive results. While early studies suggested a beneficial effect, the studies were uncontrolled, dealt with a variety of underlying diseases, type of infections and dosing of white cells.41 More recent studies have still dealt with low numbers of patients though a beneficial effect was noted.42 The overall conclusion appears to be that neutrophil transfusions remains an experimental intervention.43 If used, they should be part of experimental protocols.
Conclusion
Overall, despite the array of complicating factors including drug resistance and new patterns of infection, rates of successful therapy of neutropenic fever have been maintained at 66-80% with differences in rates being more dependent on study design than antimicrobial agents used. With more rational use of antibiotics, and new antibiotics being developed that may begin to address presently difficult-to-treat organisms, there is at last an expectation of improving the present rates of successful therapy in this population.
II. Line Sepsis in the Granulocytopenic Patient: Prevention, Diagnosis, and Management
Dennis G. Maki, MD,*
University of Wisconsin Hospital, Dept. of Medicine, Section of Infectious Diseases, 600 Highland Avenue, Room H4/572, Madison WI 53792
Dr. Maki receives grant support from Arrow, Becton-Dickinson, and Johnson & Johnson.
Reliable vascular access is one of the most essential features of modern medical care, especially in the hospitalized granulocytopenic patient requiring blood products and multiple drugs. Unfortunately, the intravascular devices (IVDs) needed to establish reliable access are associated with significant potential for producing iatrogenic bacteremia or candidemia.1 More than 250,000 IVD-related bloodstream infections (IVDR BSIs) occur in the US each year,1 each associated with 12-25% attributable mortality2,3 prolongation of hospital stay3,4 and an added cost to healthcare of $33,000-35,000.3,4
Nature of the Problem
Prospective studies show that every type of IVD carries some risk of causing BSI. The magnitude of risk varies greatly, depending on the type of device (Table 2 ).5 Historically this risk has been expressed as BSIs per 100 devices; however, the Centers for Disease Control and Prevention now recommends that rates of IVDR BSI be expressed per 1000 IVD-days. This recommendation is logical because it takes into account widely varying risks of IVDR BSI over time for different types of IVDs; in general, although rates of IVDR BSI per 100 IVDs are usually higher with long-term devices, the risk per 1000 IVD-days is usually considerably lower (Table 2).
The device that poses the greatest risk of IVDR BSI today is the central venous catheter (CVC) in its many forms (Table 2): up to 75% of IVDR BSIs originate from CVCs of various types,3–,5 and CVCs are the most important risk factor for nosocomial candidemia.6 Short-term non-cuffed, single- or multi-lumen catheters inserted percutaneously into the subclavian or internal jugular vein have shown rates of catheter-related BSI in the range of 3-5% (2-3 per 1000 IVD-days).1,5 Far lower rates of infection have been encountered with surgically-implanted cuffed Hickman or Broviac and subcutaneous central venous ports (1 and 0.2 per 1000 IVD-days, respectively) (Table 2).1,5 Recent studies suggest that peripherally inserted central catheters (PICCs) have rates of IVDR BSI no higher than surgically-implanted cuffed and tunneled CVCs (0.4 per 1000 IVD-days) and PICCs are supplanting surgically-implanted central devices on many hematology services.
Pathogenesis of IVDR BSI
There are two major sources of IVDR BSI: 1) colonization of the IVD, catheter-related infection, and 2) contamination of the fluid administered through the device, infusate-related infection.1 Contaminated infusate is the cause of most epidemic IVDR BSIs. In contrast, catheter-related infections are responsible for most endemic IVDR BSIs.
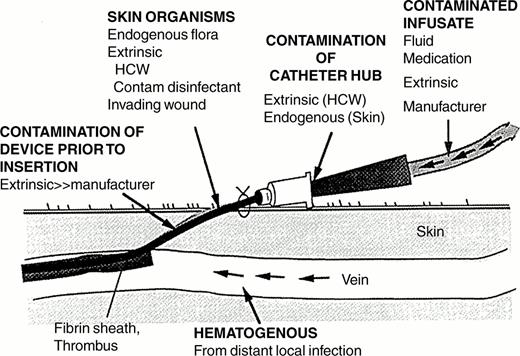
In order for microorganisms to cause catheter-related infection they must first gain access to the extraluminal or intraluminal surface of the device where they can adhere and become incorporated into a biofilm that allows sustained infection and hematogenous dissemination.1 Microorganisms gain access by one of three mechanisms (Figure 2 ): skin organisms invade the percutaneous tract, probably facilitated by capillary action, at the time of insertion or in the days following; microorganisms contaminate the catheter hub (and lumen) when the catheter is inserted over a percutaneous guidewire or later manipulated; or organisms are carried hematogenously to the implanted IVD from remote sources of local infection, such as a pneumonia.
With short-term IVDs (in place < 10 days)—peripheral IV catheters, arterial catheters and non-cuffed, non-tunneled CVCs—most device-related BSIs are of cutaneous origin, from the insertion site, and gain access extraluminally, occasionally intraluminally; in contrast, contamination of the catheter hub and lumen is the predominant mode of BSI with the long-term (in place > 10 days), permanent IVDs ubiquitous on hematology services, such as cuffed Hickman- and Broviac-type catheters, subcutaneous central ports and PICCs.1,7
Microorganisms found on patient's skin, which gain access to the IVD mainly extraluminally and occasionally intraluminally—coagulase-negative staphylococci (39%), S. aureus (26%), and Candida species (11%)—account for 76% of IVD-related BSIs with short-term, non-cuffed devices of all types; only 14% are caused by gram-negative bacilli. In contrast, with long-term IVDs, coagulase-negative staphylococci (25%) and gram-negative bacilli (45%), which most often have gained access intraluminally and contaminate infusate in the device, account for 76% of IVD-related BSIs; only 2% are caused by Candida species.5
Prevention of IVDR BSI
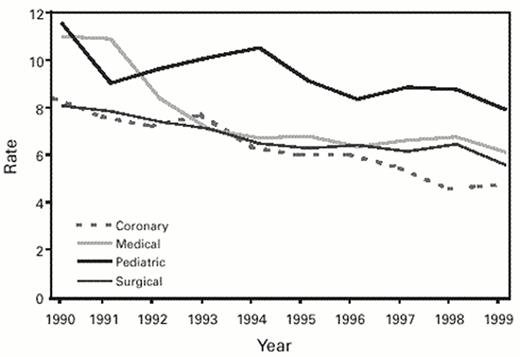
Over the past decade many investigators have evaluated strategies for the prevention of IVDR BSI, with greater success achieved than with any other form of nosocomial infection.1,8,9 Guidelines for the prevention of IVDR BSI were last issued by the Hospital Infection Control Practices Advisory Committee (HICPAC) in 1996 and recently have been revised (Table 3 ).10 Wide implementation of these measures has resulted in a substantial decline in hospital-acquired primary BSIs (Figure 3 ).11 More consistent application of control measures and wider acceptance of novel technologies shown to be effective (and cost-effective)8,9 will be needed to reduce this rate further.
Cutaneous antisepsis
Given the evidence for the importance of cutaneous microorganisms in the genesis of IVDR infection, the choice of the chemical antiseptic for disinfection of the insertion site would seem very high priority. In the US, iodophors such as 10% povidone-iodine are used very widely. Eight randomized, prospective trials have compared a chlorhexidine-containing antiseptic to povidone-iodine for preparation of the skin prior to insertion of IVDs: both agents were well tolerated in every trial, 7 of 8 found lower rates of catheter colonization, and 3 showed a significant reduction in CVC-related BSIs in the chlorhexidine-containing antiseptic group.12–,14 These studies indicate that chlorhexidine is superior to iodophors and it should be the antiseptic of first choice for vascular access.10
Innovative IVD design
Subcutaneous cuffs for long-term CVCs.
Hickman and Broviac catheters incorporate a subcutaneous Dacron® cuff which becomes ingrown by host tissue, creating a mechanical barrier against invasion of the tract by skin organisms. Rates of BSI per 1000 days with these catheters are far lower than with short-term percutaneously-inserted, non-cuffed CVCs inserted in the ICU (Table 1),1,5 and can be considered a quantum advance for safer long-term vascular access.
Subcutaneous central venous ports.
Surgically-implanted subcutaneous central venous ports, which can be accessed intermittently with a steel needle, have been associated with the lowest rates of IVDR BSI (Table 2). A prospective observational study of Hickman catheters and central ports in oncology patients showed that for patients needing intermittent central access, ports appear to safer as regards the risk of IVDR BSI.15
Peripherally-inserted central catheters (PICCs).
Antibiotic lock solutions
Prophylactic use of systemic antibiotics at the time of IVD implantation has not proven effective in reducing the incidence of IVDR BSI and is strongly discouraged.10 However, studies of continuous infusion of vancomycin, incorporated into total parenteral nutrition admixtures, have shown reduced rates of coagulase-negative staphylococcal BSI in low-birth-weight infants.17 Unfortunately, this form of prophylaxis results in prolonged low blood levels of vancomycin, which may be conducive to promoting resistance.
The “antibiotic lock” is a novel technique of local prophylaxis: an antibiotic solution is instilled into the catheter lumen and allowed to dwell for a defined period of time, usually 6-12 hours, after which it is removed. There have been 6 prospective randomized trials of antibiotic lock solutions for the prevention of BSI with long-term IVDs.18 The largest and most recent trial by Henrickson et al19 randomized 126 pediatric oncology patients (36,944 IVD-days) who had recently had a tunneled CVC implanted to 3 prophylactic lock regimens: heparin (10 U/mL) (control); heparin and vancomycin (25 μg/mL); and heparin, vancomycin and ciprofloxacin (2 μg/mL). Use of the vancomycin-ciprofloxacin-containing lock solution was associated with a markedly reduced rate of IVDR infection, compared to heparin alone (0.55 versus 1.72 per 1000 IVD-days, p = 0.005). Similarly, the rate of infection with vancomycin-containing lock solution was significantly reduced (0.37 per 1000 IVD-days, p = 0.004). The two antimicrobial lock solutions showed comparable protection against gram-positive and gram-negative IVDR infection. Unfortunately, failure to separate local infections from BSIs in the final data limits analysis of the results of this study. While rates of nosocomial colonization or infection with vancomycin-resistant enterococci, as detected by clinical cultures, were comparable in the three groups, no effort was made to proactively assess the impact of antibiotic-containing lock solutions on nosocomial colonization by vancomycin-resistant enterococci, methicillin-resistant S. aureus, and fluoroquinolone-resistant gram-negative bacilli.
Most studies utilized a lock solution containing vancomycin. It seems unlikely that microorganisms in the exposed patient's flora could develop resistance to vancomycin from the minute quantities of drug in a catheter lumen (< 15 μg), yet there is just concern over the possible effect of wide prophylactic use of vancomycin lock solutions, and more data are needed before their routine use can be recommended, specifically, randomized studies that prospectively assess the impact on nosocomial colonization by resistant microorganisms. However, because antibiotic lock solutions clearly reduce the risk of IVDR BSI with long-term IVDs, the new HICPAC Guideline considers their use acceptable in individual cases where a patient who requires indefinite vascular access continues to experience IVDR BSIs despite compliance with infection control guidelines.10
Prophylactic thrombolysis
Prophylactic anticoagulation, including mini-dose heparin and warfarin,20 has been shown to reduce catheter thrombosis with CVCs in randomized trials, but the effect on IVDR infection has not been reported. Randomized trials of prophylactic installation of urokinase (5000 IU/mL) into long-term IVDs every 1-2 or every 3-4 weeks have shown a reduced incidence of thrombosis and premature IVD loss.21,22 One trial also showed a reduction in IVDR BSIs.21 Prophylactic thrombolysis was well tolerated but the cost-benefit of this novel but expensive practice needs to be determined.
Management of Line Sepsis
Recognition of IVDR sepsis
It is essential to have a high index of suspicion of infection in the granulocytopenic patient who presents with fever or nonspecific signs, such as tachycardia, tachypnea or hypotension, signs of the systemic inflammatory syndrome. In the granulocytopenic patient, fever reflects infection more than half of the time.23,24 Yet, profoundly granulocytopenic patients often do not exhibit characteristic findings of local infection on examination.25
Clinical manifestations of underlying diseases and the various forms of therapy given to the patient, such as blood products, cytotoxic drugs or enteral feeding, can produce fever, diarrhea, respiratory distress or erythroderma, mimicking infection. Drug fever is a relatively common cause of pyrexia in the hospitalized patient and, contrary to popular dogma, is not associated in most cases with a rash or eosinophilia or a prior history of atopy and can present hours, days, or even weeks after starting the culpable agent, but averages 21 days.26 Most patients will defervesce within 24-48 hours after discontinuation of the drug. The agents most commonly implicated in drug fever are the anti-infectives, especially the β-lactams; all of the antineoplastic agents; and the lupogenic drugs, INH, methyldopa, procainamide, quinidine, hydralazine and phenytoin.
Despite the challenge in identifying the source of a granulocytopenic patient's signs of sepsis,27 several clinical, epidemiologic, and microbiologic findings point strongly towards an IVD as the source of a septic episode (Table 4 ):1,28 patients with abrupt onset of signs and symptoms of sepsis without any other identifiable source should prompt suspicion of infection of an IVD; the presence of inflammation, with or without purulence, at the IVD insertion site, while present in the minority of cases, when combined with signs and symptoms of sepsis has been shown to be predictive of IVDR bacteremia and should prompt removal of the IVD; finally, recovery of certain microorganisms in multiple blood cultures, such as staphylococci, Corynebacterium or Bacillus species, Candida or Malassezia strongly suggests infection of the IVD.
Diagnostic Studies
The importance of making every effort to confirm suspected infection microbiologically cannot be overemphasized. Failure to obtain appropriate cultures before initiating empiric therapy of suspected infection may preclude determining whether infection was present in the first place when the patient responds poorly to the antimicrobial regimen and prove deleterious over the long run; the true diagnosis may be delayed because of empiric therapy; nonbacterial infection with fungi or viruses might not be recognized sufficiently early to institute lifesaving therapy; and the patient may be subjected to unnecessarily broad-spectrum antimicrobial therapy, which greatly increases the risk of drug reactions and superinfection by resistant organisms such as antibiotic-associated colitis caused by C. difficile.
Recent evidence-based guidelines provide the best current information on the evaluation of the ICU patient with fever or other signs of sepsis.27–,29Anti-infective drugs for suspected or presumed infection should never be started in the critically ill granulocytopenic patient without first obtaining blood cultures, at least one of which is drawn from a peripheral vein by percutaneous venipuncture. Granulocytopenic patients have a very high incidence of BSI.23,24 Studies have shown that obtaining more than two 10-15 mL blood cultures provides little additional yield, but it is essential in adults that an adequate total volume of blood is cultured, at least 20 mL—ideally 30 mL—to maximize the detection of BSI.1
Standard blood cultures drawn through CVCs provide excellent sensitivity for diagnosis of BSI but are more likely to be contaminated,30,31 resulting in unnecessary or suboptimal antimicrobial therapy; isolated single positive blood cultures drawn through a CVC for coagulase-negative staphylococci reflect contaminants most of the time.31
Removal and culture of the IVD has historically been the gold standard for the diagnosis of IVDR BSI, particularly with short-term catheters.1,28 Studies have demonstrated the superiority of semiquantitative or quantitative catheter tip culture methods for the diagnosis of IVDR BSI.1 The diagnosis of IVDR BSI is completed when a colonized IVD is associated with concomitant BSI, with no other plausible source (i.e., CDC's primary BSI).28
Cultures of IVDs obviously require their removal, which is a major problem in patients with long-term IVDs. Prospective studies have shown that only 25-45% of episodes of sepsis in patients with long-term devices represent true IVDR BSI.32 Thus, it would seem that development of in situ methods for detecting IVDR BSI that do not require removal of the IVD would be of great value.
If a laboratory has available an automated quantitative system for culturing blood (e.g., Isolator® lysis-centrifugation system, Wampole Laboratories, Cranbury, NJ), quantitative blood cultures drawn through the IVD and concomitantly by venipuncture from a peripheral vein (or another IVD) can permit the diagnosis of IVDR bacteremia or fungemia to be made with sensitivity and specificity in the range of 80-95%,33,34without removal of the catheter, if empiric antimicrobial therapy has not yet been initiated. With infected IVDs, the blood culture drawn through the IVD characteristically shows a 5- to 10-fold step-up in the concentration of organisms compared to the blood culture drawn peripherally. High-grade peripheral candidemia (≥ 25 CFU/mL) reflects an infected IVD 90% of the time.33 Quantitative IVD-drawn blood cultures are most useful for diagnosis of infections with long-term devices.35 There is evidence that a single quantitative culture drawn from a long-term device, even without an accompanying peripheral culture, can accurately identify a IVDR BSI if there is > 100 CFU/mL of growth.
Quantitative blood cultures are labor intensive and cost almost twice as much as standard blood cultures. The wide availability of radiometric blood culture systems (e.g., BACTEC system®, Becton Dickinson), in which blood cultures are continuously monitored for microbial growth, has led to a clever application of this system for the detection of IVDR BSI: the differential-time-to-positivity (DTP) of blood cultures drawn through the IVD and concomitantly from a peripheral site. Detection of positivity in a blood culture drawn from the IVD more than 2 hours before positivity of the culture drawn from a peripheral site has been shown to be highly predictive of IVDR BSI, in one study with long-term catheters yielding an overall sensitivity of 94% and specificity of 91%.31
Another simple but rapid and potentially cost-effective method of detecting IVDR BSI is acridine-orange leucocyte cytospin (AOLC) staining combined with gram staining of a sample of lysed and centrifuged blood drawn from the suspected IVD. In a recent prospective study this method was found to be 96% sensitive and 92% specific.36 AOLC with gram stain will likely remain useful primarily for diagnosing BSIs with long-term IVDs.
Management of the device
Short-term IVDs. If a short-term vascular catheter is suspected of being infected because the patient has no obvious other source of infection to explain fever, there is inflammation at the insertion site, or cryptogenic staphylococcal bacteremia or candidemia has been documented, blood cultures should be obtained and the catheter should be removed and cultured. Failure to remove an infected catheter puts the patient at risk of developing septic thrombophlebitis with peripheral IV catheters, septic thrombosis of a great central vein with CVCs,37,38 or even endocarditis. Continued access, if necessary, can be established with a new catheter inserted in a new site. A new catheter should never be placed in an old site over a guidewire if the first catheter is suspected of being infected, especially if there is purulence at the site.
Long-term IVDs. BSI that might have originated from a cuffed and tunneled CVC does not automatically mandate removal of the device unless (Table 5 ): there has been persistent exit site infection; the tunnel is obviously infected;39 there is evidence of complicating endocarditis, septic thrombosis, or septic pulmonary emboli,39 the infecting pathogen is S. aureus,40Corynebacterium JK,41 a Bacillus species,42Stenotrophomonas spp., Burkholdaria cepacia and all pseudomonal species,43,44 a filamentous fungus or Malassezia species,45 or a mycobacterial species;46 or bacteremia or candidemia persists for more than three days despite adequate therapy (Table 5).39
Studies using 7 to 21 days of antibiotics infused through the infected line, primarily with BSIs caused by coagulase-negative staphylococci, have shown success rates of 60-91% without catheter removal,39,47–,49 although patient response varied significantly, depending on infecting microorganism; with coagulase-negative staphylococcal BSIs, the risk of recurrent bacteremia has been approximately 20%.39,50,51 Several studies have reported successful treatment of IVD BSIs due to Candida spp. without device removal using prolonged courses of amphotericin B (AmB) administered through the catheter;15,52,53 however, this is in contrast to the results of other prospective studies that have found an increased duration of candidemia and mortality in patients who retain their infected IVD.54–,56 Until this issue is clarified by prospective randomized studies we believe that most episodes of candidemia caused by an infected IVD should mandate early removal of the IVD. Likewise, we believe that IVDR BSI caused by S. aureus should always prompt removal of the IVD, even if signs of bacteremia have resolved following antimicrobial therapy, because of the significant risk of infectious endocarditis (IE) or other metastatic infection.40
In addition to infusion of systemic antibiotics through the infected line, which we believe is mandatory for any patient with documented IVDR BSI, instillation of a highly concentrated solution of the antibiotic or antibiotic combination, “locked” into the infected tunneled catheter may be of adjunctive value to “cure” the infected IVD. In vitro testing has proven the long-term stability of solutions of most antimicrobial agents over periods of time as long as 10 days.57
In small, uncontrolled clinical trials, “antibiotic lock therapy” (ALT), usually in conjunction with systemic antibiotic therapy, “cure” rates of infected IVDs in excess of 90% have been reported.35,5– 60 The vast majority of IVDs reported in these studies were infected with gram-positive organisms other than S. aureus and Bacillus sp.—primarily coagulase-negative staphylococci— and gram-negative bacilli other than P. aeruginosa. Data are lacking on the utility of ALT for fungal IVDR BSIs and therefore, at this time, ALT cannot be recommended for the management of long-term IVDs infected by S. aureus, Bacillus sp., Corynebacterium JK, Stenotrophomonas spp., B. cepacia, all pseudomonas species, fungi or mycobacterial species.
The use of thrombolytic agents, such as streptokinase or urokinase, has been advocated as adjunctive therapy for long-term IVDs that become infected but prospective randomized trials have failed to show demonstrable benefit.61
Historically, central ports have rarely proven to be curable with medical therapy alone if the device is clearly infected (e.g., an aspirate from the port shows heavy growth).62–,64 In vitro studies of antibiotic lock solutions in simulated models of central ports raise the possibility of using ALT to preserve the use of these long-term devices when they become infected. A recent study of patients with acquired immunodeficiency syndrome (AIDS) with central ports who developed IVDR BSIs found that ALT combined with systemic antibiotic therapy resulted in 70% of the ports being salvaged; however, long-term follow-up data was not reported. A recent large clinical study of ALT for central port infections achieved salvage rates less than 50%.65 Based on the marginal efficacy of ALT in these two studies and the historically poor cure rate achieved with systemic antibiotics alone, we believe definitive treatment of infected central ports mandates their removal.
Anti-infective therapy
In general the selection of an initial antimicrobial regimen for a septic patient is influenced by 1) whether the presumed infection was acquired in the community or is institutionally acquired, 2) the age of the patient, and 3) whether or not the patient is immunocompromised, especially granulocytopenic (< 1000 per mm3).
For the febrile granulocytopenic patient without an obvious local source of infection, an antipseudomonal penicillin or cephalosporin combined with an aminoglycoside or ciprofloxacin is yet widely used. However, monotherapy with ceftazidime,66 cefepime,67 or imipenem68 will provide reliable initial coverage, pending the results of cultures; each has been studied in randomized, comparative trials and been shown to provide efficacy comparable to combination regimens including an aminoglycoside. But if monotherapy is chosen and if cultures identify an infecting organism, it is essential that the regimen be adjusted for that organism, e.g., administer two bactericidal antibiotics of different classes shown to be effective against the organism if the bloodstream pathogen is a gram-negative rod, to provide additive—ideally synergistic—activity, which appears to improve the outcome with severe granulocytopenia.69
There has been considerable controversy regarding the inclusion of vancomycin in the initial empiric regimen for the febrile granulocytopenic patient23,24 to provide a drug active against methicillin-resistant staphylococci, enterococci and Corynebacterium species. Comparative trials have shown that beginning with empiric vancomycin does reduce the frequency of secondary nosocomial BSI with these organisms during therapy;70,71 however, these studies have also shown that not including vancomycin in the initial regimen, but giving the drug only when a β-lactamase-resistant gram-positive infection is identified, is not associated with increased morbidity or mortality, and the infection can be effectively treated.70,71 Thus, the routine use of vancomycin in the initial antimicrobial regimen for the febrile granulocytopenic patient is not recommended unless:72
Line sepsis is strongly suspected, e.g., the patient shows evidence of infection at the exit site or the catheter tunnel of a CVC.
The hospital has a high rate of nosocomial infection with MRSA or the patient is known to have previously been colonized or infected by MRSA.
There are reasons to suspect overwhelming α-hemolytic viridans streptococcal bacteremia,73 e.g., shock with respiratory distress.
The patient is at risk for endocarditis, e.g., has a prosthetic heart valve.
In most cases, vancomycin can be reserved for microbiologically confirmed infections with coagulase-negative staphylococci or other resistant gram-positive organisms.
The decision to treat a suspected IVDR BSI before microbiologic confirmation, i.e. empirically, comes down to clinical judgment, weighing the evidence suggesting BSI and the risks of delayed treatment. In general, fever or other signs of sepsis in a granulocytopenic patient must be regarded as BSI, until proven otherwise.
If IVDR BSI is suspected (Table 5), after cultures have been obtained, the combination of IV vancomycin (for staphylococci resistant to methicillin) with a fluoroquinolone, preferably ciprofloxacin or cefepime or imipenem/meropenem (for aerobic gram-negative bacilli), should prove effective against the bacterial pathogens most likely to be encountered. Initial therapy can then be modified based on the microbiologic identification and susceptibility of the infecting organisms.
How long to treat IVDR BSI will be influenced by whether the patient has underlying valvular heart disease, already has evidence of endocarditis or septic thrombosis, or shows evidence of metastatic infection. If endocarditis is suspected, transesophageal echocardiography offers superior sensitivity and discrimination for detecting vegetations, as compared with transthoracic echocardiography.74 In patients with high-grade bacteremia or fungemia, but without clinical or echocardiographic evidence of endocarditis, septic thrombosis should be suspected.37,38 Central venous thrombosis can now be diagnosed by venography,37,38 ultrasonography,75 MRI,76 or CT.75– 77
While there are no prospective data to guide the optimal duration of antimicrobial therapy for IVDR BSIs, most coagulase-negative staphylococcal infections can be cured with only 5 to 7 days of therapy,1,28,50,78 whereas most infections caused by other microorganisms can be adequately treated with 10 to 14 days of antimicrobial therapy.28,55,78,79 These recommendations hold only as long as there are no complications related to the infection—endocarditis, septic thrombophlebitis, septic thrombosis, or metastatic infection, such as osteomyelitis—and the BSI clears within 72 hours of initiating therapy. Nosocomial enterococcal bacteremia deriving from an IVD is rarely associated with persistent endovascular infection, and unless there is clinical or echocardiographic evidence of endocarditis, treatment with IV ampicillin or vancomycin alone for 7 to 14 days should suffice.80
The management of S. aureus device-related infection deserves special mention, as there have been no prospective studies to evaluate the optimal duration of therapy for IVDR BSIs due to this organism. Historically, high rates of associated IE and late complications led to a universal policy of 4 to 6 weeks of antimicrobial therapy for all patients with S. aureus bacteremia. Earlier diagnosis and initiation of bactericidal therapy of nosocomial S. aureus BSIs in recent years has been associated with lower rates of IE and metastatic complications, prompting suggestions that short-course therapy (14 days) is effective and safe for most cases of IVDR S. aureus bacteremia, as long as the patient defervesces within 72 hours and there is no evidence of metastatic infection.79,81,82 In a study of routine transesophageal echocardiography (TEE) in 103 hospitalized patients with S. aureus bacteremia, 69 related to an IVD, Fowler et al found a surprisingly high rate of late complications: 23% with IVDR S. aureus BSI.74 In a more recent report, these authors have reported that the routine use of TEE with IVDR S. aureus BSI, as a means to stratify patients into short-course or long-course therapy, is cost effective. However, at this time there are no prospective studies to affirm this approach.83 Until more data are available, short-course for IVDR S. aureus bacteremia therapy should be approached with caution and only used when a TEE is unequivocally negative and the patient has defervesced within 72 hours of starting therapy.
IVDR septic thrombosis of a great central vein, which characteristically produces high-grade bacteremia or candidemia, can be reliably cured in most cases without surgical intervention, with 4 to 6 weeks of parenteral antimicrobial therapy in cases of bacterial infection37,38 and IV amphotericin B in a daily dose of 0.7 mg/kg and a total dose of approximately 20 mg/kg in cases of candidal infection.37 Unless there are contraindications, the patient should also be anticoagulated with heparin.37,38
All patients with IVDR candidemia should be treated, even if the patient becomes afebrile and blood cultures spontaneously revert to negative following removal of the catheter, without antifungal therapy.84–,86 IVDR candidemia that responds rapidly to removal of the catheter and institution of IV AmB can be reliably treated with a daily dose of 0.3 to 0.5 mg/kg and a total dose of 3-5 mg/kg.84–,86 Fluconazole (400 mg/d) has been shown to be as effective as AmB in randomized trials in non-neutropenic patients,87 and has further been shown to be comparable to AmB in observational studies of neutropenic patients with candidal IVDR BSIs88,89 but should not be used in IVDR BSIs associated with septic thrombosis and high-grade candidemia or with infections caused by fluconazole-resistant organisms, such as Candida krusei and C. glabrata.
III. Fungal Infections in Neutropenic Patients and Newer Antifungal Agents
Peter G. Pappas, MD*
University of Alabam at Birmingham, Division of Infectious Disease, 1900 University Boulevard, 229 THT, Birmingham AL 35294
Dr. Pappas received honoraria and research support from Pfizer, Merck, Fujisawa, and Schering Plough.
Systemic fungal infections are a major are a major cause of morbidity and mortality among patients with hematologic malignancies and neutropenia. Up to 20% of patients with neutropenia may experience an invasive fungal infection,1 and autopsy studies suggest that invasive fungal infections are encountered in as many as 40% of patients with hematologic malignancies.2 Important risk factors for the development of invasive fungal infections in neutropenic patients are well described.3 The most common fungal infections in this group include superficial and invasive infections due to Candida species and invasive aspergillosis. In addition to these more common fungi, several emerging pathogens including Fusarium species, Trichosporon beigelii, Scedosporium species, and the dematiaceous fungi.4,5 The growing number of patients with disease due to these fungal pathogens has been an important factor leading to the development of the newer broad spectrum antifungal agents. The following is a brief description of the more common mycoses affecting patients with hematologic malignancies and neutropenia.
Candidiasis
Invasive candidiasis in the neutropenic patient is usually associated with well-defined risk factors including the presence of the CVC, corticosteroids, broad-spectrum antibiotic exposure, mucositis and longer duration of neutropenia.6 The most common organism associated with invasive candidiasis in the neutropenic patient is Candida albicans, followed by C. tropicalis, C. glabrata, and C. parapsilosis. C. krusei is also an important pathogen among neutropenic hosts, though this organism is not a prevalent pathogen in all centers. The increased incidence of C. krusei has been seen almost exclusively in centers where fluconazole has been widely used for prophylaxis.7 Rarely C. lusitaniae, C. dubliniensis, and C. gulliermondii are seen in this population.
The overall mortality among patients with invasive Candida infections approaches 60%, with mortality attributable to Candida ranging from 15-38%.8 In addition to increased mortality, patients with invasive Candida infection may develop visceral complications of infection including endophthalmitis and chronic disseminated (hepatosplenic) candidiasis.9,10 Both of these complications typically occur days or weeks following the initial episode of candidemia and usually present after neutrophil recovery.
The treatment of uncomplicated candidemia in this patient population involves the use of an effective antifungal agent until neutrophil recovery, but not less than 14 days.11 CVC removal is recommended when possible. Complicated Candida infections such as endophthalmitis and chronic disseminated disease usually require several weeks or months of therapy and involve initial aggressive therapy with AmB.11
Invasive Aspergillosis
Invasive infection due to Aspergillus species is among the most serious infectious complications in neutropenic patients. Risk factors that are strongly associated with invasive aspergillosis include longer duration of neutropenia, use of glucocorticosteroids and other immunosuppressive agents, and chronic graft versus host disease.12,13 The most common pathogens in this group include A. fumigatus, A. terreus, A. flavus, and A. niger. These infections often begin as unremitting fever despite broad-spectrum antibacterials and are eventually accompanied by pulmonary infiltrates in most patients. In the vast majority of cases, the lungs are the portals of entry. In neutropenic and allogeneic bone marrow transplant recipients, mortality due to invasive aspergillosis approximates 80%, and approaches 100% with central nervous system (CNS) involvement.14
Options for initial therapy for invasive aspergillosis are limited. Higher doses of AmB deoxycholate (AmB-d) (1.0-1.5 mg/kg/d) or a lipid formulation of amphotericin B (at least 5 mg/kg/d) are advised in most cases.15 Parenteral itraconazole and caspofungin are indicated for cases of refractory aspergillosis unresponsive to or intolerant of initial therapy with an AmB formulation.15
Fusariosis
Infections due to Fusarium species have become increasingly common in the neutropenic population, though the overall frequency varies widely between institutions.4,16F. solani is the most frequent pathogen isolated, followed by F. oxysporum and F. moniliforme. The most important risk factor among this group of patients is prolonged period of neutropenia, often greater than 3 weeks.4 Fusariosis may emerge in the face of empiric antifungal therapy and is associated with skin lesions and positive blood cultures in the majority of patients.4 There is no currently effective therapy for fusariosis, although high dose amphotericin is probably the drug of choice. Most cases of fusariosis have fatal outcomes unless there is rapid neutrophil recovery and an absence of graft versus host disease.16
Other Emerging Fungi
A number of fungal pathogens including Trichosporon beigelii, Blastoschizomyces capitatus, Saccharromyces cerevisiae, and Malassezia furfur have emerged as increasingly common causes of fungemia and invasive fungal infections among neutropenic patients.17 These infections are typically associated with central venous catheters and most often require catheter removal in addition to a specific antifungal therapy. In addition, the phaeohyphomycoses (pigmented fungi), including Bipolaris spp., Alternaria and Exophiala, have emerged as important pathogens in these patients.18 Infections due to the pigmented fungi may present with cutaneous, sinopulmonary or CNS involvement and are typically refractory to therapy with AmB. Among the currently available agents, itraconazole appears to have greatest activity against these pathogens.
New Antifungal Agents
Over the last decade, the growing number of immunocompromised patients at risk for invasive fungal infections, the development of antifungal resistance among older more established pathogens, and the emergence of new fungal pathogens have led to an emphasis on the development of newer antifungal agents. In this section, we will discuss some of the newer antifungal agents and their potential role in the neutropenic patient.
Polyenes
Amphotericin B Deoxycholate (AmB-d)
A brief discussion of amphotericin B deoxycholate (AmB-d) is necessary to understand and appreciate the impact of the newer lipid formulations of AmB (Table 6 ). AmB-d is a polyene antifungal agent approved for use in humans in 1959. The mechanism of action of AmB, as well as other polyenes such as nystatin, is mediated through binding to ergosterol, the principal sterol component in the cell membrane of most fungi. This binding results in defects of the cell membrane that cause depolarization and increased membrane permeability, eventually leading to cell death.19
The toxicity of AmB-d is well established.20,21 The most commonly observed infusion-related side effects include fever, chills and myalgias. Among the delayed toxicities of AmB-d, nephrotoxicity is the most significant, which may be due to either direct tubular toxicity or decreased glomerular blood flow associated with vasoconstriction. Tubular toxicity is often accompanied by wasting of potassium and magnesium. The infusion-related side effect of AmB-d can usually be ameliorated by pre-medication with acetominophen and/or meperidine. Renal and electrolyte abnormalities can be minimized by co-administration of saline (usually 500 to 1,000 cc) and replacement of potassium and magnesium.22
AmB-d is among the oldest systemic antifungal agents and has activity against most important fungal pathogens in humans, and it remains a mainstay of antifungal therapy. Notable exceptions include Candida lusitaniae, certain Aspergillus species including A. terreus, most Fusarium species, Malassezia furfur, Trichosporon beigelii, Scedosporium species, and the dematiaceous fungi.
Lipid Formulations of Amphotericin B
There are currently three lipid formulations of AmB: AmB lipid complex (ABLC), AmB colloidal dispersion (ABCD), and liposomal AmB (L-AmB). Each of these lipid formulations of AmB shares the same mechanism of action with the parent compound, and all are less nephrotoxic than AmB-d.23 The spectrum of activity is virtually identical to the parent compound. None of these agents are approved for primary therapy for an established fungal infection; however, they are approved for salvage therapy among patients unresponsive to or intolerant of AmB-d.
Amphotericin B Lipid Complex
ABLC is composed of AmB complexed with two phospholipids, dimyristoyal phophatidyl (DMPC) and dimyristoral phosphatidylglycerol choline (DMPG) in a 7:3 ratio. ABLC has a 35% molar ratio of AmB to the lipids, which are formed into ribbon-like structures complexed with AmB. Few randomized prospective studies with ABLC have been conducted. The bulk of clinical experience with ABLC prior to FDA approval was through a compassionate use program.24 Among the lipid formulations of AmB, ABLC reaches the highest concentrations in liver, spleen, lungs and reticuloendothelial tissues.23 The usual dosing of ABLC is 5 mg/kg/d, though doses of as much as 20 mg/kg/d have been given without substantial nephrotoxicity. Likewise, lower doses (e.g. 3 mg/kg/d) have been effective when given to patients with invasive candidiasis.
Amphotericin B Colloidal Dispersion
ABCD is a stable complex of AmB and cholesteryl sulfate in a 1:1 molar ratio. The in vitro activity of ABCD appears identical to that of conventional AmB-d; however, tissue distribution of ABCD differs in several aspects from AmB-d. The compound is concentrated significantly in the liver but achieves significantly lower concentrations in other organs such as the spleen, kidneys, lungs and brain.23
In a large, randomized, double-blinded clinical trial involving the empiric use of ABCD versus AmB-d for 196 persistently febrile neutropenic patients, both compounds appeared clinically equivalent with approximately 50% success rates in each arm.25 Among the ABCD recipients, there was significantly less nephrotoxicity but significantly more infusion-related adverse events including fever, chills, and hypoxia than in the AmB-d recipients. The reported frequency of these adverse events appears to be greater than that seen with the other two lipid formulations and conventional AmB-d.24 ABCD is administered at doses of 3 to 6 mg/kg and doses as high as 7.5 mg/kg have been administered safely. Thus, the major disadvantage of use of ABCD has been a high incidence of acute infusion-related side effects including chills, fever and hypoxia.
Liposomal Amphotericin B (L-AmB)
AmBisome is the only true liposomal lipid formulation of AmB. This compound consists of spherical vessels made up of hydrogenated soy phosphatidylcholine and disteaoryl phosphatidylglycerol stabilized by cholesterol and combined with AmB. Its in vitro activity is comparable to that of AmB, and the pharmacokinetics of L-AmB are quite distinct from the other two lipid formulations of AmB.23 Plasma concentrations of L-AmB are much higher since the compound remains in the circulation much longer. Like the other formulations, L-AmB concentrates in the reticuloendothelial system. L-AmB has the highest concentrations in the CNS of all these compounds.26 In addition, it appears to be the least nephrotoxic and least associated with infusion-related toxicity compared with all other formulations of AmB.
L-AmB is the most widely studied of the lipid formulations of AmB. In the largest published randomized double-blinded study to date, 687 persistently febrile and neutropenic patients received empiric antifungal treatment with either AmB-d or L-AmB.27 Results of the study indicated that the two compounds had similar efficacy (50%), though there was significantly less infusion-related toxicity, nephrotoxicity, and fewer emergent fungal infections in the L-AmB recipients when compared to patients who received AmB-d. In a subsequent randomized study of febrile neutropenic patients, L-AmB was associated with a similar low rate of infusion-related and nephrotoxic adverse events.28
The usual dose of L-AmB is between 1 and 5 mg/kg/d. Doses as high as 15 mg/kg/d have been used safely,29 and optimal dosing for specific fungal infections is not known.
Liposomal Nystatin
Liposomal nystatin is a lipid-based polyene antifungal agent composed of nystatin incorporated into liposomes containing DMPC and DMPG. It is not yet approved by the FDA. Its mechanism of action is similar to AmB. This compound has been studied in patients with candidiasis and cryptococcosis.30 To date, results of these clinical studies do not suggest an advantage over standard agents. There are no published trials examining the use of liposomal nystatin in the febrile neutropenic patient. Some have suggested that compound will become a second or third line agent for patients with refractory fungal disease including invasive aspergillosis and candidiasis unresponsive to or intolerant of other antifungal agents. Liposomal nystatin is dosed between 0.5 and 4 mg/kg, though the optimal dose is not known.
Echinocandins
Echinocandins represent a new class of antifungal drugs. These are large compounds originally derived from several fungal species. They are cell-wall active agents that presumably act through binding and inhibition of 1, 3-β glucan sythetase, thereby inhibiting production of 3-β glucan, an important structural component of the fungal cell wall of many pathogens.31 These compounds are rapidly fungicidal against most Candida species and are fungistatic versus most Aspergillus species.32 They also demonstrate good activity versus Pneumocystis carinii and limited activity versus Fusarium species, Zygomycetes, and the endemic fungi. They have little or no activity against C. neoformans. All of the echinocandins currently under development are administered parenterally and can be dosed once daily. There is little infusion-related toxicity with the echinocandins and little or no renal and hepatic toxicity. Thus, as a class, these compounds represent a new mechanism in antifungal therapeutics and provide the added advantage of significantly less toxicity than AmB. Three echinocandins are in development: caspofungin (MK991), micafungin (FK463) and anidulafungin (LY33060).
Caspofungin
Caspofungin is the only echinocandin currently approved by the FDA. This compound is approved for treatment of refractory aspergillosis and in patients intolerant of formulations of AmB. Despite its approval, there is little published clinical data on this compound, as only 56 patients with refractory aspergillosis or intolerant to therapy have been presented to date.33 Overall response rate in this group was 45%, but among patients with persistent neutropenia, only 2 of 11 (18%) had a favorable response. One large randomized blinded study is comparing caspofungin to L-AmB for persistently febrile neutropenic patients. It is anticipated that approximately 1,000 patients will be included in this ongoing study and that results from this study will provide important insights as to the role the echinocandins might play in this clinical situation.
One potential use of the echinocandins is in combination with other antifungal agents for treatment of filamentous fungal infections including aspergillosis. To date, there are few human data, but encouraging animal data suggest that there may be a synergistic effect when an echinocandin is combined with AmB or a triazole to treat either candidiasis or aspergillosis.34,35
Micafungin
Micafungin has similar broad-spectrum fungicidal activity against Candida species similar to caspofungin and anidulafungin. It also has in vitro activity against Aspergillus species at concentrations lower than AmB and itraconazole, though micafungin is not fungicidal against these organisms.31,32 The drug has not been approved by the FDA, and there are limited clinical data on this compound, though a number of clinical trials have been conducted, including studies of over 800 bone marrow transplant recipients who received either fluconazole or micafungin as primary prophylaxis for fungal infection. Optimal dosing for micafungin is not known, but doses of 50 mg per day have been used in clinical trials and appear to be effective. Toxicity is similar to the other echinocandins.
Anidulafungin
This compound is a promising echinocandin with activity similar to that of caspofungin and micafungin.31,32 Because there is limited clinical experience with this compound, its toxicities and optimal dosing are unknown, but it is likely that it has a safety profile similar to the other two compounds.
Triazoles
Three new triazoles, none of which has been approved by FDA, are in various stages of development. These include voriconazole, posaconazole, and ravuconazole. They are derivatives of fluconazole (voriconazole, ravuconazole) and itraconazole (posaconazole). They share some of the favorable pharmacokinetics features of these agents and appear to have acceptable safety profiles. Each of these agents offers broad spectrum antifungal activity including activity against most strains of Candida species and Aspergillus species.
Voriconazole
Voriconazole (Table 7 ) is a potent second-generation triazole and a derivative of fluconazole, and is currently undergoing extensive phase III clinical evaluation. This compound shows important fungicidal activity against Aspergillus species, and also demonstrates significant activity against most Candida species, C. neoformans, Scedosporium prolificans, and many dematiaceous fungi.36,37 In addition, the compound has limited activity against Fusarium but no in vitro activity against the zygomycetes. In addition, voriconazole has good activity against most endemic fungi including B. dermatitidis, H. capsulatum, and P. marneffii. It has excellent oral bioavailability and is distributed widely in tissues including the CNS. The compound is metabolized hepatically, and levels in the urine are less than 5% of unchanged drug.37 It is available in oral or parenteral form.
Voriconazole has been well tolerated in clinical trials. Its main toxicity has been transient visual disturbances (photopsia) and hepatic enzyme elevation in 20% and 10% of patients, respectively. In the largest of these studies, over 800 persistently febrile neutropenic patients were randomized in an open-label study to receive either voriconazole or L-AmB for empiric antifungal treatment.28 In this study, the two compounds demonstrated comparable efficacy, with successful outcomes in 26% and 30%, respectively, for voriconazole and L-AmB recipients. Renal toxicity was significantly greater in L-AmB recipients and visual disturbances were reported much more commonly in the voriconazole recipients. One of the most important observations in this study, however, was the significant decrease in breakthrough invasive fungal infections in the voriconazole versus L-AmB recipients (8 vs. 21 patients). In salvage studies of invasive aspergillosis, voriconazole has been associated with an overall favorable response in approximately 45% of patients, comparable to other approved agents (unpublished data).
Posaconazole
Posaconazole (Table 7) is a derivative of itraconazole and shares with itraconazole its very low water solubility. At present, the compound is only available in an oral formulation. It provides broad antifungal activity against a variety of filamentous fungi such as Aspergillus species, Scedosporium species, Bipolaris, and zygomycetes.37,38 The compound appears to have some activity against Fusarium spp., and has excellent activity against many yeasts including Candida species, C. neoformans, and the dimorphic fungi. It is currently in phase III studies, and few data have been published on its efficacy in neutropenic patients. It offers the broadest antifungal spectrum of the newer agents. The use of posaconazole may ultimately depend on its availability as a parenteral compound. It shares the same toxicity profile as the other drugs in its class.
Ravuconazole
Ravuconazole is structurally similar to fluconazole and voriconazole. At present, it is available orally and parenterally. The compound has significant activity against Candida species, C. neoformans, Aspergillus species, and the dematiaceous fungi.37,39 Its activity against Fusarium and the zygomycetes is modest. At present, this drug is undergoing investigation in phase I and phase II trials. No published efficacy data in humans is available, but the compound has good promise as an effective agent against selected filamentous fungi.
Summary
Invasive fungal infections continue to have an enormous impact on morbidity and mortality among neutropenic patients. Newer pathogens, especially the filamentous fungi, present important therapeutic challenges to the clinician. Many of the newer antifungal agents offer important advances in spectrum of activity, mechanisms of action, and are well tolerated by patients. The specific role of each agent remains undetermined, but the availability of those new compounds provides opportunities for new and innovative approaches to the treatment and prevention of fungal infections in these highly vulnerable patients.
IV. Outpatient Therapy for the Neutropenic Patient
Kenneth V. I. Rolston, MD*
M.D. Anderson Cancer Center, Dept. of Infectious Diseases, Infection Control and Employee Health, 1515 Holcombe Blvd., Box 402, Houston TX 77030
Dr. Rolston receives grant support and is on speakers' bureaus for Bayer, BMS, AstraZeneca, Pfizer, Merck, and Elan.
In a series of landmark studies published several decades ago, infections and hemorrhagic complications (often both in the same patient) were documented to be the leading causes of death in patients with neoplastic disorders, particularly those of hematological origin.1,2 Neutropenia was recognized as the leading factor predisposing toward infection, with severe (< 100 PMN/mm3) and prolonged (> 14d) neutropenia having a significant impact on both the frequency of infection and on response to therapy.3 Over the past four decades several advances in supportive care including transfusion medicine; empiric, specific, and prophylactic antimicrobial therapy; and the development of the hematopoetic growth factors, have substantially reduced the morbidity and mortality associated with hemorrhagic and infectious complications. Until recently most patients with fever and neutropenia have been managed in a hospital-based setting in order to monitor them closely and deal promptly with life-threatening complications, should they occur.4 Although hospital-based management has been effective, it has become apparent that not all neutropenic patients require or benefit from such therapy. In fact, recent information suggest that hospitalization might even be detrimental, and the assumption that the hospital is the safest place to treat such patients is increasingly being questioned. Data presented at the 4th Decennial International Conference on Nosocomial and Healthcare-Associated Infections (Atlanta, GA, March 2000) documented that each year approximately 2 million patients in the US acquire infections while hospitalized for other conditions.5 These infections account for 88,000 deaths and cost more than 4.6 billion dollars. Additionally, at least 70% of the healthcare-associated infections diagnosed in hospitals are caused by bacteria that are resistant to at least one antimicrobial agent generally used for the treatment of such infections, and an increasing proportion of hospital-acquired isolates are multidrug-resistant. Although similar infections occur in other settings (nursing homes, outpatient clinics, patients' homes), they are much less frequent in the home-care setting than in a hospital or long term care setting (1% vs. 5%).
Another recent report (“To Err is Human” issued by the Institute of Medicine) focused on the frequency of adverse events in US hospitals.6 These events ranged between 2.9% and 3.7% of hospitalizations, with between 8.8% and 13.6% of these events being fatal. Additionally, more than half of these resulted from medical errors that could have been prevented. These data again suggest that the hospital is not necessarily the safest place to deliver healthcare, especially to patients who are otherwise at very low risk for developing complications that require hospital-based monitoring.
Risk-Assessment in Febrile Neutropenia
There is uniform agreement that high-risk neutropenic patients (e.g. those with hematological malignancies and severe and prolonged neutropenia) need to be treated using standard, hospital-based, parenteral, broad-spectrum, empiric antibiotic therapy for the entire febrile episode.7 There is also general agreement that many patients with fever and neutropenia do not fall into the high-risk category. It has, however, been difficult to accurately separate high-risk from low-risk patients at the beginning of a febrile episode in order to evaluate alternatives to hospital-based antibiotic therapy. Although there is no universally accepted risk-assessment strategy, recent advances have led to the development of clinical criteria and statistically derived risk prediction rules, which are reasonably accurate in distinguishing low-risk from high-risk patients.8– 12 Although misclassifications occasionally occur, these risk-prediction rules have enabled investigators to evaluate endpoints other than response rates to antimicrobial regimens, adverse events, and mortality. Issues such as routes of drug administration, time to clinical response, site(s) and cost of care, and quality of life have become important considerations. Table 8 lists the various risk-groups and associated patient characteristics. In general, low-risk patients (in whom early discharge after initial stabilization or outpatient therapy are a potential options) are patients with solid tumors receiving conventional chemotherapy, with expected duration of neutropenia ≤ 7 days, who are clinically stable and present with unexplained fever or simple infections.
Therapeutic Options in Febrile Neutropenic Patients
The various treatment options for febrile neutropenic patients are listed in Table 9 . As indicated earlier, several factors regarding empiric antimicrobial therapy need to be considered including a) the nature of the antimicrobial regimen (combination vs. monotherapy), b) the route of drug administration (parenteral, sequential [IV →PO], or oral), and c) the site or setting of therapy (hospital based, early discharge after initial stabilization in the hospital, and outpatient/home treatment). All are important considerations and require constant re-evaluation of the clinical situation and readjustment of the initial regimen and/or setting.
Initial antimicrobial regimen
The specific agent(s) chosen for empiric therapy will depend on local microflora and susceptibility/resistance patterns. Several choices are available including the following:
aminoglycoside + anti-pseudomonal penicillin
aminoglycoside + extended spectrum cephalosporin
aminoglycoside + quinolone
vancomycin + anti-pseudomonal penicillin
vancomycin + quinolone
double β-lactam combinations
carbapenem or extended spectrum cephalosporins
All of these are standard regimens and are associated with response rates of 65-85%, without modification of the initial regimen.
Routes of administration
Parenteral therapy is indicated in most hospitalized, high-risk patients and in those who have chemotherapy-induced mucositis or nausea/vomiting. A switch to an effective oral regimen (generally a quinolone combined with an agent active against gram-positive organisms) can be made after an initial response to parenteral therapy has occurred in patients able to tolerate an oral regimen. A substantial number of low-risk patients are eligible for oral antimicrobial therapy for the entire febrile episode.
Site or setting of therapy
The site of therapy depends largely on the patients' risk group and the complexity of the initial infection or clinical situation (Table 10 ). The remainder of this discussion will focus on empiric outpatient therapy. Parenteral outpatient regimens (for stable low-risk patients with some mucositis or nausea) include long-acting agents such as ceftriaxone ± once daily amikacin, and combination regimens such as a quinolone or aztreonam + an agent with gram-positive activity. Oral outpatient regimens generally include a quinolone in combination with amoxicillin/clavulanate, clindamycin, or a macrolide.13 Monotherapy with some of the newer, broader-spectrum quinolones (gatifloxacin, moxifloxacin) is currently being evaluated, but is not yet recommended. A large number of clinical trials have evaluated outpatient regimens in both adult and pediatric cancer populations with initial response rates varying between 77% and 95%.12,14–,17 Most failures have been in patients misclassified as “low-risk,” but the overall mortality rate has been < 2%. This is comparable to oral, hospital-based therapy, suggesting that hospitalization would not necessarily have prevented such mortality.10,11 Further improvements in risk assessment strategies might reduce even this low, outpatient mortality rate. Outpatient therapy is associated with several advantages over standard hospital-based therapy, if it can be administered safely. These are outlined in Table 11 . Several clinical trials have demonstrated the economic benefits of this approach, particularly if oral regimens can be used. Enhanced quality of life for patients and increased convenience for family or other caretakers (factors which do not get much press) have also clearly been demonstrated. Eliminating or reducing exposure to multidrug resistant nosocomial pathogens has been associated with fewer secondary superinfections with such organisms, further reducing the cost of care and having a positive impact on morbidity and mortality. Additionally, a reduction in hospital associated adverse events and iatrogenic problems might also be expected with outpatient therapy.
Some potential hazards or disadvantages do exist. Serious complications (septic shock, significant bleeding in thrombocytopenic patients, or seizures) although distinctly uncommon, may occur, and delays in management while patients are being transported to the hospital are possible. Non-compliance with oral regimens or infusion-related problems may also occur, but can be minimized with meticulous monitoring and follow-up.
A successful outpatient therapy program requires considerable commitment from all parties involved (Table 12 ). Institutional support to create and/or maintain an adequate infrastructure to deal with substantial numbers of febrile neutropenic patients being treated in the outpatient setting is critical. This includes a dedicated team of healthcare providers (physicians, nurses, pharmacists, infusion therapists, home healthcare providers) who are interested and experienced in such a program, and 24 hour access to the team, should complications requiring urgent interventions occur. The patients and their families (or other support personnel) need to be motivated, and compliant, and have adequate communication and transportation facilities. Appropriate antimicrobial therapy based on local epidemiologic and susceptibility/resistant patterns will ensure that outpatient therapy is associated with high response rates. Frequent monitoring of response, lack of response, development of complications, toxicity, and compliance is also critical and cannot be over-stressed. All these issues need to be worked out in advance to ensure a successful outpatient treatment program.
Risk-based therapy of the febrile neutropenic patient (including outpatient management) is being increasingly accepted as the standard of care. Several organizations including the National Comprehensive Cancer Network (NCCN) and the Infectious Diseases Society of America (IDSA) have included the options discussed above in their guidelines for the management of febrile neutropenic patients.7,18 All institutions dealing with such patients need to consider risk-based therapy for their patients.
| Organism . | Microbiologic Features . | Type of Infection . | Therapy . | Comments . |
|---|---|---|---|---|
| Abbreviations: ARDS, acute respiratory distress syndrome; CNS, central nervous system; gi, gastrointestinal; gu, genito-urinary | ||||
| viridans streptococci | from oral flora | bacteremia | Vancomycin until susceptibility is determined | associated with mucositis and use of certain prophylactic antibiotics |
| toxic shock-like syndrome with ARDS | increased resistance to penicillins and some cephalosporins | |||
| 6-30% mortality rates | ||||
| Enterococcus sp | from gi flora | bacteremia | no”best” therapy | associated with outbreaks |
| linezolid or quinopristin/dalfopristin may be useful | mortality rates >70% noted | |||
| Stomatococcus mucilaginous | slime producing encapsulated organism | catheter associated sepsis | vancomycin | infection may be slow to resolve or may recur even with appropriate treatment |
| from oral flora | CNS infection | |||
| bacteremia | ||||
| Bacillus cereus | slime producing bacillus | pneumonia | vancomycin | remove central line in presence of bacteremia |
| line-related sepsis | clindamycin | |||
| skin and soft tissue infection | ||||
| fasciitis | ||||
| meningitis | ||||
| Rhodococcus equi | pleiomorphic gram-positive bacillus | necrotizing pneumonia | macrolides | more commonly seen in AIDS |
| lung abscesses | vancomycin | |||
| empyema | ||||
| Corynebacterium sp | non-hemolytic, coccobacillus from skin, rectal flora | line-sepsis | vancomycin | remove central line in presence of bacteremia |
| endocarditis | ||||
| Leuconostoc sp | fastidious cocci may be mistaken for viridans streptococci | fever | clindamycin | combination therapy with penicillins + clindamycin may be best therapy |
| line sepsis | aminoglycoside | |||
| colitis | ||||
| Lactobacillus sp | bacillus from oral, gi, gu flora | bacteremia | penicillin plus aminoglycoside | mortality may be as high as 45% |
| endocarditis | ||||
| meningitis | ||||
| intrabdominal abscess | ||||
| pneumonia | ||||
| Organism . | Microbiologic Features . | Type of Infection . | Therapy . | Comments . |
|---|---|---|---|---|
| Abbreviations: ARDS, acute respiratory distress syndrome; CNS, central nervous system; gi, gastrointestinal; gu, genito-urinary | ||||
| viridans streptococci | from oral flora | bacteremia | Vancomycin until susceptibility is determined | associated with mucositis and use of certain prophylactic antibiotics |
| toxic shock-like syndrome with ARDS | increased resistance to penicillins and some cephalosporins | |||
| 6-30% mortality rates | ||||
| Enterococcus sp | from gi flora | bacteremia | no”best” therapy | associated with outbreaks |
| linezolid or quinopristin/dalfopristin may be useful | mortality rates >70% noted | |||
| Stomatococcus mucilaginous | slime producing encapsulated organism | catheter associated sepsis | vancomycin | infection may be slow to resolve or may recur even with appropriate treatment |
| from oral flora | CNS infection | |||
| bacteremia | ||||
| Bacillus cereus | slime producing bacillus | pneumonia | vancomycin | remove central line in presence of bacteremia |
| line-related sepsis | clindamycin | |||
| skin and soft tissue infection | ||||
| fasciitis | ||||
| meningitis | ||||
| Rhodococcus equi | pleiomorphic gram-positive bacillus | necrotizing pneumonia | macrolides | more commonly seen in AIDS |
| lung abscesses | vancomycin | |||
| empyema | ||||
| Corynebacterium sp | non-hemolytic, coccobacillus from skin, rectal flora | line-sepsis | vancomycin | remove central line in presence of bacteremia |
| endocarditis | ||||
| Leuconostoc sp | fastidious cocci may be mistaken for viridans streptococci | fever | clindamycin | combination therapy with penicillins + clindamycin may be best therapy |
| line sepsis | aminoglycoside | |||
| colitis | ||||
| Lactobacillus sp | bacillus from oral, gi, gu flora | bacteremia | penicillin plus aminoglycoside | mortality may be as high as 45% |
| endocarditis | ||||
| meningitis | ||||
| intrabdominal abscess | ||||
| pneumonia | ||||
Rates of intravascular device-related bloodstream infection (IVDR BSI) associated with the major types of devices used in clinical practice.*
| . | Rates of IVDR BSI . | |||
|---|---|---|---|---|
| . | per 100 IVDs . | per 1000 IVD-days . | ||
| Type of IVD (no. studies) . | Pooled Mean . | 95% CI . | Pooled Mean . | 95% CI . |
| * Based on 206 published prospective studies where every device was evaluated for infection.5 | ||||
| Abbreviations: CVCs, central venous catheters | ||||
| Peripheral venous catheters (13) | 0.2 | 0.1-0.3 | 0.6 | 0.3-1.2 |
| Arterial catheters (6) | 1.5 | 0.9-2.4 | 2.9 | 1.8-4.5 |
| Short-term, noncuffed, nonmedicated CVCs (61) | 3.3 | 3.3-4.0 | 2.3 | 2.0-2.4 |
| Hemodialysis catheters | ||||
| Noncuffed (15) | 16.2 | 13.5-18.3 | 2.8 | 2.3-3.1 |
| Cuffed (5) | 6.3 | 4.2-9.2 | 1.1 | 0.7-1.6 |
| Peripherally-inserted central catheters (PICCs)(8) | 1.2 | 0.5-2.2 | 0.4 | 0.2-0.7 |
| Long-term tunneled and cuffed CVCs (18) | 20.9 | 18.2-21.9 | 1.2 | 1.0-1.3 |
| Subcutaneous central venous ports (13) | 5.1 | 4.0-6.3 | 0.2 | 0.1-0.2 |
| . | Rates of IVDR BSI . | |||
|---|---|---|---|---|
| . | per 100 IVDs . | per 1000 IVD-days . | ||
| Type of IVD (no. studies) . | Pooled Mean . | 95% CI . | Pooled Mean . | 95% CI . |
| * Based on 206 published prospective studies where every device was evaluated for infection.5 | ||||
| Abbreviations: CVCs, central venous catheters | ||||
| Peripheral venous catheters (13) | 0.2 | 0.1-0.3 | 0.6 | 0.3-1.2 |
| Arterial catheters (6) | 1.5 | 0.9-2.4 | 2.9 | 1.8-4.5 |
| Short-term, noncuffed, nonmedicated CVCs (61) | 3.3 | 3.3-4.0 | 2.3 | 2.0-2.4 |
| Hemodialysis catheters | ||||
| Noncuffed (15) | 16.2 | 13.5-18.3 | 2.8 | 2.3-3.1 |
| Cuffed (5) | 6.3 | 4.2-9.2 | 1.1 | 0.7-1.6 |
| Peripherally-inserted central catheters (PICCs)(8) | 1.2 | 0.5-2.2 | 0.4 | 0.2-0.7 |
| Long-term tunneled and cuffed CVCs (18) | 20.9 | 18.2-21.9 | 1.2 | 1.0-1.3 |
| Subcutaneous central venous ports (13) | 5.1 | 4.0-6.3 | 0.2 | 0.1-0.2 |
Healthcare Infection Control Practices Advisory Committee (HICPAC) recommendations for the prevention of intravenous device related bloodstream infections (IVDR BSI).*
| * Adapted from the draft of the Healthcare Infection Control Practices Advisory Committee (HICPAC) guideline for the prevention of intravascular catheter-related infections.10 |
| General Measures |
| Education of all healthcare workers involved with vascular access regarding indications for use, proper insertion technique and maintenance of IVDs |
| Surveillance: |
| Institutional rates of IVDR BSI monitored routinely |
| Rates of central venous catheter (CVC)-related BSI using standardized definitions and denominators, expressed per 1000 CVC-days |
| At Insertion |
| Aseptic technique: |
| Hand washing before inserting or manipulation of any IVD |
| Clean or sterile gloves during insertion or manipulation of non-central IVD |
| Maximal barrier precautions (mask, cap, long-sleeved sterile gown, sterile gloves, and sterile sheet-drape) during insertion of CVCs |
| Dedicated IV teams strongly recommended |
| Cutaneous antisepsis: chlorhexidine preferred, however, an iodophor, such as 10% povidone-iodine, tincture of iodine or 70% |
| Sterile gauze or a sterile semipermeable polyurethane film dressing |
| Systemic antibiotics at insertion strongly discouraged |
| Maintenance |
| Remove IVDs as soon as their use is no longer essential |
| Monitor the IVD site on regular basis, ideally daily |
| Change dressing of CVC insertion site at least weekly |
| Topical antibiotic ointmentsnot recommended |
| Systemic anticoagulation with low-dose warfarin (1 mg daily) for patients with long-term IVDs and no contraindication. |
| Replace PIVCs every 72 hours |
| Replace administration sets every 72 hours unless lipid-containing admixture or blood products given, then every 24 hours |
| Technology |
| Consider use of chlorhexidine-impregnated sponge dressing with adolescent and adult patients with non-cuffed central venousor arterial catheters expected to remain in place for 4 days or more. |
| If after consistent application of basic infection control precautions, the institutional rate of IVDR BSI is yet high with short-term CVCs(≥ 3.3 BSIs per 1000 IVD-days), consider the use of an anti-infective coated CVC (chlorhexidine-silver sulfadiazine or minocycline-rifampin). |
| In individual patients with long-term IVDs who have had recurrent IVDR BSIs despite consistent application of infection control practices, consider the use of a prophylactic antibiotic lock solution (i.e., heparin with vancomycin (25 μg/mL), with or without, ciprofloxacin (2 μg/mL). |
| * Adapted from the draft of the Healthcare Infection Control Practices Advisory Committee (HICPAC) guideline for the prevention of intravascular catheter-related infections.10 |
| General Measures |
| Education of all healthcare workers involved with vascular access regarding indications for use, proper insertion technique and maintenance of IVDs |
| Surveillance: |
| Institutional rates of IVDR BSI monitored routinely |
| Rates of central venous catheter (CVC)-related BSI using standardized definitions and denominators, expressed per 1000 CVC-days |
| At Insertion |
| Aseptic technique: |
| Hand washing before inserting or manipulation of any IVD |
| Clean or sterile gloves during insertion or manipulation of non-central IVD |
| Maximal barrier precautions (mask, cap, long-sleeved sterile gown, sterile gloves, and sterile sheet-drape) during insertion of CVCs |
| Dedicated IV teams strongly recommended |
| Cutaneous antisepsis: chlorhexidine preferred, however, an iodophor, such as 10% povidone-iodine, tincture of iodine or 70% |
| Sterile gauze or a sterile semipermeable polyurethane film dressing |
| Systemic antibiotics at insertion strongly discouraged |
| Maintenance |
| Remove IVDs as soon as their use is no longer essential |
| Monitor the IVD site on regular basis, ideally daily |
| Change dressing of CVC insertion site at least weekly |
| Topical antibiotic ointmentsnot recommended |
| Systemic anticoagulation with low-dose warfarin (1 mg daily) for patients with long-term IVDs and no contraindication. |
| Replace PIVCs every 72 hours |
| Replace administration sets every 72 hours unless lipid-containing admixture or blood products given, then every 24 hours |
| Technology |
| Consider use of chlorhexidine-impregnated sponge dressing with adolescent and adult patients with non-cuffed central venousor arterial catheters expected to remain in place for 4 days or more. |
| If after consistent application of basic infection control precautions, the institutional rate of IVDR BSI is yet high with short-term CVCs(≥ 3.3 BSIs per 1000 IVD-days), consider the use of an anti-infective coated CVC (chlorhexidine-silver sulfadiazine or minocycline-rifampin). |
| In individual patients with long-term IVDs who have had recurrent IVDR BSIs despite consistent application of infection control practices, consider the use of a prophylactic antibiotic lock solution (i.e., heparin with vancomycin (25 μg/mL), with or without, ciprofloxacin (2 μg/mL). |
Clinical features of intravascular line-related sepsis.1
| Nonspecific . | Highly Suggestive of Line-Related Etiology . |
|---|---|
| Fever | Source of sepsis inapparent |
| Chills, shaking rigors | Patient unlikely candidate for sepsis |
| Hypotension, shock | Intravascular line in place (or recently placed) |
| Hyperventilation | Inflammation or purulence at insertion site |
| Gastrointestinal | Abrupt onset, associated with shock |
| Abdominal pain | Sepsis refractory to antimicrobial therapy or dramatic improvement with serendipitous removal of device and infusion |
| Vomiting | |
| Diarrhea | |
| Neurologic | Cryptogenic bloodstream infection with: |
| Confusion | Staphylococcus aureus |
| Seizures | Coagulase-negative Staphylococcus |
| Corynebacterium spp. | |
| Bacillus spp. | |
| Candida spp. | |
| Malassezia spp. |
| Nonspecific . | Highly Suggestive of Line-Related Etiology . |
|---|---|
| Fever | Source of sepsis inapparent |
| Chills, shaking rigors | Patient unlikely candidate for sepsis |
| Hypotension, shock | Intravascular line in place (or recently placed) |
| Hyperventilation | Inflammation or purulence at insertion site |
| Gastrointestinal | Abrupt onset, associated with shock |
| Abdominal pain | Sepsis refractory to antimicrobial therapy or dramatic improvement with serendipitous removal of device and infusion |
| Vomiting | |
| Diarrhea | |
| Neurologic | Cryptogenic bloodstream infection with: |
| Confusion | Staphylococcus aureus |
| Seizures | Coagulase-negative Staphylococcus |
| Corynebacterium spp. | |
| Bacillus spp. | |
| Candida spp. | |
| Malassezia spp. |
Algorithm for diagnosis and management of line sepsis with long-term intravenous devices (IVDs).
| * Per 1000 days a central line was used. |
| • Examine the patient thoroughly to identify unrelated sources of infection. |
| • Carefully examine all catheter insertion sites; gram stain and culture any expressible purulence. |
| • Obtain two 10-15 mL cultures: |
| • If standard (nonquantitative) blood cultures, draw one by percutaneous peripheral venipuncture and one through the suspect IVD. |
| If quantitative blood culture techniques are available (e.g., the Isolator® system), catheter-drawn cultures can enhance the diagnostic specificity of blood culturing in diagnosis of line sepsis. However, a peripheral percutaneous quantitative blood culture must be drawn concomitantly. |
| • Option regarding a peripheral IV or arterial catheter: remove and culture catheter. |
| • Options regarding a short-term central venous catheter: |
| •Purulence at insertion site |
| or |
| No purulence, but patient floridly septic, without obvious source: |
| Remove and culture catheter. |
| Gram stain purulence. |
| Re-establish access at new site. |
| •No purulence, patient not floridly septic: |
| • Leave catheter in place, pending results of blood cultures. |
| or |
| •Remove and culture catheter, re-establish needed access at new site. |
| • Options regarding surgically-implanted, cuffed Hickman-type catheters. |
| •Remove at outset if: |
| • Infecting organism known to be S. aureus, Bacillus spp., JK Diptheroid, Mycobacterium species or filamentous fungus. |
| • Refractory or progressive exit site infection, despite antimicrobial therapy, especially with Pseudomonas aeruginosa. |
| • Tunnel infected. |
| • Evidence of septic thrombosis of cannulated central vein or septic pulmonary emboli. |
| • Evidence of endocarditis. |
| •Remove later on if: |
| • Any of the above become manifest. |
| • BSI persists ≥ 3 days, despite IV antimicrobial therapy through catheter. |
| • Options regarding surgically implanted subcutaneous ports (e.g., Portacath): |
| • Cellulitis without documented bacteremia: begin antimicrobial therapy, withhold removing port. |
| • Aspirate from port shows organisms on gram-stain or heavy growth in quantitative culture, or documented port-related bacteremia: remove port. |
| • Decision on whether to begin antimicrobial therapy, before culture results available, based on clinical assessment and/or gram stain of exit site or the blood drawn from a long-term IVD. |
| • With no microbiologic data to guide antimicrobial selection in a septic patient with suspected line sepsis, consider: IV vancomycin and ciprofloxacin, cefepime, or imipenem. |
| * Per 1000 days a central line was used. |
| • Examine the patient thoroughly to identify unrelated sources of infection. |
| • Carefully examine all catheter insertion sites; gram stain and culture any expressible purulence. |
| • Obtain two 10-15 mL cultures: |
| • If standard (nonquantitative) blood cultures, draw one by percutaneous peripheral venipuncture and one through the suspect IVD. |
| If quantitative blood culture techniques are available (e.g., the Isolator® system), catheter-drawn cultures can enhance the diagnostic specificity of blood culturing in diagnosis of line sepsis. However, a peripheral percutaneous quantitative blood culture must be drawn concomitantly. |
| • Option regarding a peripheral IV or arterial catheter: remove and culture catheter. |
| • Options regarding a short-term central venous catheter: |
| •Purulence at insertion site |
| or |
| No purulence, but patient floridly septic, without obvious source: |
| Remove and culture catheter. |
| Gram stain purulence. |
| Re-establish access at new site. |
| •No purulence, patient not floridly septic: |
| • Leave catheter in place, pending results of blood cultures. |
| or |
| •Remove and culture catheter, re-establish needed access at new site. |
| • Options regarding surgically-implanted, cuffed Hickman-type catheters. |
| •Remove at outset if: |
| • Infecting organism known to be S. aureus, Bacillus spp., JK Diptheroid, Mycobacterium species or filamentous fungus. |
| • Refractory or progressive exit site infection, despite antimicrobial therapy, especially with Pseudomonas aeruginosa. |
| • Tunnel infected. |
| • Evidence of septic thrombosis of cannulated central vein or septic pulmonary emboli. |
| • Evidence of endocarditis. |
| •Remove later on if: |
| • Any of the above become manifest. |
| • BSI persists ≥ 3 days, despite IV antimicrobial therapy through catheter. |
| • Options regarding surgically implanted subcutaneous ports (e.g., Portacath): |
| • Cellulitis without documented bacteremia: begin antimicrobial therapy, withhold removing port. |
| • Aspirate from port shows organisms on gram-stain or heavy growth in quantitative culture, or documented port-related bacteremia: remove port. |
| • Decision on whether to begin antimicrobial therapy, before culture results available, based on clinical assessment and/or gram stain of exit site or the blood drawn from a long-term IVD. |
| • With no microbiologic data to guide antimicrobial selection in a septic patient with suspected line sepsis, consider: IV vancomycin and ciprofloxacin, cefepime, or imipenem. |
Summary of Amphotericin B (AmB) formulations.
| . | AmB . | L-AmB . | ABLC . | ABCD . |
|---|---|---|---|---|
| Abbreviations: HPC, hydrogenated phosphatidylcholine; CHOL, cholesterol; DMPC, dimyristoyl phosphatidylcholine; DMPG, dimyristoyl phosphatidylglycerol | ||||
| Trade Name (US) | Fungizone | AmBisome | Abelcet | Amphotec |
| Lipid components | Deoxycholate | HPC/CHOL/DSPG | DMPC/DMPG | Cholesterylsulfate |
| Mol % AmB | 34% | 10% | 35 % | 50% |
| Standard dose | 0.5 – 1.5 mg/kg | 3-5 mg/kg | 3-5 mg/kg | 3-5 mg/kg |
| Relative nephrotoxicity | ++++ | + | ++ | ++ |
| Infusion related toxicity | +++ | + | ++ | ++ |
| . | AmB . | L-AmB . | ABLC . | ABCD . |
|---|---|---|---|---|
| Abbreviations: HPC, hydrogenated phosphatidylcholine; CHOL, cholesterol; DMPC, dimyristoyl phosphatidylcholine; DMPG, dimyristoyl phosphatidylglycerol | ||||
| Trade Name (US) | Fungizone | AmBisome | Abelcet | Amphotec |
| Lipid components | Deoxycholate | HPC/CHOL/DSPG | DMPC/DMPG | Cholesterylsulfate |
| Mol % AmB | 34% | 10% | 35 % | 50% |
| Standard dose | 0.5 – 1.5 mg/kg | 3-5 mg/kg | 3-5 mg/kg | 3-5 mg/kg |
| Relative nephrotoxicity | ++++ | + | ++ | ++ |
| Infusion related toxicity | +++ | + | ++ | ++ |
Selected pharmacologic features of voriconazole and posaconazole.
| . | Voriconazole . | Posaconazole . |
|---|---|---|
| Abbreviations: CSF, cerebrospinal fluid | ||
| T ½ (hrs) | 6 | 25 |
| % protein binding | 65% | >90% |
| Metabolism | Hepatic | Hepatic |
| Oral bioavailability | 90% | 35% |
| CSF / serum | 50% | <1% |
| Urine / serum | 5% | <1% |
| Administration | p.o. / i.v. | p.o. |
| . | Voriconazole . | Posaconazole . |
|---|---|---|
| Abbreviations: CSF, cerebrospinal fluid | ||
| T ½ (hrs) | 6 | 25 |
| % protein binding | 65% | >90% |
| Metabolism | Hepatic | Hepatic |
| Oral bioavailability | 90% | 35% |
| CSF / serum | 50% | <1% |
| Urine / serum | 5% | <1% |
| Administration | p.o. / i.v. | p.o. |
Risk groups in febrile neutropenic patients.
| Risk Group . | Patient Characteristic . |
|---|---|
| High-risk | Severe (ANC < 100) and prolonged (> 14d) neutropenia. Hematological malignancy; allogeneic bone marrow/stem cell transplantation; significant medical co-morbidity or poor performance status; presentation with shock, complex infection (e.g. pneumonia, meningitis) |
| Intermediate (moderate) risk | Solid tumors → intensive chemotherapy → autologous hematopoetic stem cell transplantation. Moderate duration of neutropenia (7-14 days). Minimal medical comorbidity. Clinical/ hemodynamic stability. |
| Low-risk | Solid tumors → conventional chemotherapy. No comorbidity. Short duration of neutropenia (≤ 7 days). Clinical and hemodynamic stability. Unexplained fever (FUO) or simple infection (eg. UTI, simple cellulitis). |
| Risk Group . | Patient Characteristic . |
|---|---|
| High-risk | Severe (ANC < 100) and prolonged (> 14d) neutropenia. Hematological malignancy; allogeneic bone marrow/stem cell transplantation; significant medical co-morbidity or poor performance status; presentation with shock, complex infection (e.g. pneumonia, meningitis) |
| Intermediate (moderate) risk | Solid tumors → intensive chemotherapy → autologous hematopoetic stem cell transplantation. Moderate duration of neutropenia (7-14 days). Minimal medical comorbidity. Clinical/ hemodynamic stability. |
| Low-risk | Solid tumors → conventional chemotherapy. No comorbidity. Short duration of neutropenia (≤ 7 days). Clinical and hemodynamic stability. Unexplained fever (FUO) or simple infection (eg. UTI, simple cellulitis). |
Treatment options for febrile neutropenic patients.
| Based on the nature of the initial regimen → | Combination therapy Monotherapy |
| Based on the route of antibiotic administration → | Parenteral Sequential (IV → PO) Oral |
| Based on site of care → | Hospital based |
| Early discharge (step down) | |
| Outpatient therapy |
| Based on the nature of the initial regimen → | Combination therapy Monotherapy |
| Based on the route of antibiotic administration → | Parenteral Sequential (IV → PO) Oral |
| Based on site of care → | Hospital based |
| Early discharge (step down) | |
| Outpatient therapy |
Treatment options based on risk and site of therapy.
| Risk Group . | Treatment Options . |
|---|---|
| High-risk | Hospital-based, broad-spectrum, parenteral therapy for duration of febrile episode |
| Intermediate (moderate) risk | Initial hospital-based parenteral therapy followed by early discharge on a parenteral or oral regimen |
| Low-risk | Outpatient therapy (parenteral, sequential, or oral) for the entire episode |
| Risk Group . | Treatment Options . |
|---|---|
| High-risk | Hospital-based, broad-spectrum, parenteral therapy for duration of febrile episode |
| Intermediate (moderate) risk | Initial hospital-based parenteral therapy followed by early discharge on a parenteral or oral regimen |
| Low-risk | Outpatient therapy (parenteral, sequential, or oral) for the entire episode |
Outpatient therapy: advantages and disadvantages.
| Advantages |
| • Lower cost of care (particularly using oral regimens) |
| • Enhanced quality of life (patients) |
| • Increased convenience (family or caretakers) |
| • Reduced rates of nosocomial resistant superinfections |
| • Reduction in iatrogenic complications and other adverse events associated with hospitalization |
| • More efficient overall resource utilization |
| Disadvantages |
| • Potential for serious complications (septic shock, hemorrhage, seizures) occurring in an unsupervised setting |
| • Potential for non-compliance (oral therapy) |
| • Infusion-related problems |
| • Need to create and maintain an infrastructure; requires institutional commitment |
| Advantages |
| • Lower cost of care (particularly using oral regimens) |
| • Enhanced quality of life (patients) |
| • Increased convenience (family or caretakers) |
| • Reduced rates of nosocomial resistant superinfections |
| • Reduction in iatrogenic complications and other adverse events associated with hospitalization |
| • More efficient overall resource utilization |
| Disadvantages |
| • Potential for serious complications (septic shock, hemorrhage, seizures) occurring in an unsupervised setting |
| • Potential for non-compliance (oral therapy) |
| • Infusion-related problems |
| • Need to create and maintain an infrastructure; requires institutional commitment |
Requirements for a successful outpatient program.
| • Adequate institutional infrastructure |
| • Dedicated team of healthcare providers |
| • Availability of local epidemiologic and susceptibility/resistance data |
| • Selection of appropriate (not just convenient) empiric regimens |
| • Adequate follow-up and monitoring of patients in the outpatient setting (clinic or office) |
| • Motivated, compliant patients and family (or other support personnel) |
| • Adequate transportation and communication |
| • Access 24 hours a day to management team and ambulatory care facility (Emergency department; hot-line to answer questions) |
| • Adequate institutional infrastructure |
| • Dedicated team of healthcare providers |
| • Availability of local epidemiologic and susceptibility/resistance data |
| • Selection of appropriate (not just convenient) empiric regimens |
| • Adequate follow-up and monitoring of patients in the outpatient setting (clinic or office) |
| • Motivated, compliant patients and family (or other support personnel) |
| • Adequate transportation and communication |
| • Access 24 hours a day to management team and ambulatory care facility (Emergency department; hot-line to answer questions) |
Sources of infection of a percutaneous intravascular device. The major sources are the skin flora, contamination of the catheter hub, contamination of infusate, and hematogenous colonization of the intravascular device and its fibronectin-fibrin sheath from distant, unrelated sites of infection.1
Sources of infection of a percutaneous intravascular device. The major sources are the skin flora, contamination of the catheter hub, contamination of infusate, and hematogenous colonization of the intravascular device and its fibronectin-fibrin sheath from distant, unrelated sites of infection.1
Trends in rates of central line-associated bloodstream infection, by type of intensive care unit. National Nosocomial Infection Surveillance System, United States, 1990-1999.11
Trends in rates of central line-associated bloodstream infection, by type of intensive care unit. National Nosocomial Infection Surveillance System, United States, 1990-1999.11