Abstract
Plasminogen can be converted to plasmin either via the tissue-type plasminogen activator (t-PA) or via the urokinase-type plasminogen activator (u-PA)/u-PA receptor (u-PAR) pathway. A dual role for these pathways is now well established: 1) t-PA is involved in fibrin homeostasis and 2) u-PA is primarily involved in cell migration and tissue remodeling. t-PA mediated activation is used for thrombolytic therapy of acute myocardial infarction and some other thromboembolic diseases. The u-PA mediated pathway, in concert with the matrix metalloproteinase (MMP) system, plays a pleiotropic role in arterial neointima formation, atherosclerosis, angiogenesis, tumor growth metastasis, and infarction. However, therapeutic interventions in the u-PA/MMP system remain to be further defined.
The plasminogen (fibrinolytic) system (Figure 1 ) comprises an inactive proenzyme, plasminogen, that can be converted to the active enzyme, plasmin, that degrades fibrin and that activates matrix metalloproteinases (MMPs), which in turn degrade extracellular matrix (ECM) (for references, cfr. 1, 2). Two physiological plasminogen activators (PA) have been identified: tissue-type PA (t-PA) and urokinase-type PA (u-PA), which binds to a cellular u-PA receptor (u-PAR). Inhibition of the plasminogen/MMP system occurs at the level of the PAs, by specific plasminogen activator inhibitors (PAIs), at the level of plasmin, primarily by α2-antiplasmin, or at the level of MMPs, by tissue inhibitors of MMPs (TIMPs). A dual role of the plasminogen system is presently well established: 1) the t-PA mediated pathway is primarily involved in fibrin homeostasis and 2) the u-PA mediated pathway is primarily involved in phenomena such as cell migration and tissue remodeling. Consequently the terminology “fibrinolytic system” has become inadequate and should be replaced by “plasminogen system.” The elucidation of the biochemistry, the (patho)physiology and the therapeutic applications of the plasminogen system has been catalyzed by the emergence of powerful molecular biological technologies, including recombinant DNA techniques for the expression of heterologous proteins, and targeted gene manipulation in vivo for the elucidation of the (patho)physiological role of their translation products. This review summarizes the main developments in the plasminogen field over the last two decades. Reference is primarily made to review articles, in which more details and citations to original work can be found.
Components of the Plasminogen System
All enzymes of the plasminogen system are serine proteinases, i.e. their active site consists of a “catalytic triad” composed of the amino acids serine, aspartic acid and histidine. This active site is located in the COOH-terminal region of the molecules (serine proteinase part), while the NH2-terminal regions contain one or more structural/functional domains (modules). The inhibitors of the plasminogen system are members of the serpin (serine proteinase inhibitor) superfamily. They have in their COOH-terminal region a specific reactive site peptide bond (Arg-X or Lys-X), which is cleaved by their target enzyme, resulting in the formation of an inactive enzyme-inhibitor complex. The physicochemical and genetic properties of the main components of the plasminogen system3 and of the MMP system, and their interactions2 are described in detail elsewhere.
Plasminogen consists of 791 amino acids as determined by cDNA sequencing, although originally 790 amino acids were identified by protein sequencing. It is organized in seven structural domains, comprising a “preactivation peptide” (amino acid residues 1-77), 5 sequential homologous kringle domains (disulfide bonded triple loop structures of about 80 residues each), and the proteinase domain (residues 562-791). The kringle domains contain lysine-binding sites that play a crucial role in the specific binding to fibrin, cell surfaces and α2-antiplasmin. Plasminogen is converted to plasmin by cleavage of a single Arg561-Val562 peptide bond.
Tissue-type plasminogen activator (t-PA) consists of 530 amino acids, although originally 527 were identified. It is composed of several domains with homologies to other proteins: a finger domain (residues 4-50), a growth factor domain (residues 50-87), two kringles of about 80 residues, and the protease domain (residues 276-527), comprising the catalytic triad. Binding of t-PA to fibrin is most likely mediated via the finger and the second kringle domains.
Urokinase-type plasminogen activator (u-PA) is composed of an epidermal growth factor domain, one kringle domain and a protease domain containing the catalytic triad. The epidermal growth factor domain is responsible for the binding of u-PA to its receptor, which is present on the surface of a variety of cells. Single chain u-PA is converted to two chain u-PA by cleavage of the Lys158-Ile159 peptide bond.
α2-Antiplasmin (α2-AP, α2-plasmin inhibitor) was originally isolated as a glycoprotein containing 452 amino acids but it was later shown that native α2-AP contains 464 amino acids. It is unique among serpins by having a COOH-terminal extension of 51 amino acid residues, which contains a binding site that reacts with the lysine-binding sites of both plasminogen and plasmin. The NH2-terminal Gln14 residue of α2-AP (Gln2 in the original numbering system) can cross-link to Aα-chains of fibrin, in a process which requires Ca2+ and is catalyzed by activated coagulation factor XIII.
The two most important plasminogen activator inhibitors (PAIs) are PAI-1 and PAI-2. PAI-1 is stabilized by a tight binding to the cell adhesion protein vitronectin. PAI-2 exists in two different forms with comparable kinetic properties, a 47 kDa intracellular non-glycosylated form with pI 5.0 and a 60 kDa secreted glycosylated form.
The specific cell surface u-PA receptor (u-PAR) is synthesized as a 313 amino acid polypeptide, which is posttranslationally processed at the COOH-terminus into a protein of 283 amino acids anchored to the plasma membrane by a glycosyl phosphatidylinositol (GPI) moiety. It binds all forms of u-PA containing an intact growth factor domain, with high affinity. It is composed of three distantly related homologous structural domains, of which the NH2-terminal one binds u-PA.
Phenotype of Mice Deficient in Plasminogen System Components
Targeting of genes via homologous recombination in embryonic stem cells has allowed the generation of deficiencies of specific gene products in transgenic mice. Mice with single deficiency of t-PA, u-PA, PAI-1, u-PAR, plasminogen or α2-AP survived embryonic development and were apparently normal at birth, while no effects on health and survival were observed in most gene-inactivated animals. However, plasminogen deficient and combined t-PA:u-PA deficient mice developed chronic ulcerations and rectal prolapse and suffered a progressive wasting syndrome and a significantly shortened life span due to generalized thrombosis and organ failure (for references cfr. 4). Contrary to patients with low or absent plasma PAI-1 or α2-AP levels, PAI-1 or α2-AP deficient mice did not reveal spontaneous or delayed rebleeding, even after trauma. Thus the plasminogen system is important in maintaining vascular patency, and t-PA and u-PA are the only physiologically significant plasminogen activators in vivo which appear to cooperate in fibrin surveillance in the mouse.
The Plasminogen System and Fibrin Homeostasis
Physiological fibrinolysis
Molecular mechanism: Physiological fibrinolysis appears to be regulated by specific molecular interactions between components of the plasminogen system.5 t-PA has a weak affinity for plasminogen in the absence of fibrin (KM = 65 μM) but a much higher affinity in the presence of fibrin (KM between 0.15 and 1.5 μM). This increased affinity appears to be the result of a “surface assembly” of plasminogen activator and plasminogen on the fibrin surface. In this reaction t-PA binds via the finger domain and kringle 2 to fibrin, and plasminogen binds primarily via the “lysine-binding site” in kringle 1. Thus one way of regulating fibrinolysis is at the level of plasminogen activation localized at the fibrin surface. Plasmin is very rapidly inactivated by α2-AP (k1∼ 107 M-1 sec-1); the initial half-life of free plasmin in the blood is therefore estimated to be approximately 0.1 sec. Plasmin with an occupied lysine-binding site is however inactivated 50 times more slowly by α2-AP. Reversible blocking of the active site of plasmin with substrate also markedly reduces the rate of inactivation by α2-AP.
From these findings one can extrapolate that plasmin molecules generated on the fibrin surface, which are bound to fibrin through their lysine-binding sites and involved in fibrin degradation, are protected from rapid inactivation by α2-AP. Plasmin released from the fibrin surface would, however, be rapidly inactivated.
Control of plasminogen activation: Rapid removal of t-PA from the blood occurs by clearance in the liver via two different recognition systems.6 Hepatocytes bind t-PA via the low density lipoprotein receptor-related protein (LRP) and endothelial cells via a mannose-dependent receptor. PAI-1 reacts with t-PA with a second order rate constant of about 107 M-1s-1. PAI activity is very rapidly cleared from the circulation via the liver.7
The synthesis and secretion of t-PA and PAI-1 by endothelial cells is highly regulated. Histamine and thrombin bind to specific receptors and activate phospholipase C which acts on phosphatidyl-inositol bisphosphate to produce diacylglycerol, which activates membrane-bound protein kinase C, that regulates t-PA synthesis. Synthesis and secretion of PAI-1 can be modulated by various agonists.7,8
Most cells bind plasminogen via its lysine binding sites with a high capacity (> 107 sites per cell) but a relatively low affinity (Kd of 1 μM). Gangliosides as well as membrane proteins with COOH-terminal lysine residues such as α-enolase, also bind plasminogen (for references, cfr. 9). Endothelial cells bind t-PA and plasminogen via annexin II and thereby may play a role in maintaining blood fluidity. Lp(a) competes with plasminogen for binding and may play a role in the regulation of fibrinolysis at the endothelial cell surface. Thrombin activatable fibrinolysis inhibitor (TAFI) may have an antifibrinolytic effect by removing COOH-terminal lysine residues from the fibrin surface.
Pathophysiology of Fibrinolysis
Impaired fibrinolysis and thrombosis: A deficient fibrinolytic response may be caused by impaired release of t-PA from the vessel wall or by an increased rate of neutralization (for references cfr.10). A causal relationship between deficient synthesis and/or release of t-PA and thrombosis has however not been conclusively established in man. Transgenic mice which totally lack functional t-PA lyse experimental pulmonary emboli at a markedly reduced rate, but are healthy under basal conditions.
The PAI-1 concentration in plasma is increased in several diseases including venous thromboembolism, obesity, sepsis and coronary artery disease. There is a clear correlation between the circadian variation in the time of onset of myocardial infarction, with the highest incidence at about 8 a.m., and the circadian rhythm of plasma PAI-1 activity, which is also highest early in the morning.
Enhanced fibrinolysis and bleeding: Increased levels of t-PA or deficiency of α2-AP or PAI-1 may cause a bleeding tendency. Homozygous α2-AP deficiency may be associated with a severe and heterozygosity with no or only a mild hemorrhagic diathesis (for references cfr. 10). Excessive fibrinolysis due to decreased PAI-1 levels has been reported in a few cases and was apparently associated with bleeding complications.
Thrombolytic Therapy
Major developments since 1980: Acute myocardial infarction is the first cause of death and disability in Western societies. It is caused by thrombosis, triggered by rupture of an atheromatous plaque in the wall of a coronary artery. Occlusive thrombosis results in loss of blood flow to vital organs producing local oxygen deprivation, cell necrosis and loss of organ function. The hypothesis underlying thrombolytic therapy of thromboembolic disease is that early and sustained recanalization prevents cell death, reduces infarct size, preserves organ function, and reduces early and late mortality. One approach to treatment consists of the pharmacological dissolution of the blood clot by intravenous infusion of plasminogen activators that activate the plasminogen system. Primary angioplasty, when applied under optimal circumstances, may provide a similar or better alternative, although the relative clinical benefits of the pharmacological and interventional approaches remain debated.
The modern era of thrombolytic therapy started around 1980 with the demonstration by De Wood et al that myocardial infarction in its early stage was invariably associated with thrombotic coronary artery occlusion and the demonstration by Rentrop et al that infusion of streptokinase within the infarct-related coronary artery early after symptom onset induced rapid recanalization. Randomized clinical trials with short term intravenous streptokinase demonstrated moderate but significant potency for coronary artery recanalization and culminated in 1986 in the GISSI trial, which demonstrated a significant overall reduction in mortality with intravenous streptokinase (for references cfr. 11).
In a parallel development in the early 1980s, elucidation of biochemical mechanisms that regulate physiological fibrinolysis provided the conceptual framework for fibrin-selective thrombolysis with t-PA, which fueled the hope that more specific and efficacious thrombolytic agents could be developed.5 With the development of recombinant DNA technology, recombinant human t-PA could concurrently be produced in sufficient amounts to test its clinical efficacy against the non-fibrin-selective streptokinase.
Initially coronary patency studies (TIMI-1 and ECSG-1) supported the higher efficacy of fibrin-selective recombinant t-PA (rt-PA) over non fibrin-selective streptokinase, and several mechanistic trials of the TIMI organization led by E. Braunwald, the ECSG organization chaired by M. Verstraete and the TAMI group of E. Topol and R. Califf validated and extended the hypothesis of fibrin-selective thrombolytic therapy for acute myocardial infarction (for references cfr. 12). However, two subsequent megatrials (GISSI-2 and ISIS-3) could not confirm that this translated into a mortality benefit. Finally, the GUSTO trial13 and its angiographic substudy14 conclusively demonstrated that brisk full perfusion of the infarct vessel (TIMI grade 3 flow) with early and persistent coronary artery recanalization are the primary determinants of clinical benefit.15 The beneficial effect of fibrin-selectivity with respect to bleeding is less convincing, but in aggregate, fibrin-selectivity is a desirable property of thrombolytic agents, as discussed in more detail elsewhere.11
Currently used thrombolytic agents: Thrombolytic agents that are generally approved for use in patients with acute myocardial infarction include streptokinase, recombinant tissue-type plasminogen activator (rt-PA or alteplase), and the rt-PA derivatives reteplase and tenecteplase (TNK-rtPA). The beneficial effects of thrombolytic therapy in acute myocardial infarction have been well established in placebo controlled clinical trials (for references cfr. 16) and it has become routine treatment. It is given to more than 750,000 patients per year worldwide, while at least three times that number could potentially benefit from this treatment. The current indications and contraindications to thrombolytic therapy in patients with acute myocardial infarction and the currently used regimens for coronary thrombolysis are also briefly summarized in Table 1 .
Streptokinase is a bacterial protein that, when added to human plasma, forms a complex with plasminogen; this complex activates other plasminogen molecules to plasmin. The streptokinase-plasmin(ogen) complex is insensitive to circulating proteinase inhibitors and activates circulating and fibrin-bound plasminogen relatively indiscriminately, producing the so-called “systemic lytic state,” characterized by fibrinogen degradation and α2-AP depletion in circulating blood. The standard dose in patients with acute myocardial infarction is 1.5 million U intravenously infused over 60 minutes. Streptokinase causes transient hypotension in many patients and significant allergic reactions in a small percentage of patients. Its administration causes a rapid rise in anti-streptokinase antibody titer after about 4 to 7 days, which is sufficient to neutralize (in vitro) a standard dose of streptokinase and to make repeated treatment of uncertain efficacy.
t-PA is a human protein produced by recombinant DNA technology (recombinant t-PA, rt-PA, alteplase). t-PA is a poor enzyme in the absence of fibrin, which enhances the activation rate of plasminogen by at least 100 times. Activation of the fibrinolytic system thus seems to be triggered by and largely confined to fibrin. The preferred dosage regimen of fibrin-selective alteplase at present consists of a weight-adjusted, accelerated (“front-loaded”) regimen over 90 minutes (15 mg bolus, 0.75 mg/kg over 30 minutes [not to exceed 50 mg], and 0.5 mg/kg over 60 minutes [not to exceed 35 mg]), because of the survival benefit of this accelerated regimen over streptokinase demonstrated in the GUSTO trial.13,17
Comparative trials of streptokinase and alteplase have shown significant differences in efficacy for early coronary artery recanalization (for references cfr. 11). In the angiographic substudy of the GUSTO trial,14 “accelerated” alteplase with intravenous heparin produced somewhat over 50% complete recanalization (TIMI grade 3 flow) at 90 minutes compared to around 30% with streptokinase and aspirin. The 30 day mortality in over 40,000 patients was 6.3% for rt-PA and 7.3% for streptokinase (p = 0.001). A combined end point of death or disabling stroke was also significantly lower in the accelerated alteplase group than in the streptokinase-only groups (6.9% versus 7.8%, p = 0.006). This difference was maintained after one year. The slightly but significantly (p = 0.03) increased risk of intracranial hemorrhage with rt-PA remains, however, a concern.
Toward improved thrombolytic therapy: Thrombolytic therapy could be improved by: 1) earlier and accelerated treatment to reduce the duration of ischemia; 2) the use of plasminogen activators with increased thrombolytic potency to enhance coronary thrombolysis, which can be administered by bolus injection; and 3) the use of more specific and potent anticoagulant and antiplatelet agents to accelerate recanalization and prevent reocclusion (for references cfr. 16).
There is compelling evidence that patients should receive thrombolytic therapy as soon after the onset of symptoms as possible. Continued and intensified education of the public, paramedical personnel and physicians, together with the development of rapid and efficient triage systems and out of hospital treatment are essential to achieve these goals.
Variants of rt-PA with reduced clearance, altered binding to fibrin, and resistance to plasma protease inhibitors have been constructed. Two variants, reteplase and tenecteplase have been approved for clinical use. Reteplase is a deletion mutant, consisting of the kringle 2 and protease domains of rt-PA, given as a double bolus in patients with acute myocardial infarction, which is probably equipotent to alteplase as demonstrated in the GUSTO III trial. Tenecteplase, an rt-PA mutant in which Thr103 is substituted by Asn, Asn117 by Gln, and the sequence Lys296-His-Arg-Arg by Ala-Ala-Ala-Ala has an 8-fold slower clearance and a 200-fold enhanced resistance to PAI-1 as compared to alteplase. Given as a single bolus it is equivalent to alteplase, as demonstrated in the ASSENT II trial. These trials indicate that rt-PA mutants can be produced with significantly reduced plasma clearance, but with similar thrombolytic potency as rt-PA.17 Because these variants have the same catalytic machinery as rt-PA, they probably should not have been expected to outperform rt-PA. In order to obtain thrombolytic agents with an increased thrombolytic potency for coronary recanalization, it will probably be necessary to turn to other plasminogen activators with higher specific activity and different mechanisms of fibrin-selectivity. Staphylokinase could constitute such an alternative, as further discussed below.
Aspirin and heparin have a limited impact on the speed of coronary thrombolysis and on the resistance to lysis, and do not consistently prevent reocclusion. This could have been anticipated on the basis of the unselective inhibition by aspirin of the synthesis of both proaggregating and antiaggregating prostaglandins, and of the relative inefficacy of heparin to inhibit clot-associated thrombin. Several more specific approaches to reduce platelet aggregation including monoclonal antibodies against the platelet GPIIb/IIIa receptor and small synthetic arginine-glycine-aspartic acid (RGD)-containing peptides, are presently being explored (for references cfr. 18). The concomitant administration of potent platelet GPIIb/IIIa antagonists with aspirin, heparin and reduced dose reteplase was found to be comparable but not better than full dose reteplase in the recent GUSTO V trial. Another approach consists of the use of selective thrombin inhibitors, including hirudin and its derivatives, or synthetic thrombin inhibitors. An ongoing megatrial, HERO 2, comparing hirulog with heparin, both in combination with thrombolytic therapy has demonstrated a marginal benefit of the former.
Staphylokinase, a potential highly fibrin-selective thrombolytic agent: Staphylokinase is a single polypeptide chain of 136 amino acids without disulfide bridges secreted by certain strains of Staphylococcus aureus. Like streptokinase, staphylokinase is not an enzyme but it forms a 1:1 stoichiometric complex with plasmin(ogen) that activates other plasminogen molecules. Staphylokinase added to human plasma containing a fibrin clot will react poorly with plasminogen in plasma, but will react with high affinity with traces of plasmin at the clot surface, where the plasmin.staphylokinase complex efficiently activates plasminogen to plasmin. Both plasmin-staphylokinase and uncomplexed plasmin bound to fibrin are protected from rapid inhibition by α2-AP, whereas their unbound counterparts, liberated from the clot or generated in plasma, are rapidly inhibited by α2-antiplasmin. The process of plasminogen activation is thereby confined to the thrombus, preventing excessive plasmin generation, α2-AP depletion and fibrinogen degradation in plasma. The biochemical pathways governing these fibrin-selective interactions are summarized elsewhere (for references cfr. 20).
In an effort to reduce the antigenicity of staphylokinase, over 350 plasmids encoding mutants of SakSTAR (product of the gene cloned from the genomic DNA of a lysopenic S. aureus strain) were constructed and expressed in E. coli, and the expression products were purified and characterized.21 A comprehensive analysis of combination variants led to the identification of SakSTAR (K35A,E65Q,K74Q, D82A,S84A, T90A,E99D,T101S,E108A,K109A,K130T,K135R) with a maintained fibrinolytic potency and fibrin selectivity in a human plasma milieu, and a markedly reduced reactivity with anti-SakSTAR antibodies in pooled immunized patient plasma.
Staphylokinase disappears from plasma in a biphasic mode with a t1/2α of 6.3 min and plasma clearance of 270 ml/min. The clearance can, however, be reduced 5- to 30-fold by selective chemical substitution with single polyethylene glycol molecules with Mr 5,000 to 20,000. A recombinant staphylokinase variant, substituted with a single polyethylene glycol molecule with Mr 5,000, has been investigated in a pilot study in patients with acute myocardial infarction22 and a formal phase II trial in 500 patients has recently been concluded. In essence, bolus doses of 0.0185 to 0.0375 mg/kg were found to be comparable to front-loaded alteplase in terms of coronary artery recanalization at 60 minutes.
The Plasminogen System and Tissue Remodeling
Proteinases play an essential role in cell migration and tissue remodeling, occurring in many biological processes. They degrade extracellular matrix components, a prerequisite for endothelial, smooth muscle, inflammatory, or cancerous cells to migrate to distant sites, and activate cytokines, or liberate sequestered growth factors. Recent gene targeting and gene transfer studies in the mouse have revealed a pleiotropic role of the plasminogen and the matrix metalloproteinase (MMP) systems in arterial neointima formation, in atherosclerosis, aneurysm formation and myocardial ischemia, in angiogenesis, tumor growth and metastasis and in infection. These studies will be briefly reviewed here but have been discussed in more detail elsewhere.23
Neointima formation
u-PA, t-PA and, to a lesser degree, PAI-1 activity in the vessel wall are significantly increased after injury, coincident with the time of smooth muscle cell proliferation and migration; expression of MMP-3, MMP-7, MMP-9, MMP-12 and MMP-13 is induced in injured, transplanted or atherosclerotic arteries (for references cfr. 23). Neointima formation and neointimal cell accumulation after injury were significantly reduced in mice deficient in u-PA, plasminogen or combined t-PA:u-PA due to impaired migration but not proliferation of medial and neointimal smooth muscle cells (for references cfr. 23). u-PAR deficient arteries developed a similar degree of neointima formation as wild type arteries, suggesting that sufficient pericellular plasmin proteolysis is present in the absence of binding of u-PA to its cellular receptor. Similar levels of proMMP-2 and active MMP-2 but significantly lower levels of active MMP-9 were present in extracts of plasminogen deficient arteries than of wild type arteries. Since MMP-9 is primarily expressed by leukocytes, which are involved in the healing of the injured arteries, the lower active MMP-9 levels may contribute to the impaired medial and adventitial remodeling and to the reduced neointima formation.
Atherosclerosis
Expression of t-PA, u-PA and several MMPs in plaques is increased, but a causative role of the plasminogen (Plg) and/or MMP system in atherosclerosis has not been conclusively demonstrated. No differences in the size or the predilection site of plaques have been observed between mice with a single deficiency of alipoprotein E (apoE) or with a combined deficiency of apoE and t-PA, or of apoE and u-PA, suggesting that plasmin is not essential for subendothelial infiltration by macrophages. However, destruction of the media with resultant aneurysmal dilatation and rupture of the vessel wall were more frequent and severe in mice lacking apoE or apoE and t-PA than in mice lacking apoE and u-PA.24 Macrophages were absent in the media of uninvolved arteries and were only able to infiltrate into and destroy the media of atherosclerotic arteries after they degraded the elastin fibers. A dramatic increase of free u-PA activity (which is minimal in quiescent arteries) was generated by the infiltrating plaque macrophages, which abundantly expressed MMP-3, MMP-9, MMP-12 and MMP-13, colocalizing with u-PA in plaque macrophages and suggesting that plasmin is a likely activator of proMMPs in vivo.24
Myocardial ischemia
Recently, a mouse model of chronic myocardial infarction has been used to evaluate the role of the plasminogen system in cardiac healing.25 Following ligation of the left anterior descending coronary artery, wild type or t-PA deficient mice heal their ischemic myocardium within two weeks via scar formation, i.e. the ischemic myocardium becomes infiltrated by leukocytes, endothelial cells and fibroblasts with resultant deposition of collagen. In a fraction of these mice, rupture of the ischemic myocardium occurs shortly after infarction due to excessive u-PA-generated plasmin proteolysis by infiltrating wound cells. In sharp contrast, mice lacking u-PA or plasminogen are protected against ventricular wall rupture, but fail to heal the ischemic myocardium, which remains largely devoid of infiltrating leukocytes, endothelial cells and fibroblasts. Thus u-PA-generated plasmin proteolysis is required for healing, but needs to be carefully balanced to avoid tissue destruction and ventricular wall rupture (for references cfr. 23).
Angiogenesis
Migration of endothelial cells involves proteolysis of the extracellular matrix. When endothelial cells migrate, they significantly upregulate u-PA, u-PAR, and, to a lesser extent, t-PA at the leading edge of migration (for references cfr. 26). Although PAI-1 is also increased, its expression at different locations and times allows a net increase in fibrinolytic activity. Surprisingly however, mice deficient in u-PA and/or t-PA, PAI-1, u-PAR, plasminogen or α2-AP develop normally without overt vascular anomalies.
Migration of endothelial cells alongside a denuded vessel does not require u-PA-generated plasmin, whereas invasion of endothelial cells through an anatomic barrier of extracellular matrix may (ischemic myocardium, polyoma tumor model) or may not (cornea, skin healing) require plasmin proteolysis. Whether these differences relate to the composition and/or thickness of the extracellular matrix, or to the expression pattern of proteinases by endothelial cells during these different conditions, remains to be determined.
Tumor growth and dissemination.
Pericellular plasmin proteolysis has been proposed to play a role in tumor invasion and metastasis by facilitating the migration of malignant cells through anatomic barriers via degradation of extracellular matrix constituents. u-PA generally exerts a positive effect on tumorigenesis, although its mode of action (e.g. on the growth, dissemination or progression of the tumors) may differ according to the models studied.
Based on its ability to block u-PA proteolysis, PAI-1 would be anticipated to impair tumorigenesis. The role of PAI-1 in tumor growth and metastasis remains, however, controversial as epidemiologic studies indicate that PAI-1 is a marker of poor prognosis for survival of patients suffering from a variety of different cancers. Recent studies indicate that tumor angiogenesis (and secondarily tumor invasion) were markedly reduced to absent in PAI-1 deficient hosts, whereas adenoviral PAI-1 gene transfer restored the invasive behavior of the tumor cells, suggesting a role for PAI-1 in vessel stabilization.27
Wound healing
Migration of keratinocytes is associated with expression of proteinases at the leading edge of their migration front. Plasminogen deficient mice exhibited delayed and impaired closure of skin wounds, whereas combined plasminogen and fibrinogen deficient mice had normal wound healing,28 indicating that fibrin mediates the effects of plasminogen deficiency. Although MMPs are likely involved in similar processes, to date little is known about their in vivo role as deduced from the gene targeting studies.
Infection
The expression of proteinases (in particular of the u-PA:u-PAR system) is thought to be critical for the ability of leukocytes to degrade matrix proteins and to traverse tissue planes during recruitment to inflammatory sites. u-PA has, however, also been implicated in the modulation of cytokine and growth factor expression. It is required for TNF-α expression by mononuclear phagocytes, for activation of latent TGFβ-1, and may also be involved in the release of interleukin-1 (IL-1). In contrast to wild-type mice, u-PA deficient mice were unable to mount an adequate pulmonary inflammatory response to a challenge with the non-lethal 52D Cryptococcus neoformans pathogen, which disseminated widely and ultimately infected the brain, leading to death. This pattern of wide dissemination and death with strain 52D has only been seen in profoundly immuno-incompentent mice (for references cfr. 23).
A number of invasive bacteria can interact with the host plasminogen system by expressing endogenous plasminogen activators and by binding plasminogen directly through bacterial cell-surface receptors, thereby allowing them to utilize the plasminogen activators of the host for activation. Studies in plasminogen-deficient mice indicate that plasminogen is required for efficient dissemination of the spirochete Borrelia burgdorferi within the tick and for enhancement of spirochetemia in mice.29 A similar requirement of host-derived plasminogen by Yersinia pestis for its dissemination was recently reported. Bacterial strains expressing a plasminogen activator (pla+) escaped elimination by the host immune system and were almost a million-fold more pathogenic than pla- strains (not expressing such plasminogen activator) in wild type but not in plasminogen deficient hosts.
Current indications/contraindications and currently used regimens for thrombolytic therapy in acute myocardial infarction.
| * Recent evidence (ASSENT III) suggests that low molecular weight heparin may be preferable over unfractionated heparin. |
| A. Indications and contraindications |
| Indications |
| Patients with chest pain consistent with the diagnosis of acute myocardial infarction and at least 0.1 mm of ST-segment elevation in at least two contiguous ECG leads in whom treatment can be initiated within 12 hours of pain onset, provided there are no contraindications to thrombolytic therapy. |
| Contraindications |
| History of a serious bleeding tendency. |
| Recent acute internal hemorrhages. |
| Major surgery, trauma, or delivery within 10 days. |
| Traumatic cardiopulmonary resuscitation. |
| Vascular puncture in a noncompressible site. |
| Uncontrolled hypertension. |
| Previous use of streptokinase is a contraindication for its repeated administration. |
| B. Currently used regimens |
| Streptokinase and aspirin |
| Streptokinase 1.5 million U IV over 30 to 60 minutes, combined with acetylsalicylic acid (ASA) 160 to 325 mg daily started as soon as possible and continued indefinitely. |
| Alteplase and intravenous heparin* |
| Alteplase (recombinant tissue-type plasminogen activator; rt-PA) 100 mg IV over 90 minutes (15 mg bolus, 0.75 mg/kg not exceeding 50 mg over 30 minutes, and 0.5 mg/kg not exceeding 35 mg over the next hour) combined with 160 to 325 mg ASA and immediate intravenous heparin (5000 U bolus and 1000 U per hour, preferably monitored with activated partial thromboplastin time). |
| Selection of regimen |
| In GUSTO, the accelerated alteplase regimen was associated with a statistically significant lower mortality than streptokinase (6.3% vs 7.3%, p= .001) but with a slightly higher incidence (0.1%) of survival with disabling stroke. |
| The t-PA congeners (reteplase and tenecteplase) have been shown in comparative megatrials to be equivalent to alteplase but they can be administered as a double or single bolus, respectively. |
| * Recent evidence (ASSENT III) suggests that low molecular weight heparin may be preferable over unfractionated heparin. |
| A. Indications and contraindications |
| Indications |
| Patients with chest pain consistent with the diagnosis of acute myocardial infarction and at least 0.1 mm of ST-segment elevation in at least two contiguous ECG leads in whom treatment can be initiated within 12 hours of pain onset, provided there are no contraindications to thrombolytic therapy. |
| Contraindications |
| History of a serious bleeding tendency. |
| Recent acute internal hemorrhages. |
| Major surgery, trauma, or delivery within 10 days. |
| Traumatic cardiopulmonary resuscitation. |
| Vascular puncture in a noncompressible site. |
| Uncontrolled hypertension. |
| Previous use of streptokinase is a contraindication for its repeated administration. |
| B. Currently used regimens |
| Streptokinase and aspirin |
| Streptokinase 1.5 million U IV over 30 to 60 minutes, combined with acetylsalicylic acid (ASA) 160 to 325 mg daily started as soon as possible and continued indefinitely. |
| Alteplase and intravenous heparin* |
| Alteplase (recombinant tissue-type plasminogen activator; rt-PA) 100 mg IV over 90 minutes (15 mg bolus, 0.75 mg/kg not exceeding 50 mg over 30 minutes, and 0.5 mg/kg not exceeding 35 mg over the next hour) combined with 160 to 325 mg ASA and immediate intravenous heparin (5000 U bolus and 1000 U per hour, preferably monitored with activated partial thromboplastin time). |
| Selection of regimen |
| In GUSTO, the accelerated alteplase regimen was associated with a statistically significant lower mortality than streptokinase (6.3% vs 7.3%, p= .001) but with a slightly higher incidence (0.1%) of survival with disabling stroke. |
| The t-PA congeners (reteplase and tenecteplase) have been shown in comparative megatrials to be equivalent to alteplase but they can be administered as a double or single bolus, respectively. |
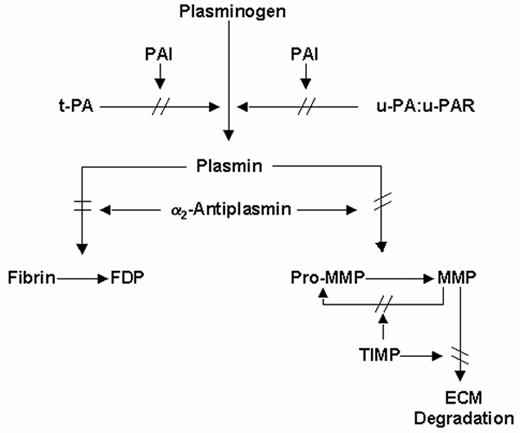
Schematic representation of the plasminogen (fibrinolytic) system.
The proenzyme, plasminogen, is converted to the active enzyme plasmin by tissue-type plasminogen activator (t-PA) or by urokinase-type plasminogen activator (u-PA), which binds to a cellular u-PA receptor (u-PAR). Plasmin degrades fibrin and can convert latent matrix metalloproteinases (pro-MMPs) into active MMPs, which in turn degrade extracellular matrix (ECM). Pro-MMPs may also be activated directly by u-PA, or by other MMP. t-PA mediated plasminogen activation is primarily involved in fibrin homeostasis, while plasmin generation via u-PA, complexed with u-PAR plays a role in tissue remodeling. Inhibition may occur at the level of the plasminogen activators by plasminogen activator inhibitors (mainly PAI-1 and possibly PAI-2), at the level of plasmin by α2-antiplasmin and at the level of the MMPs by tissue inhibitors of MMP's (TIMPs).
Schematic representation of the plasminogen (fibrinolytic) system.
The proenzyme, plasminogen, is converted to the active enzyme plasmin by tissue-type plasminogen activator (t-PA) or by urokinase-type plasminogen activator (u-PA), which binds to a cellular u-PA receptor (u-PAR). Plasmin degrades fibrin and can convert latent matrix metalloproteinases (pro-MMPs) into active MMPs, which in turn degrade extracellular matrix (ECM). Pro-MMPs may also be activated directly by u-PA, or by other MMP. t-PA mediated plasminogen activation is primarily involved in fibrin homeostasis, while plasmin generation via u-PA, complexed with u-PAR plays a role in tissue remodeling. Inhibition may occur at the level of the plasminogen activators by plasminogen activator inhibitors (mainly PAI-1 and possibly PAI-2), at the level of plasmin by α2-antiplasmin and at the level of the MMPs by tissue inhibitors of MMP's (TIMPs).
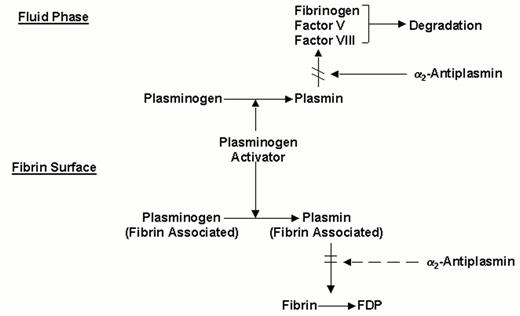
Molecular interactions determining the fibrin-specificity of plasminogen activators.
Non-fibrin-specific plasminogen activators (streptokinase, two chain urokinase, anisoylated plasminogen-streptokinase activator complex or anistreplase) activate both plasminogen in the fluid phase and fibrin-associated plasminogen. Fibrin-specific plasminogen activators (t-PA, scu-PA and staphylokinase) preferentially activate fibrin-associated plasminogen
Molecular interactions determining the fibrin-specificity of plasminogen activators.
Non-fibrin-specific plasminogen activators (streptokinase, two chain urokinase, anisoylated plasminogen-streptokinase activator complex or anistreplase) activate both plasminogen in the fluid phase and fibrin-associated plasminogen. Fibrin-specific plasminogen activators (t-PA, scu-PA and staphylokinase) preferentially activate fibrin-associated plasminogen
Author notes
Afdeling Moleculaire & Vasculaire Biologie, K.U. Leuven, O & N Gasthuisberg, Herestr. 49, B-3000 Leuven, Belgium
Acknowledgments: The experimental studies of the author referred to in this review could not have been performed without essential contributions of many collaborators from several laboratories, which are gratefully acknowledged.
The author is a party to a royalty bearing licensing agreement on recombinant tissue-type plasminogen activator between the University of Leuven and Genentech Inc., South San Fransisco, CA and has an equity interest in ThromboGenics Ltd., a spin-off company involved in the development of recombinant staphylokinase for thrombolytic therapy. Neither of these organizations nor the university have exerted any influence on the statements made in this review.