Abstract
Erythropoietin (EPO) is an endogenous hormone produced in the kidney that regulates red blood cell production within the body. Since the cloning and first clinical introduction of recombinant erythropoietin (epoetin) in the late 1980s indications and usage of epoetin have expanded significantly. It is estimated that as many as one third of patients with substantial anemia (hemoglobin less than 10.0 g/dL) resulting from chemotherapy for cancer are treated with epoetin. Though use of epoetin may avoid the inconvenience and infectious risk of blood transfusions, it is expensive and its benefit in some clinical scenarios has been modest. Like many new technologies, strong evidence suggesting situations where the benefit is high has lagged behind its adoption by patients and practitioners. As well, epoetin is expensive and third party payers do not always reimburse it. Research suggests there is considerable variation in epoetin usage in practice.
To provide guidance to hematology/oncology specialists regarding use of epoetin, the American Society of Hematology (ASH) and the American Society of Clinical Oncology (ASCO) proposed that the Agency for Healthcare Research and Quality (AHRQ) fund an evidence review by one of the Evidence-based Practice Centers (EPC) that would be used to develop evidence-based guidelines for members of the society. This review highlights principles of evidence-based medicine, distills and appraises the evidence in the published literature that supports the use of epoetin, and presents evidence-based recommendations for use of epoetin in situations where benefit is substantiated by high-quality studies. As well, this review addresses some of the difficulties of performing clinical research in this area, provocative research findings that will require further study, and suggestions regarding epoetin in those areas where further strong evidence has yet to be developed.
I. Evidence-Based Medicine and Evidence-Based Practice Guidelines
J. Douglas Rizzo, MD*
IBMTR/ABMTR, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee WI 53226-4801
Erythropoietin is an endogenous hormone produced in the kidney that regulates red blood cell production within the body. The human gene for erythropoietin was cloned in the early 1980s,1,2 and the recombinant form was developed shortly thereafter. The recombinant alfa form of erythropoietin (epoetin) became available after approval for marketing by the US Food and Drug Administration (FDA) in 1989. Its use has expanded dramatically since then. Epoetin rapidly became the mainstay of treatment for anemia of endstage renal disease (ESRD) in the US, somewhat related to its reimbursement by the Medicare system. It is estimated that sales each year in the US for oncology indications are about $2.5 billion, with approximately 200,000 patients treated yearly. This paper will review the principles of evidence-based medicine, which will be applied to the use of epoetin in subsequent papers.
The current applications of epoetin are many. The initial indication for use by the FDA was in end-stage renal dialysis patients to decrease transfusion requirements. The current FDA approved indications for its use are 1) the treatment of anemia associated with chronic renal failure; 2) the treatment of anemia related to therapy with zidovudine in HIV infected patients; 3) the treatment of anemia in patients with non-myeloid malignancies where anemia is due to the effect of concomitantly administered chemotherapy; and 4) the treatment of anemic patients scheduled to undergo elective, non-cardiac, non-vascular surgery to reduce the need for allogeneic blood transfusions, and in patients at high risk for peri-operative transfusions with significant, anticipated blood loss. Its use has grown to include a broad range of applications outside of the indicated clinical settings, including cancer-related or cancer treatment-related anemia (such as stem cell transplantation), anemia of chronic disease and sickle cell anemia.
Though epoetin can be effective in reducing anemia, and in general is an extremely well tolerated drug, it is not without risks. The most common clinically relevant side effect is hypertension, especially in patients with ESRD.3 Hypertensive encephalopathy, seizures, and thrombotic/vascular events are rare but have been described.3 Inappropriate erythropoietic stimulation in normal persons (e.g. “doping in athletes”) may be life threatening.4,5 In order to maximize the benefit of epoetin therapy and potentially minimize the costs, significant research has been done regarding the usage of epoetin. It is our goal in this education session to summarize and appraise the evidence for the use of epoetin in patients with malignancy, and in doing so provide evidence-based guidelines for its use, as well as suggestions for research in areas where strong evidence is currently lacking. The goal of this article is to review the value of an evidence-based approach to clinical decision-making and briefly describe an approach to critical appraisal of the medical literature. The articles that follow will apply the principles of evidence-based medicine (EBM) to usage of epoetin in patients with malignant diseases.
What Is Evidence-Based Medicine?
Although the term ‘evidence-based medicine’ has recently achieved prominence, it reflects principles in epidemiology and medicine that are not novel. EBM is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients.6 It combines clinical judgement and experience, best available scientific evidence, and patient preferences to improve medical decision-making. All components of this decision-making triad are important. Clinical experience and judgement are critical to recognizing clinical problems, understanding diagnostic testing, establishing accurate diagnoses, identifying treatment alternatives, and appropriately applying research evidence to individual patients' circumstances. Contrary to the criticism that EBM represents an effort to undermine clinical expertise, many proponents suggest it emphasizes the importance of good clinical judgement. The quality and relevance of research evidence to a particular patient's problems determines its influence on medical decision making. Patient preferences are an important component of medical decision making. Manifestations of disease, comorbid conditions and value systems vary widely between individuals, and their preferences regarding treatment must be included in shared decision-making. These three components of evidence-based decision making are highly interactive. Greater understanding of research evidence and patients preferences may enhance clinical experience and judgement. Realization of strengths and weaknesses of research evidence is likely to affect physician considerations of patient values. Because evidence-based decision making incorporates clinical judgement, best evidence, and patient preferences, it acknowledges that no single “cookbook” approach fits any given patient's situation.
Why Is Evidence-Based Medicine Important?
Many factors appear to be involved in the increasing adoption of the principles of EBM. The desire to provide high quality care in an era of cost containment is one strong factor. Patients, physicians and payers are all interested in high quality care. EBM makes efficacy one of the most important determinants of quality.
The medical literature continues to expand, with increasing numbers of textbooks and medical journals in print. Though increasing the number of research articles might appear beneficial, the greater number of articles may not advance medical knowledge significantly, and physicians may find fewer of value to their practice. In fact, with more journals searching for articles to publish, work that may routinely be declined because of quality issues may eventually find outlets. Though electronic databases such as Medline provide rapid access to the literature and address some of the problems of greater numbers or research articles and journals, effective use of these databases requires skill and practice. The literature appraisal skills of EBM arm the clinician with an ability to be more selective regarding research that may benefit patients, decreasing the often time-consuming search for relevant research evidence.
A second and paradoxical barrier to acquiring relevant scientific information that may be addressed by EBM is the opinion of experts. Expert opinion has traditionally been a popular source of medical knowledge. Though physicians frequently seek the opinion of experts to resolve medical dilemmas, experts frequently provide conflicting recommendations. Experts may be biased in their interpretation of the available research evidence, either because of the “micro-specialized” nature of their practice population or their own participation in focused research in a particular field. The essence of their expertise may be because of the biased niche they have created in a field. Experts may practice based upon subjective judgement rather than rigorous analysis of available scientific evidence. A greater understanding of the principles of EBM empowers physicians to evaluate expert recommendations in the appropriate context of the hierarchy of research evidence and determine their usefulness and applicability to particular patient problems. In situations where the research evidence is weak, expert recommendations may have a role in clinical decision-making even though they are founded on experience and opinion.
Wide, unexplained geographic variation in practice patterns, best demonstrated for surgical procedures,7–,9 is another problem in medicine that may be improved with greater use of the principles of EBM. There are many instances where practice patterns show variability far beyond what may be expected based on clinical, demographic or regional variations in patient populations. Such variation is likely to represent some level of inappropriate care (over- or under-use, for instance). Variation would be expected to decrease with greater implementation of evidence-based clinical practice. There is substantial variation in the use of erythropoietin, which may be influenced by practice setting (fee-for-service versus academic or HMO), country of residence, reimbursement denial, and patient demand (via direct to consumer advertising efforts).10
As health care spending continues to rise in the US, appropriate resource utilization has become an important issue in the daily practice of medicine. Finite medical resources are available to all patients in the health care system, and appropriate allocation of these resources is the responsibility of physicians and society. Though patients are concerned primarily with their own health and payers may wish to constrain health care expenditures, physicians and society must take a perspective that maximizes the allocation of health care resources for the benefit of all patients. An evidence-based approach may offer some solutions to this inherent conflict. As physicians and patients make greater use of strong evidence to select among intervention alternatives, they are more likely to be choosing interventions that improve patient outcomes. Evidence-driven interventions may not have the lowest cost, but they are likely to have the greatest benefit or efficacy. With a greater emphasis on strength of evidence pervading medical culture, it is likely that health care payers (employers and insurers), policy makers and even patients will demand better evidence to substantiate high-cost treatment alternatives, constraining resource utilization that does not yield high quality outcomes. Though cost-benefit ratios of health care interventions may vary across health care systems and payers, EBM offers the opportunity to evaluate efficacy independent of health care system financing.
Practicing Evidence-Based Medicine
This section briefly discusses the fundamentals of evidence-based medicine. Readers who are interested in more comprehensive reviews of the topic are directed to books by Sackett11 and Geyman12 or the ongoing series “Users' Guides to the Medical Literature” published in JAMA.13– 45
Sackett has proposed the following steps in practicing EBM:11
Formulate a focused clinical question that can be answered.
Search for the relevant evidence from the literature.
Critically evaluate the evidence for its usefulness and validity.
Implement the evidence into decision-making.
Framing the question
Though superficially this step may seem self-evident, it involves some experience and clinical judgement to frame questions that will answer the needs of patients and be amenable to literature review. Questions of interest may be about clinical findings, differential diagnosis or accuracy of diagnostic testing, causality, prognosis, therapy, prevention, quality of life or other topics. When defining a question, the basic elements to consider include the population, the intervention of interest, the comparison (treatment alternative) of interest (if relevant), and the outcomes to be evaluated. It is important to be as precise as possible in outlining the question to expedite the literature search and improve the applicability of the results of the search. When more than one question arises, clinical experience will help define the question most essential to the patient's care that can be answered in the limited time available.
Searching for evidence
The usual source of evidence is electronic databases, which are increasingly available to most clinicians. Electronic databases offer advantages of speed, breadth of search (number of searchable sources), ability to specifically narrow the search criteria to suit the question, and the ability to review either the abstract or, in many cases, the entire article on-line.
Primary publications can be reviewed through Medline. A second set of electronic databases gives the user access to secondary publications of the relevant clinical literature. These databases include the Cochrane Database of Systematic Reviews and the ACP Journal Club and make available evidence-based reviews of the literature (systematic reviews of randomized trials) on specific topics. The Agency for Healthcare Research and Quality (AHRQ, www.ahcpr.gov) maintains evidence-based reviews performed by Evidence-based Practice Centers (EPCs) as well as guidelines that have been developed from the evidence based reviews. Some authors suggest45 that an initial search be performed on these secondary publication databases to efficiently gather evidence, when available, that has already undergone rigorous methodological and relevance review.
Evaluating the evidence
The critical appraisal of the literature for validity and usefulness is essential to deciding whether the evidence fits the scenario and can give useful guidance to the clinician. It is at this step in the process that methodological rigor must be evaluated. The fundamentals of assessing methodological rigor come from the disciplines of epidemiology and biostatistics, and thus may require some additional instruction for individuals without research expertise.
A comprehensive review of the principles of critical appraisal is beyond the scope of this article. Appraisal tools have been developed by various research teams and encompass a broad range of research categories that users are likely to encounter. Perhaps the most comprehensive of these tools is the JAMA series “Users' Guides to the Medical Literature.”13– 45 These tools are designed to assist the user to ask key questions about the validity of the evidence presented and its relevance to the clinical question.
Practitioners of EBM must understand the limitations and potential for bias in clinical research. Clinical research studies are frequently limited by small sample sizes, differences in selection criteria and prognostic characteristics in the treatment and control groups, short length of follow-up, and researchers' expectations of the intervention group. These are particularly evident in observational studies, which are often not controlled (e.g. no comparison group). Clinical observations play a critical role in medicine; they are often the genesis of research projects/findings that eventually lead to clinical trials. However, practitioners of EBM must recognize that unsystematic clinical observation, physiologic rationale and intuition are, by themselves, insufficient grounds for clinical decision making.45 For this reason, a hierarchy of strength of research designs is important to understand when evaluating research articles (Table 1 ).46 A well-designed randomized trial offers the greatest opportunity to minimize bias from clinical observations and, therefore, is generally considered the strongest study design. The Evidence-based Medicine Working Group suggests that systematic reviews of randomized trials are even stronger than single randomized trials.45 Among those skilled in EBM, skepticism surrounds interventions documented to be effective in research designs that are not randomized clinical trials. Unfortunately, randomized clinical trials are rare in the literature. There is often a lengthy lag time between technical innovation and completion and reporting of quality clinical trials demonstrating efficacy. In some cases, randomized trials are not feasible or ethical. In other cases, observational studies where the treatment effect is large and consistent may be sufficient. Therefore, the hierarchy is not absolute, leaving room for the experienced clinician to judge the value of the evidence for the clinical situation in question.
Implementing the evidence
The strength and validity of the evidence is but one part of the decision to implement the evidence. Clinical expertise and patient preferences should also play a fundamental role at this stage. In the shared decision-making model, physicians gather, collate, and present the necessary evidence in an understandable manner to patients, who then apply their belief systems and preferences to reach a satisfactory choice. For instance, in circumstances where evidence in support of treatment can only be drawn from limited observational studies, a clinician and patient may decide to undergo treatment based on patient preferences and a common-sense mechanism of action of the intervention, acknowledging the limitations. On the other hand, the same pair may use evidence from limited observational studies demonstrating harm to decide to forgo a particular treatment. Understanding the strength and validity of the evidence gives the physician the opportunity to tailor the research findings to individual patients and more appropriately incorporate patient values.
An additional advantage to understanding EBM is a greater understanding of where the evidence is weak. For clinicians, this provides an area to focus future evidence reviews to remain up-to-date. For researchers, areas of weakness in the current evidence may suggest future research topics that would be of high value.
Translating Evidence into Practice
Recognizing that epoetin is an expensive intervention with increasing use and significant variation in its use, the American Society of Hematology (ASH) and the American Society of Clinical Oncology (ASCO) sought to develop an evidence-based guideline to provide clinicians direction on the usage of epoetin for anemic patients with hematologic malignancies or undergoing chemotherapy for cancer. ASH and ASCO submitted a proposal to the AHRQ to support a systematic review of the literature by an Evidence-based Practice Center regarding epoetin. The proposal was funded, and the evidence report became available in early 2001. A publication summarizing the evidence for epoetin for anemia of cancer therapy is now complete.47 Evidence-based guidelines are being developed from the evidence review, and should be available at the time of the Education Session at the ASH Annual Meeting in Orlando. The role of erythropoietin is still being defined.
II. Building the Case: What Evidence Exists for Usage of Epoetin and How Good is the Evidence?
Jerome Seidenfeld, PhD,*
Blue Cross and Blue Shield Association, Technology Evaluation Center, 225 N Michigan Ave, Chicago IL 60601
This work was developed under contract with the Agency for Healthcare Research and Quality, Rockville, MD (AHRQ contract number 290-97-0015). The authors of this article are responsible for its contents, including any clinical or treatment recommendations. No statement in this article should be construed as an official position of the Agency for Healthcare Research and Quality or of the U.S. Department of Health and Human Services.
The AHRQ EPC Program and Its Approach to Systematic Reviews
Program overview
In 1997, the Agency for Health Care Policy and Research, now known as the AHRQ, launched an initiative to promote evidence-based practice in everyday care through establishment of 12 Evidence-based Practice Centers (EPCs). The EPCs develop evidence reports and technology assessments on clinical topics that are common, expensive, and/or are significant for the Medicare and Medicaid populations. Through this program, AHRQ partners with private and public organizations to improve the quality, effectiveness, and appropriateness of clinical care by facilitating the translation of research evidence into clinical practice. In 1998, ASH and ASCO jointly nominated the topic “Use of Epoetin in Oncology” to AHRQ for an EPC systematic review and evidence report.
The EPCs develop evidence reports and technology assessments based on rigorous, comprehensive syntheses and analyses of relevant scientific literature, emphasizing explicit and detailed documentation of methods, rationale, and assumptions. These scientific syntheses may include meta-analyses and cost analyses. Each EPC collaborates with other medical and research organizations so that a broad range of experts is included in the development process. More detailed information on the EPC program, the topic nomination process, and the list of EPCs is available at http://www.ahrq.gov/clinic/epcix.htm.1 Summaries and completed reports (with bibliographies and evidence tables) are available at the same URL for viewing or downloading. Printed copies also may be obtained from the AHRQ Publications Clearinghouse (1-800-358-9295).
Systematic review methods
Protocols for systematic review are prospectively designed to define study objectives; search strategy; patient populations of interest; study selection criteria and methods to determine study eligibility; outcomes of interest; data elements to be abstracted and abstraction methods; and methods to assess study quality. Usually, two independent reviewers complete each step of the protocol. Reviewers individually evaluate studies against selection criteria, abstract data separately, and compare their results after each step. Disagreements are generally resolved by consensus but may require resolution by a third reviewer.
A technical advisory group provides ongoing guidance on all phases of each EPC review. Six technical advisors participated in the evidence report on use of epoetin in oncology patients. ASCO and ASH each appointed two of the six advisors, and the EPC staff of the Blue Cross and Blue Shield Association Technology Evaluation Center (TEC) recruited the remaining two.
EPC reviews begin with a comprehensive literature search that attempts to identify all publications of relevant controlled trials. The search strategy for the review on epoetin is described briefly in the Summary posted on the AHRQ web site1 and more completely in the full evidence report.2 The MEDLINE, Cancerlit, and Embase databases, last searched in December 1998, yielded 2,915 references. Supplementary searches (e.g., Current Contents, bibliographies from manufacturers) through October 30, 1999 yielded 28 additional published reports for a total retrieval of 2,943 references.
Next, studies are selected for data abstraction using criteria specified in the protocol. The primary study selection criteria for the epoetin review required that studies be designed as controlled trials comparing the outcomes of managing anemia with and without epoetin in a patient population relevant to one of four clinical settings. These were anemia due primarily to cancer therapy; anemia due primarily to malignancy; myeloablation and autologous stem cell rescue; or myeloablation and allogeneic stem cell rescue. We defined the setting as anemia primarily due to cancer therapy if trials limited enrollment to patients undergoing concurrent chemotherapy or radiation therapy with conventional non-myeloablative doses while on study. We defined the setting as anemia primarily due to malignancy if some enrolled patients did not receive concurrent chemotherapy or radiation therapy while on study.2 Trials were excluded if there were < 10 similarly treated evaluable patients in each arm.
In the available trials, epoetin treatment (with transfusion used as necessary) was always compared to red blood cell (RBC) transfusion alone; no trials compared epoetin to any other alternative. All randomized controlled trials relevant to any of the four clinical settings were included. Studies that used nonrandomized concurrent or historical controls were included if the reviewers could determine that similar patients were included in the treatment and control groups. Nonrandomized trials were considered to be of lesser quality than randomized controlled trials.
The systematic review addressed the following questions separately for each clinical setting:
What were the relative effects on outcomes of managing anemia with epoetin compared to transfusion alone? In settings other than stem cell transplants, what were the relative effects of epoetin treatment when different Hb thresholds were used to initiate treatment?
In the included studies, did varying the characteristics of the administration of epoetin affect the outcomes of treatment? Were the characteristics of epoetin administration likely to confound interpretation of the evidence on the relative effects of epoetin treatment according to the alternative Hb thresholds for initiating treatment?
Were there populations or subgroups of patients more or less likely to benefit from epoetin treatment? Were there laboratory measurements that either predicted or permitted early identification of patients whose anemia responded to epoetin?
What were the incidence and severity of adverse effects associated with the use of epoetin and how did these compare with the adverse affects of transfusion?
Abstraction of data on adverse events was also limited to controlled trials because our objective was to estimate the frequency of occurrence in the oncology setting of the common adverse effects of epoetin. This precluded analysis of uncontrolled series, because adverse events related to disease progression and cancer therapy could not be distinguished from those related to epoetin.
To supplement the systematic review, we conducted a literature-based meta-analysis of the effect of epoetin on the odds of transfusion for patients with anemia or at risk of anemia due primarily to cancer therapy. A random effects model was used to calculate the combined odds ratio of transfusion for the 12 randomized controlled trials that reported numbers or percentages of patients transfused with or without epoetin administered subcutaneously for treatment-related anemia. The odds ratio expresses the relative likelihood that epoetin-treated patients will be transfused compared to the likelihood for controls. Published data were insufficient for literature-based meta-analysis of other outcomes or of odds of transfusion in other clinical settings.
Sensitivity analysis compared results of higher quality trials to those of lesser quality trials. A trial was classified as higher quality when it was randomized and double-blinded and met our criteria concerning limits on the number of subjects excluded from the analysis of results. We required that < 10% of subjects within each study arm were excluded from the analysis and that the ratio of exclusions from each arm was less than 2:1; or, alternatively, that results were reported as an intention to treat analysis.
External review
AHRQ requires that EPC reports undergo extensive review by external experts and representatives of stakeholder organizations. Early in each project, these individuals review and provide input for modifying the study protocol. Later, they review and comment on the initial draft. However, each EPC has ultimate responsibility for the final draft of its reports, subject to AHRQ review.
For the epoetin report,2 a preliminary analysis of the evidence base was reviewed by the Blue Cross and Blue Shield Association Medical Advisory Panel (MAP), which includes nationally recognized experts in technology assessment and hematology/oncology. Additionally, 20 external reviewers critiqued the study protocol and draft report, and revisions were made based on their comments. Eight were invited by TEC based on their expertise in medical oncology, hematology, transfusion medicine, quality of life, and systematic review methodology. One reviewer directs another AHRQ EPC and is a medical oncologist. Ten reviewers were appointed by professional organizations other than ASCO or ASH and by patient advocacy groups. These reviewers included clinical and research specialists involved in the treatment of cancer and/or management of cancer-related anemia and patient advocacy representatives. One external reviewer was from the technical staff of Ortho Biotech, Inc. Lists of the MAP members, external reviewers and technical advisors are included in the appendices of the full evidence report.2
Evidence on Outcomes of Epoetin for Anemia Primarily Due to Cancer Therapy
Our evidence review is based on data abstraction and analysis of 22 controlled trials with a total enrollment of 1,927 patients.3–,24 All trials compared the outcomes of managing anemia in patients undergoing therapy for a malignancy using epoetin treatment or RBC transfusion alone. Eighteen trials with a total 1,698 enrolled patients (88%) were randomized,3–,20 and 7 of these (853 patients; 44%) were placebo-controlled and double-blind.3–,9 For all 22 trials, the number of patients reported as evaluable is 1,838, which is 95% of all enrolled patients. We classified the 22 trials into 3 categories defined by the study patients' mean Hb at enrollment: Hb ≤ 10 g/dL;3–,7,9,11,15,16,23 Hb > 10 but < 12 g/dL;8,10,12,17,21,22,24 and Hb ≥ 12 g/dL.13,14,18– 20 No trial directly compared the outcomes of initiating epoetin treatment at different Hb thresholds.
Epoetin versus transfusion and relative effects at different Hb thresholds
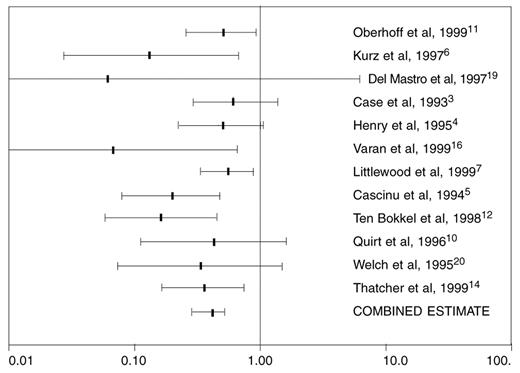
The systematic review found adequate and consistent evidence that epoetin increased Hb levels and percentage of patients demonstrating hematologic response when compared with controls managed by transfusion alone. This was true for pediatric patients as well as adults. For all randomized studies that gave epoetin subcutaneously,3–,7,10–,12,14,16,19,20 meta-analysis showed that epoetin reduced the odds of transfusion by a factor of 0.38 compared to controls not given epoetin (Figure 1 ). This indicated that the likelihood of transfusion for epoetin-treated patients was 38% of the likelihood for controls. The overall number needed to treat (NNT) calculated for this group of studies was 4.4 (95% CI, 3.6-6.1), which suggested four to five patients must be treated with epoetin to spare one patient from transfusion.
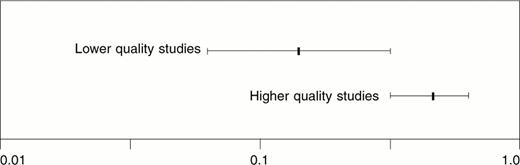
Sensitivity analysis found a smaller magnitude of risk reduction for higher quality studies,3–,7 which were double-blinded (Figure 2 ). For higher quality studies, the calculated NNT was 5.2 (95% CI, 3.8 to 8.4), and for lower quality studies10–,12,14,16,19,20 the calculated NNT was 2.6 (95% CI, 2.1 to 3.8). Thus, higher quality studies predicted one patient would avoid transfusion for every 5-6 patients treated with epoetin, while lesser quality studies predicted one for every 2-3 treated. There was evidence that in unblinded studies, physicians may have transfused patients in the control arm more aggressively, yielding an overestimate for the effect of epoetin.
The strongest evidence for an effect of epoetin on quality of life outcomes was a randomized, double-blinded, placebo-controlled trial that was available only as an abstract when the systematic review was conducted but later was published in full.a
The report published by Littlewood et al 7 was reviewed in detail; there were no substantial changes in results that affected the analyses or conclusions in the systematic review, which were based on an earlier version.
The most robust evidence that epoetin reduced use of RBC transfusions for patients undergoing therapy for malignancy was from trials in groups with baseline Hb < 10 g/dL. 3–,7,9,11,15,16,23 Transfusion outcomes did not appear to be superior in trials where epoetin treatment was initiated in groups with mean Hb > 10 g/dL compared to trials where mean Hb was < 10 g/dL. Among trials on adults with baseline Hb ≤ 10 g/dL, the range of differences between epoetin and control arms for percentage of patients transfused was 9-45%.3–,7,11,15 For baseline Hb > 10 but < 12 g/dL 8,10,12,17,21,22,24 the range was 7-47%; the range was 7-39% for baseline Hb ≥ 12 g/dL.13,14,18– 20 However, these ranges are wide and it is uncertain whether the three groups of studies compare patient populations that are similar except for baseline Hb.
The available evidence was inadequate to determine whether outcomes of epoetin treatment were superior when treatment was initiated in groups with mean Hb > 10 g/dL, compared to groups where mean Hb is ≤ 10 g/dL. Randomized controlled trials, double blinded and adequately powered, are needed to compare the outcomes of epoetin treatment initiated at various Hb thresholds. Inferences from indirect comparison of the results of the available trials cannot resolve this question.
While it is possible that adequately powered comparative trials might demonstrate the superiority of epoetin intervention at the higher Hb levels, our examination of the evidence suggests two reasons why that may not prove to be true. First, patients with Hb at entry below the mean may have accounted for most of the transfusions among epoetin-treated patients in trials where baseline Hb was ≤ 10 g/dL. Thus the greatest yield for reducing the number of patients transfused in this population might come from initiating epoetin before the Hb level falls substantially below 10, rather than by initiating epoetin treatment at a level substantially above 10 g/dL. Second, in all trials, patients who were unresponsive to epoetin may have accounted for a substantial proportion of patients transfused. Initiating epoetin treatment at a higher Hb level is unlikely to reduce transfusions for this subgroup.
Effects of different methods for administering epoetin
The meta-analysis examined whether the characteristics of epoetin administration (dosing regimen, treatment duration, and dose range) had an effect on the estimate of the summary odds ratio for transfusion. Only epoetin dose appeared to have an independent effect on transfusion outcomes, but this was potentially confounded by study quality. However, the results of two randomized controlled trials that directly compared lower and higher doses of epoetin (450 vs. 900 units/kg/week) did not provide convincing evidence that the higher dose was more effective in preventing transfusions.12,14
Effects of patient characteristics
Age: Epoetin was effective in preventing transfusion in pediatric patients.16,23 No studies reported outcomes stratified specifically for geriatric patients, but adults up to age 90 were included in some trials.3,4,7
Malignant Disease: There was evidence that epoetin produced hematologic responses and probably reduced transfusions in patients with non-myeloid hematologic malignancies7,15 to a similar degree as in patients with tumors of solid organs or tissues.
Radiotherapy: Although epoetin increased Hb levels for patients managed with radiotherapy alone, mean Hb levels of control patients did not decrease from baseline values.13,17,22 The radiotherapy regimens utilized apparently did not contribute to or exacerbate pre-existing mild anemia.
Platinum Regimens: The evidence demonstrated benefit from epoetin for patients receiving chemotherapy regimens with or without either cisplatin or carboplatin (see the full evidence report2 for citations).
Predictors of Response: The 22 trials included in this evidence base reported no significant predictors of response to epoetin therapy.2 In particular, neither baseline serum erythropoietin nor the ratio of observed to predicted serum erythropoietin levels (O/P ratio) predicted response in any analysis.
Adverse effects
Limited evidence on adverse events was available from the studies included in this review, but the frequencies of those reported did not appear to differ markedly between epoetin-treated patients and controls.2 The only statistically significant difference was a greater frequency of fatigue reported by patients in the control arms.
Evidence on Outcomes of Epoetin for Anemia Due Primarily to Malignant Disease
The literature search identified 6 controlled trials, all randomized, with a total enrollment of 693 patients that met inclusion criteria for this systematic review.25–,30 Three trials were placebo-controlled and double-blind (n = 332; 48%).25–,27 Of the 693 patients enrolled, 648 (93.5%) were reported as evaluable. Patients in this evidence base had diagnoses known to have a high occurrence of anemia of malignancy (multiple myeloma, non-Hodgkin's lymphoma, chronic lymphocytic leukemia [CLL], and myelodysplastic syndromes [MDS]). With the exception of one trial on patients with MDS,25 the preponderance of patients in these trials received concurrent therapy for their malignancy.
Epoetin versus transfusion and relative effects at different Hb thresholds
There was consistent evidence that epoetin increased Hb levels and percentage of patients demonstrating hematologic response in patients with anemia of malignancy. The evidence on transfusion outcomes was sparse, but suggested a favorable effect of epoetin treatment. The only report on measurements of quality of life was an abstract that did not provide sufficient detail for interpretation of the results.27 All patients included in these studies had baseline hemoglobin ≤ 10 g/dL. The evidence did not address alternative thresholds for initiating epoetin treatment in patients with anemia of malignancy.
Effects of different methods for administering epoetin
The studies suggested that starting doses in the 200-450 units/kg per week range were adequate to achieve hematologic response. However, the only study of patients with MDS used a much higher dose, 1050 units/kg per week, yet obtained a smaller increase in response rate.25 The distinct mechanism of anemia in this clonal disorder probably contributed to the reduced response rate.
Effects of patient characteristics
Malignant disease: A statistically significant hematologic response in the epoetin arm was reported for all hematologic malignancies included in this review. However, the limited evidence available suggested that hematologic response rates were lower for patients with MDS.
Age: All studies were of adults; there were no studies of pediatric patients or studies that separately reported on geriatric patients.
Prior Transfusion: Epoetin increased hematologic responses or Hb levels for patients with either multiple myeloma or non-Hodgkin's lymphoma (NHL), irrespective of history of prior transfusion.28–,30 A single study of MDS patients reported that epoetin increased hematologic responses for patients without previous history of transfusion but not for those previously transfused.25 However history of prior transfusion may be associated with other characteristics, such as duration and progression of disease, which may have affected erythropoiesis in MDS patients.
Predictors of Response: This group of studies did not provide sufficient evidence to draw conclusions on predictors of response. Only the serum concentration of endogenous erythropoietin at baseline25,30 and the ratio of observed to expected concentrations of serum erythropoietin28,30 were reported as significant predictors of response in at least two trials.
Adverse effects
There was a statistically significant increase in hypertension (10% versus 1%, p = 0.011) and a non-significant increase in thromboembolic events (3% versus zero, p = 0.55) among those treated with epoetin. The reported frequency of other adverse events did not appear to differ between epoetin-treated patients and controls.
Evidence on Outcomes of Epoetin for Anemia Due to Myeloablation and Allogeneic Stem Cell Rescue
Evidence concerning the use of epoetin after high dose chemotherapy and allogeneic stem cell transplantation (alloSCT) was derived from 7 controlled studies31–,37 (total enrollment, 493) of patients with malignancies representative of those undergoing alloSCT in clinical practice. Of the 7 controlled trials, all but 2 were randomized (total enrollment in randomized studies, 400);31–,35 non-randomized trials compared epoetin-treated patients to historical controls.36,37 The largest study enrolled and evaluated 215 patients;31 all other studies enrolled fewer than 100 patients.
These studies compared the outcomes of controls managed with RBC transfusion initiated at a pre-defined threshold to the outcomes of epoetin treatment supplemented with RBC transfusion when necessary. One study exclusively enrolled pediatric patients.37 Enrolled patients had a variety of hematologic tumors. All studies used bone marrow as the stem-cell source, and all studies administered epoetin intravenously.
Epoetin versus transfusion
Epoetin consistently resulted in a statistically significant decrease in the time to RBC engraftment, as indicated by achievement of a pre-determined Hb level independent of transfusion support.31–,37 The range of reduction reported was 1-2 weeks. Reticulocyte measures, which tend to predict RBC engraftment, also suggested more rapid engraftment with epoetin administration.31,33–,37 Outcomes for day of last transfusion were related to and correlated with RBC engraftment by Hb level results, with statistically significant results that favored the epoetin-treated study arm.31,32,36 Epoetin administration is unlikely to spare anyone from transfusion as recipients of alloSCT are uniformly anemic following the procedure and response to erythropoietin, whether endogenous or exogenous, is not immediate. The evidence suggested that epoetin treatment may have decreased the number of RBC units transfused.32,34,35,37 Limited evidence suggested that epoetin treatment had no significant effect on length of hospital stay.32,33 This is not surprising given the number of complications from alloSCT that are unrelated to anemia.
Effects of different methods for administering epoetin
Transfusion outcomes appeared to be associated with the duration of follow-up for reporting and statistical comparison; shorter follow-up was more often associated with a significant beneficial effect,34,35,37 whereas longer follow-up may have been complicated by transfusions for graft-versus-host disease and resulted in non-significant differences in outcomes for the epoetin-treated arms.31,33 For both RBC engraftment and RBC transfusion outcomes, results obtained with epoetin dose extremes (525 or 3500 U/Kg/week)35,37 did not appear to differ from those obtained with the moderate doses (700-1050 U/kg/week) used in the majority of studies.
Effects of patient characteristics
Age: Although only one small study37 (non-randomized, historical controls) specifically examined the use of epoetin in a pediatric population, results were consistent with those obtained in all other studies, which enrolled primarily adult populations. Additionally, significant results were obtained in this study using a dose per kg per week that was half or less the doses used in studies of adult patients.
Adverse effects
There did not appear to be significant adverse events associated with epoetin treatment in patients receiving allogeneic stem-cell transplants; however, reporting was sparse. The available evidence showed no depression of platelet engraftment with epoetin treatment.
Evidence on Outcomes of Epoetin for Anemia Due to Myeloablation and Autologous Stem Cell Rescue
The literature search and review for studies of epoetin use after autologous transplantation identified 6 controlled trials (total enrollment, 321).31,37–,41 Three of the 6 trials were randomized (total enrollment, 169);31,38,39 non-randomized trials compared epoetin-treated patients to historical controls.37,40,41 Studies ranged in size from 2038 to 11431 enrolled patients. All of the studies used bone marrow as the exclusive source of stem cells except for one39 in which patients with Hodgkin's lymphoma were given pooled bone marrow and peripheral blood stem cells. Nevertheless, it appears that results from these studies can be generalized to patients transplanted with peripheral blood stem cells, the current standard of care.
Epoetin versus transfusion
The evidence did not support a beneficial effect of epoetin administration on RBC engraftment, RBC transfusion, or length of hospital stay outcomes. It is particularly noteworthy that 2 studies31,37 that used the same epoetin protocol for both allogeneic and autologous stem cell transplant patients reported several outcomes significantly improved for allogeneic stem cell transplant patients, but not for autologous stem cell transplant patients.
Effects of different methods for administering epoetin
Since the available evidence did not show a clear benefit for epoetin treatment, there was no evidence to favor a particular dose, dosing regimen, or treatment duration. Although it is possible that treatment duration was too short in all included studies to significantly improve outcomes, reticulocyte measures, an early indicator of RBC engraftment, did not indicate a probable response.31,37,39
Effects of patient characteristics
Epoetin did not show a beneficial effect for the entire population of patients treated in these studies. Results among the subpopulations were consistent with overall results, and no subpopulation that derived benefit from epoetin treatment could be identified. The lack of response to epoetin in patients given bone marrow stem cells suggests that patients given peripheral blood stem cells also would be unlikely to respond. Preparations of peripheral blood stem cells mobilized with growth factors contain progenitor cells from the erythroid (and other) lineages. These progenitors are farther along the maturation pathway to functional end-stage cells, and may be less dependent on erythropoietin, than are unstimulated stem cells harvested from the bone marrow. The time to recovery of red cell counts and correction of anemia thus seems less likely to be shortened by epoetin therapy after infusion of peripheral blood stem cells than after infusion of bone marrow stem cells.
Adverse effects
There did not appear to be significant adverse events associated with epoetin treatment in patients receiving autologous stem-cell transplants; however, reporting was sparse. The available evidence showed no depression of platelet engraftment with epoetin treatment.
III. American Society of Hematology/ American Society of Clinical Oncology Guidelines on the Use of Erythropoietin
Alan Lichtin, MD*
Dept. of Hematology/Oncology, Cleveland Clinic Founda-tion, 9500 Euclid Avenue, Cleveland OH 44195-0002
At the time of the writing, the joint American Society of Hematology/American Society of Clinical Oncology (ASH/ASCO) erythropoietin (EPO) guidelines are not yet completed. Thus, the following discussion of the guidelines is preliminary. Once it is published, this review should be discarded in favor of the actual manuscript.
Subjects to Be Addressed by the Guidelines
The previous chapters in this section discuss the value of evidence-based medicine, and an evidence-based review of the medical literature regarding erythropoietin usage in cancer and cancer chemotherapy.
The Blue Cross and Blue Shield TEC evidence review has been accepted for publication and is internet accessible.1,2 Using the evidence-based review performed by the TEC center, ASCO and ASH have undertaken development of an evidence-based guideline to provide guidance to clinicians making decisions between treatment alternatives for patients with anemia. Our guidelines will focus on the following clinical questions thought to be important to clinicians and patients.
Is the use of EPO appropriate and is it recommended to reduce the need for red blood cell transfusions for patients with chemotherapy-associated anemia and a declining hemoglobin to a level of 10 g/dL?
For patients with declining hemoglobin concentrations but for hemoglobin between 10-12 g/dL, is it appropriate to use EPO? Are there clinical situations, such as underlying cardiac or pulmonary compromise, for which use of erythropoietin in the 10-12 g/dl range may be appropriate?
Is a dose of erythropoietin appropriate at 150 U/kg thrice weekly for a minimum of 4 weeks with consideration for dose escalation to 300 U/kg for an additional 4-8 weeks?
At what point should non-responding erythropoietin treated patients have their erythropoietin discontinued?
Is the use of erythropoietin associated with an improvement in the health related quality of life (QOL) for patients with clinically significant anemia (with baseline hemoglobin > 10 g/dL)?
Does erythropoietin therapy help patients with MDS?
What is the role of erythropoietin in myeloma, non-Hodgkin's lymphoma or chronic lymphocytic leukemia patients?
What is the role of iron in supporting the use of erythropoietin?
The Blue Cross and Blue Shield TEC performed an exhaustive literature review and determined criteria for “high quality studies.” Articles selected as “high quality” were generally randomized controlled trials (RCT) with large numbers, with adequate power to result in statistically significant differences between randomized groups. Non-randomized, observational, and uncontrolled studies were generally excluded from analysis. The ASH/ASCO guideline will primarily use these “high quality studies” in its analysis of the literature, though evidence from non-randomized studies will also be considered, when appropriate.
Strength of Current Evidence
We expect the guidelines will help physicians distill the evidence for the use of erythropoietin and make informed decisions regarding treatment alternatives for anemia. Erythropoietin is generally convenient and low risk, though it is expensive. Transfusions are also expensive, have infectious and infusion-related risks, and are time consuming. In an era of financial constraints, a societal goal is to maximize health benefits from health care expenditures. Weighing the risks and benefits of both erythropoietin and transfusion is a part of the daily life of a practicing hematologist-oncologist, and it is our hope that the guideline will help inform the clinician about the evidence supporting either of these treatment alternatives.
Hemoglobin levels at entry into trials, duration of therapy, and populations being treated vary from study to study. Overall, however, there appears to be sufficient evidence supporting a rise in hemoglobin level and decrease in red cell transfusion requirements in erythropoietin-treated patients, especially solid tumor chemotherapy patients who are receiving platinum-based therapy (guideline items 1 and 2). The size of this effect is modest. The meta-analysis presented in the TEC paper states that about five patients need to be treated with erythropoietin to save one patient from needing a transfusion.
Many studies have used doses of EPO of 150 U/kg TIW. Gabrilove's widely quoted abstract3 and subsequent publication4 recommend dosing 40,000 units once per week based upon hemoglobin improvement in a large single-arm study. The convenience of this approach for patients has led to wide clinical practice. Unfortunately, there is no randomized controlled trial comparing dosing with 40,000 units once per week with weight-based dosing (guideline item 3). Many physicians currently use 40,000 units once per week and, if there is no response after 4 to 6 weeks, will raise the dose. Though this is common practice, there is not strong evidence in the literature supporting a dose-effect relationship for EPO response in patients on chemotherapy, or who have anemia secondary to underlying malignancy (guideline item 4).
There are multiple non-randomized studies, and one randomized clinical trial to support an improvement in QOL for patients treated with EPO. However, QOL is difficult to assess (see discussion below) and it is likely the guidelines will focus on this as one area where substantial further research is needed to establish conclusively that overall QOL is improved with usage of EPO (guideline item 5).
Likewise, the benefit of erythropoietin to treat anemia for patients with MDS, myeloma, non-Hodgkin lymphoma and CLL is an area where research opportunities exist (guideline items 6 and 7).
Areas of Weakness in Current Evidence
There are shortcomings common to many of the studies evaluating erythropoietin, including some of the RCTs. These include methodologic concerns, appropriate consideration of adjunctive therapy for anemia, and difficulties inherent in assessing quality of life.
Methodologic concerns in some erythropoietin studies may reduce the strength and validity of their conclusions. For example, in the Littlewood Study,5 there is an abandonment of the intention to treat analysis for some outcomes, including quality of life (QOL). Nearly 23% of patients were not evaluable for QOL outcomes because of incomplete data or language barriers. If these patients were not similar to those evaluated for QOL, the improvement in QOL among patients treated with EPO demonstrated in this RCT may be confounded by other factors (effectively nullifying the goal of randomization) and may therefore be less valid.
Nutritional deficiency is one of the most common causes of anemia and is common in patients with malignancies. There is non-uniformity in these studies as to how carefully iron status is monitored and how aggressively iron deficiency is treated. Tolerance of oral iron may be poor in a population already suffering from the gastrointestinal side effects of chemotherapy. No study has documented compliance with iron therapy or demonstrated whether iron-deficient patients on EPO, treated with iron, responded to the iron before EPO was initiated. Intuitively, one must optimize all non-erythropoietin related erythron stimulants (i.e., iron, Vitamin B12 and folic acid) in order for EPO to work. No matter what dose of EPO is given, if a patient has absent iron stores, one would expect a blunted response to EPO. Ferritin alone is a poor barometer of iron status. Patients with malignancy receiving chemotherapy will have an elevated ferritin level, because it is an acute phase reactant. Iron saturation alone is also a poor barometer, since the anemia of malignancy may represent the “anemia of chronic disease” with misleadingly low percentage saturation. In one randomized study, iron deficiency was noted, but patients were not given iron supplementation.6 The authors relied on EPO alone to stimulate erythropoiesis, even when iron deficiency was identified.6 Response to EPO may have been underestimated in these patients.
Evaluation of the effect of EPO on quality of life presents special difficulties. QOL is a subjective judgement of an individual's existence and is generally considered to encompass physical, functional, social, emotional, and economic aspects (often referred to as domains). Work capacity, sleep habits, depression, family relationships, etc. affect a patient's perception of QOL. QOL is important, and one goal of any therapy is to help patients to feel better. However, raising hemoglobin does not always equate with increased strength or QOL.
Because QOL is subjective, special instruments are necessary for its measurement. The QOL instruments presently used in the EPO literature are linear analog scales (LASA) or the Functional Assessment of Cancer Therapy–Anemia (FACT-An) scoring system. One debate in assessing QOL is whether to use a “general” instrument that has been widely used in various diseases, or to use an instrument “specific” to the disease process being evaluated. While the former gives the opportunity to compare changes in quality of life across diseases (like treatment for renal failure with treatment for lung cancer), it may not be as sensitive as the latter for changes expected with some diseases. Many investigators handle this by including both general and specific instruments in their study. Whichever type of instrument is used, it is essential that changes in quality of life be benchmarked to correlate significant clinical changes with significant changes in QOL. This is to be certain we understand which changes, as measured by any QOL instrument, represent clinically meaningful changes. For example, many would agree that raising hemoglobin from 5 to 14 g/dL is likely to make a person stronger, feel better, and increase QOL. A legitimate question is whether a rise in hemoglobin from 9.8 to 10.7, or even to 11.3 g/dL, translates into meaningfully improved global QOL, versus an improvement in fatigue as one component of overall QOL. The science of correlating rise in hemoglobin with a rise in QOL is still developing.
EPO studies have not blinded the patient and/or investigators to the changes in hemoglobin level. Patients in a trial measuring EPO's effect on hemoglobin who know their hemoglobin is rising may translate that physiologic success into higher scores on a LASA or a FACT-An scoring system, whether or not they have experienced symptomatic improvement. The only way to control for this would be to blind both the investigator and the patient to hemoglobin changes, yet this might be impractical. Patients' response to chemotherapy may affect their QOL, regardless of response to EPO. As well, depression, neuropathy, inattention, and lack of exercise may also affect patients' assessment of QOL.7– 11
Opportunities for Further Research
The problems with the EPO literature as mentioned above may be addressed by future studies. Can studies be designed to help physicians predict who will respond to erythropoietin? There are data suggesting that there is a correlation between baseline serum erythropoietin level and response to EPO. Unfortunately, it is not the strongest of correlations, but generally, the higher serum erythropoietin level, the less likely for the patients to respond. Outliers exist, so it is impossible to be definitive when recommending the erythropoietin level as a predictor of response. Unfortunately, in many of the previous chemotherapy studies, pre-treatment erythropoietin levels were not drawn on all patients. Another problem with some studies, especially in myeloma, lymphoma, and CLL, is the lumping together of patients with the same diagnosis but who may have very different prognoses or histologic sub-types. Lymphoma is a clear example where necessity for treatment, steroid responsiveness, and perhaps response to erythropoietin, differs based on histologic sub-type, but none of the current studies separate indolent disease from intermediate or high-grade lymphoma. Future research may differentiate the role of disease subtype, stage and prior therapy on the responsiveness of a patient to EPO.
Amgen is evaluating a new form of erythropoietin called “Novel erythropoiesis stimulating protein (NESP).” This is glycosylated in such a way that it can be dosed once per week (as many clinicians are already dosing EPO) or even once every two weeks. Data have been presented in abstract form12 that suggest a response profile similar to EPO. It is too early to speculate on what role NESP will occupy in the treatment of anemic cancer patients.
Hopefully, the ASH/ASCO evidence-based review will help patients and physicians know how to optimize use of this drug. It will also point out how future studies can contribute answers to some of the questions for which there is not strong evidence in the current literature.
IV. The Efficacy of Recombinant Erythropoietin in Cancer-Related Anemia: Where Do We Go From Here?
Timothy J. Littlewood, MD*
Hematology Department, John Radcliffe Hospital, Oxford OX39DU, United Kingdom
Dr. Littlewood is on the speaker's bureau for three companies.
Incidence and Symptoms of Anemia
Anemia is very common in patients with hematological malignancy or solid tumors. It can be attributed to the anemia of chronic disease, to tumor infiltration of the bone marrow and to the myelosuppressive effects of the chemotherapy.1 For example, in patients with myeloma approximately 50% will have a hemoglobin of < 10.5g/dL at presentation and most of the remainder will develop anemia during their initial chemotherapy.2,3 Successful control of the myeloma will usually improve the hemoglobin level, but anemia will recur with disease progression. For patients with lymphoma, anemia (hemoglobin < 12.0 g/dL) is present in approximately 40% at diagnosis, and this figure increases towards 70% after 3-4 cycles of chemotherapy.4
Common symptoms of anemia include fatigue, breathlessness, swollen feet, chest pain and loss of mental acuity. Fatigue is very common in patients with hematological malignancy and may be caused by physiological factors (such as anemia) or psychological factors. Approximately 75% of patients with cancer experience fatigue during their treatment, which is often not fully appreciated by their physicians, and approximately one half reported that the fatigue had an important negative impact on their daily activities.7
Erythropoietin production is impaired in patients with malignant disease. Miller et al5 demonstrated the relative decrease in erythropoietin production, which occurs in patients with malignant disease, by contrasting serum erythropoietin levels in patients with iron deficiency anemia with the levels in patients with a variety of cancers. Miller showed that for any hemoglobin concentration, serum erythropoietin levels were lower in patients with cancer than for patients with iron deficiency. Although the patients studied had solid tumors, the same relative erythropoietin deficiency has been demonstrated in approximately 75% of patients with hematological malignancies such as CLL, lymphoma and myeloma.6
Treatment of Anemia
The possible treatments for the anemia are to do nothing, to transfuse with red cells or to treat with recombinant human erythropoietin (rHuEpo). The majority of anemic cancer patients do not get treated,8 probably because symptoms of fatigue and lethargy are attributed to the underlying malignancy and/or treatment.
Blood transfusion is the most commonly used treatment for anemia in patients receiving chemotherapy. The hemoglobin level at which a transfusion will be suggested varies considerably.8 Some doctors recommend transfusion when the hemoglobin falls below 10.0 g/dL; others believe that transfusion should be withheld until the hemoglobin is less than 8.0g/dL. Blood transfusion is costly, inconvenient for the patient, usually must be given in a hospital environment and is, of course, not completely safe. However, for the majority of patients it is an effective way to safely increase the hemoglobin concentration and make them feel better. The beneficial effects usually last for 2-4 weeks, at which point another blood transfusion may be needed if the underlying cause of anemia has not been reversed.
RHuEpo is an alternative treatment option for anemic patients with hematological malignancy. Substantial data detail its use in patients with lymphoproliferative disorders, in myelodysplasia and following bone marrow transplantation. RHuEpo is not commonly used in patients with acute leukemia, probably because serum erythropoietin levels tend to be high and because the intensive myelotoxic chemotherapy regimens mitigate against a treatment response.
RHuEpo in Anemic Patients with Malignant Disease
In 1990 Ludwig et al9 published a report on rHuEpo treatment of 13 anemic patients with advanced myeloma. The treatment dose of rHuEpo was 150 U/kg body weight by subcutaneous injection three times per week. The median baseline hemoglobin was 10.2 g/dL, and 11 (85%) of the patients responded to treatment with a hemoglobin rise of > 2.0g/dL. The time to response ranged from 3-20 weeks with a median of 5 weeks. As a result of this important initial study, several randomized trials were conducted to establish the effectiveness of rHuEpo in anemic patients with CLL, non-Hodgkin's lymphoma and myeloma.
A summary of the results from 4 trials is shown in Table 2 .9– 12 Response was defined as an increase in hemoglobin of > 2.0g/dL above baseline independently of blood transfusion. This was achieved in 58-85% of patients in the treatment arms and in 7-24% in the placebo arms. These differences were statistically significant. The proportion of patients requiring transfusion decreased by approximately 50% in the treatment compared to the placebo arms. Two of these studies randomized patients to different doses of rHuEpo and were able to demonstrate that a starting dose of, or equivalent to, 150 U/kg subcutaneously 3 times per week produced a superior response to a lower starting dose. Doubling the dose to 300 U/kg 3 times per week in non-responders after 4 weeks produces a response in a further quarter of patients. All of the studies confirmed that rHuEpo is safe with a side effect profile similar to that found in the placebo treated arms.
In a study by Rose et al13 221 anemic patients with CLL were randomized to treatment with rHuEpo or placebo. The baseline hematocrits were 27.5% on the treatment group compared to 27.7% in the placebo group. The mean hematocrit increased by 5.7% in the rHuEpo group compared to 1.5% in the placebo group (p < 0.0001), and 50% of the rHuEpo group had a hematocrit increase of > 6 points above baseline compared to 15% of the placebo group (p < 0.0001). rHuEpo-treated patients who achieved a hematocrit above 38% had a significant improvement in many domains of quality of life compared to the patients treated with placebo.
These important findings have been supported by three very large, single-arm, community-based studies in the United States.14–,16 The observational study by Gabrilove16 was notable for showing that a once weekly dosing schedule of rHuEpo was equivalent to a 3 times per week regimen.
In an attempt to confirm these findings a large, multicenter, placebo controlled, double blind, randomized trial was instigated.17 Three hundred seventy-five patients with solid or nonmyeloid hematological malignancies and hemoglobin levels ≤ 10.5 g/dL or > 10.5 g/dL but ≤ 12.0 g/dL following a decrease in hemoglobin ≥ 1.5 g/dL per cycle or month since starting chemotherapy were randomized 2:1 to 150-300 IU/kg epoetin alfa (251 patients) or placebo (124 patients) t.i.w. subcutaneously for at least 12-24 weeks or 3-6 chemotherapy cycles plus 4 weeks after chemotherapy. The primary efficacy endpoint was proportion of patients transfused; secondary endpoints included hemoglobin level and QOL measurements. The protocol was amended before study end to collect survival data up to 12 months after the last patient completed study.
Epoetin alfa, compared with placebo, significantly decreased transfusion requirements (p = .0057) and increased hemoglobin (p < .001); also, epoetin alfa provided significantly (p < .01) greater improvement of all primary cancer- and anemia-specific QOL domains including energy level, ability to do daily activities, and fatigue. Although the study was not powered for survival as an endpoint, Kaplan-Meier estimates showed a trend in overall survival favouring epoetin alfa (p = .13, log rank test). Side effects were comparable between groups.
The conclusions drawn from this, and other studies described above, are that rHuEpo is indicated for the treatment of anemic patients with non-myeloid hematological malignancies and solid tumors and will, in addition to reducing transfusion need, result in an important improvement in QOL.
Myelodysplasia
Anemia is the most common hematological abnormality in patients with MDS. Treatment of MDS with rHuEpo has been investigated in a number of relatively small studies.18,19 A meta-analysis of 17 trials20 including 205 patients was published in 1995. In the majority of these studies, patients were eligible for treatment with rHuEpo if they had a hemoglobin of < 10.5g/dL or were transfusion dependent. Response was generally defined as abolition of the need for transfusion or a rise in hemoglobin of > 1.5g/dL in non-transfusion dependent patients. The overall response rate in the 17 trials was 16% (33/205 patients) using quite a range of rHuEpo doses given either subcutaneously and intravenously. The median time to response was 8 weeks, and some of the responses, with continued rHuEpo administration, were durable. The patients most likely to respond were those who did not have sideroblastic anemia, those who were not transfusion dependent and those whose serum erythropoietin level was less than 200 U/L.
Three groups have investigated the impact of combination treatment with rHuEpo and G-CSF on the anemia of patients with MDS.21–,23 When a complete response (CR) was defined as achieving a hemoglobin of > 11.5 g/dl and partial response (PR) a rise in hemoglobin of > 1.5 g/dL or a 100% reduction of transfusion need, a response rate of 36% (CR 21.4%; PR 14.4%) was seen when the results of the Scandinavian and US studies were pooled.24 In the German study,21 a good erythroid response (GER) was defined as a complete loss of transfusion need or as an increase of > 2.0 g/dL in hemoglobin, and a partial erythroid response (PER) as a reduction in transfusion need of > 50% or an increase in hemoglobin of 1-2 g/dL. After 12 weeks of treatment the response rate was 61% (GER 43%; PER 18%) and after 36 weeks the response rate was 80% (GER 56%; PER 24%). If the Scandinavian response criteria were applied to this study the overall response rate would have been 50% at 12 weeks and 56% at 36 weeks. Responses have been durable with continued treatment in most patients,23 and the treatment has been well tolerated. No increase in risk of transformation to acute myeloid leukemia was noted, but a non-significant fall in platelet counts was seen in some patients.24 There was no significant difference in erythroid response rates for patients with refractory anemia, refractory anemia with ringed sideroblasts or refractory anemia with excess blasts in these studies. A predictive model for response in patients with MDS has been proposed by Hellström-Lindberg.24 This model was based on a retrospective analysis from the combined results of the Scandinavian and US groups. Using multivariate analysis the serum erythropoietin level and red cell transfusion need were used to create a scoring system that allowed patients with a high, intermediate, or low chance of response to be identified. This model has recently been confirmed and simplified in a prospective study.25
Patients with a transfusion need of < 2 units per month and a serum erythropoietin concentration of < 500 U/l had a 74% response rate to combined erythropoietin/G-CSF compared to a response rate of 23% and 7% for those patients with a > 2 units per month transfusion need or serum erythropoietin concentration > 500 U/l or both of these risk factors, respectively.
With these data available where should we go from here with treatment of an individual patient in the clinic and in terms of future clinical studies?
Blood Transfusion Need
Treatment with rHuEpo will reduce transfusion need by approximately 50%, excluding transfusions required during the first month of treatment before the rHuEpo has had the opportunity to work. This will be important for patients who are enthusiastic about avoiding blood transfusions on safety grounds and also where there are concerns about the adequacy of the blood supply. Some patients who are red cell transfusion dependent (perhaps in a palliative care setting) can benefit enormously from rHuEpo with a significant reduction or abolition of transfusion need.
Are further studies in this field likely to be of value? The mean hemoglobin has been < 10.0 g/dl for patients entered into most of the randomized trials of rHuEpo to date and approximately one fifth to one third of entered patients have already required transfusion at enrollment. Whether treating patients at a higher hemoglobin concentration (say > 12.0 g/dL) would influence hemoglobin response or the need for transfusion is unknown and could usefully be addressed by future studies.
Quality of Life
QOL in patients treated with rHuEpo was reported to improve by several investigators. Demetri15 showed that the improvement in QOL as a function of a rise in hemoglobin level occurred independently of tumor response. An analysis of the Glaspy and Demetri data by Cleeland26 demonstrated a statistically significant, non-linear relationship between hemoglobin level and QOL. RHuEpo related increases in hemoglobin were associated with quality of life improvements for the hemoglobin range of 8.0-14.0 g/dL. The largest QOL improvement for each 1 g/dl increment in hemoglobin occurred when the hemoglobin increased from 11.0-12.0 g/dL, and little benefit was noted when the hemoglobin increased between 7.0-8.0 g/dL. This is somewhat counterintuitive and a confirmatory prospective study would be enormously helpful, albeit logistically difficult.
Is it possible then to tell fatigued, anemic patients with cancer that treatment with rHuEpo is guaranteed to make them feel better? The short answer is no. What you can say is that those patients who have a hemoglobin response to treatment with rHuEpo tend to have an improvement in their quality of life and some patients will benefit enormously. Given that there are so many factors that influence a patient's perception of QOL (and that there is no fully accepted definition for the term), it is not surprising that correcting one parameter (hemoglobin) will not make all the patients feel wonderful all of the time. Further, large randomized trials of rHuEpo, powered to detect a priori meaningful differences in QOL, at different entry levels of hemoglobin would also be very useful in determining the point at which treatment with rHuEpo would provide optimal improvement or maintenance of QOL.
There has been a suspicion for some time that the improvement in a patient's QOL precedes any increase in hemoglobin and may not be just a placebo effect. Proponents of this idea will be fascinated to read that erythropoietin has neuroprotective effects in a mouse model.27 Perhaps some QOL effect from rHuEpo is centrally mediated?
Does Treatment with rHuEpo Improve Survival?
Anemia at presentation has an adverse impact on prognosis for patients with hematological malignancies such as lymphoma, myeloma and CLL and similar findings have been reported for patients with some solid tumors. More advanced disease is likely to be associated with a lower hemoglobin, but this may not be the only explanation for the poor outcome noted in anemic patients. Several non-randomized studies of chemo- and radiotherapy in patients with solid tumors have suggested that correcting anemia during the course of treatment improves prognosis compared to similar patients with anemia left untreated.28– 30
A recent randomized, placebo controlled, double-blind clinical trial of erythropoietin was reported in which the rHuEpo treated patients had a median survival of 17 months compared to a median survival of 11 months in the placebo treated patients.17 A subgroup analysis according to whether the patients had a hematological malignancy (173 patients) or a solid tumor (202 patients) showed that the survival advantage for the rHuEpo treated group held true irrespective of the underlying tumor type. The investigators concluded that although the study was neither designed nor powered for survival as an endpoint, the results suggest a survival benefit with erythropoietin. Further trials are underway to determine if a survival benefit is apparent, since other uncontrolled variables may have influenced survival such as tumor stage, intensity of chemotherapy, extent of bone marrow involvement, and disease progression.
If treatment with rHuEpo truly increases survival in anemic patients with cancer this would be very exciting and the mechanism producing the effect would be eagerly sought. Data from patients with solid tumors suggest that many tumors are hypoxic31 and that anemia contributes to that hypoxia.32 Hypoxic tissues are less radiosensitive than normoxic ones,33 and many cytotoxic drugs may be less effective in hypoxic compared to normoxic conditions.34 Studies show that regardless of treatment, patients with hypoxic tumors are likely to have less local disease control and less chance of cure compared to patients with better oxygenated tumors of the same size and stage.35,36 Three explanations for the adverse impact of tumor hypoxia on survival have been postulated.37 First, hypoxia may induce changes within the tumor cells of the expression of oxygen-dependent proteins such as vascular endothelial growth factor (VEGF), which stimulates angiogenesis and increases the potential for tumor growth and metastases. Secondly, ionizing radiation results in the formation of free radicals within cells. In the presence of oxygen, the free radicals are fixed and interact with DNA and cell membranes to cause cell death. When cells are hypoxic, free radicals are not fixed and cell death may not occur.38 Thirdly, hypoxia may produce a growth advantage for tumor cells that are resistant to apoptosis, with a decrease in the potential for cure or control. In addition, hypoxic tumors may overexpress the tumor suppressor gene p53, a cell phenotype with a higher malignant potential. Anemia may also impair survival by influencing the patient's overall well-being which may, in turn, impact the delivery of appropriate doses of chemo and or radiotherapy.
A new insight into the mechanisms by which rHuEpo treatment might prolong survival was described this year.39 In a mouse myeloma model, the investigators showed that treatment with rHuEpo produced complete tumor responses in 51% of mice compared with 5.4% complete responses in control mice. Further detailed laboratory studies suggested that rHuEpo triggered a tumor specific immune response mediated predominantly by CD8+ T cells. How rHuEpo caused this effect is not understood.
Data from patients with HIV infection also show an adverse impact of anemia on survival and also suggest that treatment with rHuEpo can prolong survival compared to patients receiving no treatment or blood transfusion.40 Whether rHuEpo stimulates a T cell response in these patients is not known.
What Can Be Recommended?
From the available data there is excellent evidence to recommend the use of erythropoietin in anemic patients with lymphoproliferative disorders who are receiving chemotherapy, with the aim of maintaining the hemoglobin at > 12.0 g/dL.10,26
Similarly, most research has been conducted in patients with malignancies who are receiving concurrent chemo or radiotherapy. However, anemic patients with malignant disease who are not being actively treated may also benefit from treatment with erythropoietin.41
The possibility that treatment with erythropoietin might enhance survival is intriguing, but it would be premature to advise treatment with rHuEpo on this basis. Future studies, both in the laboratory and the clinic, are vital.
In MDS there are no placebo controlled, randomized trials. Nevertheless, the data indicate that treating patients with the combination of erythropoietin and G-CSF is effective in the majority of patients whose baseline red cell transfusion need is < 2 U/month and who have a serum erythropoietin concentration of < 500 U/l. Patients meeting neither or just one of these criteria respond much less well. A trial of treatment in such patients, who have complications from blood transfusion (such as the development of multiple red cell antibodies), making the provision of blood difficult, is a reasonable approach. There are few data on QOL benefits in MDS patients treated with erythropoietin/G-CSF and no studies to determine whether such treatment might have any impact on life expectancy. The lack of such data makes recommendations about the use of rHuEpo in patients with MDS uncertain. Further research is needed.
Many physicians were trained that anemia is not important in patients with malignant disease until it became very severe. This thinking results in patients experiencing substantial morbidity and possible impairment in survival. Effective treatment is available for many patients using rHuEpo, but uncertainties about its real worth and its high cost still precludes its use in many health care systems.
Hierarchy of evidence.
| I. | Evidence obtained from at least one properly randomized trial. |
| II. | Evidence obtained from well-designed controlled trials without randomization. |
| III. | Evidence obtained from well-designed cohort or case-controlled analytic studies, preferably from more than one center or research group. |
| IV. | Evidence obtained from multiple time series with or without intervention. Dramatic results in uncontrolled experiments (e.g., the results of the introduction of penicillin treatment in the 1940's) could also be regarded as this type of evidence. |
| V. | Opinions of respected authorities based on clinical experience, descriptive studies and case reports, or reports of expert committees. |
| I. | Evidence obtained from at least one properly randomized trial. |
| II. | Evidence obtained from well-designed controlled trials without randomization. |
| III. | Evidence obtained from well-designed cohort or case-controlled analytic studies, preferably from more than one center or research group. |
| IV. | Evidence obtained from multiple time series with or without intervention. Dramatic results in uncontrolled experiments (e.g., the results of the introduction of penicillin treatment in the 1940's) could also be regarded as this type of evidence. |
| V. | Opinions of respected authorities based on clinical experience, descriptive studies and case reports, or reports of expert committees. |
Clinical studies of recombinant human erythropoietin (rHuEpo) in patients with myeloma and lymphoma.
| Author . | Year . | No. Patients . | Treatment Response % . | Control Response % . |
|---|---|---|---|---|
| * indicates studies where only patients with myeloma were investigated | ||||
| Ludwig (*) | 1990 | 13 | 85 | |
| Cazzola | 1995 | 146 | 61 | 7 |
| Osterborg | 1996 | 121 | 60 | 24 |
| Dammaco (*) | 2001 | 145 | 58 | 9 |
| Author . | Year . | No. Patients . | Treatment Response % . | Control Response % . |
|---|---|---|---|---|
| * indicates studies where only patients with myeloma were investigated | ||||
| Ludwig (*) | 1990 | 13 | 85 | |
| Cazzola | 1995 | 146 | 61 | 7 |
| Osterborg | 1996 | 121 | 60 | 24 |
| Dammaco (*) | 2001 | 145 | 58 | 9 |
Meta-Analysis: Effect of epoetin on the odds of transfusion in patients with anemia due to cancer therapy.
Meta-Analysis: Effect of epoetin on the odds of transfusion in patients with anemia due to cancer therapy.
Sensitivity Analysis: Effect of study quality on the odds ratio of transfusion.
Sensitivity Analysis: Effect of study quality on the odds ratio of transfusion.