Abstract
Cytomegalovirus (CMV) infection continues to be a problem in selected populations following hematopoietic stem cell transplantation (SCT). Although there have been no new antiviral agents for management of this infection in recent years, the methods for using the existing agents have improved with newer assays for detection of virus. In addition, our understanding of immunity to CMV has undergone considerable expansion. This paper will address these new aspects relating to CMV infection in the setting of SCT.
In Section I Dr. Zaia reviews the pathogenesis of CMV and the current epidemiology of CMV disease following marrow or blood allo-SCT with emphasis on late-onset disease. The current lab tests available for preemptive management are summarized including the role for conventional shell vial cultures, and a comparison of the CMV antigenemia assay with the new nucleic acid-based assays, including the hybrid capture assay, the NASBA assay, and “real-time” PCR assays. Use of antiviral agents with these tests in the preemptive management of CMV infection is discussed.
Ultimately, what is necessary is restoration of adequate CMV immunity, and that requires understanding the basics of the CMV-specific immune response. In Section II, Dr. Sissons traces the evolution of the CTL response from primary infection into memory and reviews recent advances in the understanding of cytotoxic T cell based immunity to CMV, based on the use of T cell clonotypic analysis and markers of T cell memory and activation, with conventional CTL functional assays.
In Section III Dr. Riddell presents approaches to correction of the problem of CMV pathogenesis, namely direct restoration of the CMV-specific cellular immune deficiency. Attempts at passive therapies will be reviewed with the focus on current problems and approaches to these problems.
In Section IV, Dr. Diamond presents work on the identification of multiple HLA-allele specific cytotoxic T cell epitopes specific for CMV-pp65 and - pp150. Specific epitopes are recognized by CMV-seropositive individuals including healthy donors, SCT recipients, and AIDS patients, indicating their potential usefulness as vaccines. One of these epitopes is recognized by most individuals who express the HLA A*0201 Class I allele. Pre-clinical evaluation in HLA2.1 transgenic mice of vaccine structures utilizing this epitope, and alternative delivery systems are described. Possible methods for vaccination of donor and/or recipient of a SCT as well as their limitations, utilizing synthetic or viral vaccines, are discusseed.
I. Overview: Current Problem of Cytomegalovirus in 2000
John A. Zaia, M.D.*
Beckman Research Institute of the City of Hope, Department of Virology, 1500 E Duarte Road, Duarte CA 91010
The 1990s have seen a dramatic change in the occurrence of CMV-associated disease following allogeneic hematopoietic cell transplantation (HCT). It is noteworthy, however, that no conceptually new antiviral drugs appeared during that decade, and the only new developments during this time were the timing (indication) for drug use and the methods for detection of CMV infection. In regards to the use of these agents, because of inherent toxicities, a preemptive strategy of antiviral therapy emerged that was based on the ability to selectively treat patients based on early detection of reactivated CMV post-HCT (for review see 6 ). This strategy required that a patient be monitored for virus and that institutions have facilities for relatively rapid and sensitive detection. What has emerged is a variety of approaches to the prevention of CMV in this setting, based primarily on local hospital resources and on the logistics chosen for implementation of this treatment strategy. The effect has been, nevertheless, to minimize the amount of CMV-associated disease during the first three months after transplantation and to move the onset of disease to a period of 6-12 months after HCT. Nevertheless, there is still a risk of severe CMV disease in approximately 10% of allogeneic HCT recipients. Thus, in the absence of new antiviral agents, we must now focus on the ultimate requirement for the prevention and management of CMV infection, namely, the reconstitution of CMV-specific immunity after HCT. The ultimate problem remaining for CMV prevention and management is the restoration of host factors necessary for control of CMV infection. The next decade will require that we develop new approaches to patient support, which will permit an immunologic method of infection control. This review is devoted to exploring such possibilities.
Current Preemptive Antiviral Strategies for CMV Prevention after HCT
Ganciclovir (GCV) is the standard agent used to prevent progression to CMV disease following HCT.1,2,3,4,5 Because of the marrow toxicity associated with GCV, antiviral chemotherapy is administered at a time of particularly high risk for CMV disease but prior to symptomatic disease (preemptive therapy). The two risk factors that are most significant in defining the population for treatment are presence of CMV infection and presence of graft-versus-host disease (GVHD). Treatment is most often started at the time of first positive CMV blood culture (see below), but it has also been demonstrated that treatment at first steroid use for GVHD can be effective.4 In general, however, preemptive GCV use requires that patients be monitored once or twice weekly during the period of maximum risk (weeks 3-10 post-HCT). At the time of documented CMV reactivation in blood, GCV is administered at a dose of 5mg/kg twice daily for 7 days and then 5mg/kg once daily 5 days per week for an additional 4-5 weeks. If marrow suppression is a problem, foscarnet (FCN) can be substituted at a dose of 60mg/kg 3 x daily infused over 2 hours, with concomitant administration of 500 ml/m2 saline for hydration.
Logistics of Surveillance for Monitoring CMV Reactivation
The key to efficient and effective prevention and management of CMV infection in the transplant population is the use of monitoring tests that can quickly and accurately detect the presence of CMV. This is essential for application of preemptive strategies of antiviral therapy.6 The purpose of monitoring for CMV is to restrict exposure to GCV to those at highest risk of CMV disease. As indicated above, some patients will be at such high risk for CMV reactivation, e.g. those receiving steroid therapy for accelerated GVHD, that treatment can be used prophylactically and no surveillance is necessary.4 But for most patients, the goal is to avoid the risks of GCV or FCN therapy, and for these patients blood specimens should be monitored at least weekly from weeks 3-10 post HCT. The currently available CMV assays frequently used in this setting include shell vial culture,7 CMV antigenemia assay,1 polymerase chain reaction (PCR) for CMV DNA,2 hybrid capture assay (HCA) for quantitative CMV DNA,8,9 and detection of CMV RNA by nucleic acid sequence-based amplification (NASBA).10 At this time, there is no one best assay method for such monitoring, but those that permit earliest detection of CMV, such as PCR, HCA, or NASBA, promise earlier treatment and have been shown to have very good results.11 But assays such as antigenemia and shell vial culture remain reliable methods for CMV detection and preemptive therapy. Each institution must determine an optimal monitoring system based on patient numbers and available diagnostic resources.
Effect of New Transplant Methods on the Epidemiology of CMV-Associated Disease
The principal effect of preemptive antiviral treatment strategies on the epidemiology of CMV disease has been to increase the time to onset of disease. In the 1980s, CMV-associated diseases occurred at a median time of 50-60 days post-bone marrow transplant (BMT),3,12,13 but with the introduction of preemptive GCV therapy, the median time of onset was closer to 180 days post-BMT and often occurred late in the first year after BMT.1,5,14,15 The therapeutic maneuvers used to prevent early CMV disease have altered the natural history of infection and resulted in late-onset disease.
The pathogenesis of CMV-associated disease is defined by three critical elements: exposure to reactivated (or newly introduced) CMV, presence of GVHD, and absence of T cell immunity. Thus, new methods of HCT, including mini-transplants with donor lymphocyte infusion that can often be associated with GVHD and use of selected CD34+ HCT or of cord blood HCT with T-depleted immune status, can increase the risk of CMV disease. The critical need in certain of these methods is to develop a reconstituted immunity that can control CMV infection. At present, it is not likely that new antiviral drugs will be developed for use in the transplant population, and, the available agents are incapable of solving the problem of continued infection in the patient with chronically impaired immunity. For this reason, the solution to control of CMV must be sought in methods for restoring the anti-CMV host immunity.
II. The Generation and Maintenance of Cytotoxic T Cell Immunity to Human Cytomegalovirus
J.G. Patrick Sissons, M.D,*with assistance from M.R. Wills, A.J. Carmichael, M.P. Weekes, and M. Gandhi
University of Cambridge Clinical School, Department of Medicine, Addenbrooke's Hospital, Box 157, Hills Road, Cambridge CB2 2QQ, UK
Initial Immune Response
Following primary infection, CMV persists for the lifetime of the infected carrier. The principal site of virus latency in the peripheral blood is the monocyte. CMV can probably enter myeloid precursors in the bone marrow and be maintained during differentiation to monocytes. However, only when these cells differentiate into macrophages does the cellular environment become permissive for lytic infection with CMV, and virus reactivation is consequently possible. Virus gene expression occurs in three regulated phases typical of herpes viruses: immediate early, early and late genes. As yet, there is no definite evidence for expression of any specific virus genes during latency. There is a high frequency of CMV specific CD8+ cytotoxic T lymphocytes (CTL) in peripheral blood, and there is inferential evidence that their presence limits the extent of dissemination following virus reactivation from latency.
Induction and Maintenance of Virus-Specific Memory CD8+ T Cells—The Current View
T cell activation
Activation of naïve CTL involves stimulation through the T cell receptor (TCR), which recognizes viral peptide antigen bound to class I MHC molecules, and co-stimulation through CD28, which binds members of the B7 family (CD80, CD86) on the antigen-presenting cell (APC). Upon activation, a proportion of human CD8+ T cells downregulate their surface expression of CD28 and upregulate expression of the CD28 homologue ICOS (inducible costimulator protein) and CTLA-4 (cytotoxic T lymphocyte-associated protein 4). Activated virus-specific CTL undergo clonal proliferation and many become effector CTL that kill virus-infected cells. After the clearance of an acute virus infection, most virus-specific CTL die by apoptosis, but some survive as a population of memory cells that lack direct cytotoxic activity but upon restimulation with antigen become cytotoxic and proliferate. Recent evidence in mice suggests that there is a direct lineage relationship between effector and memory cells, because some but not all effector CTL can directly give rise to memory cells. The factors that determine whether an activated virus-specific T cell dies or survives as a memory cell include the relative concentration of antigen, the nature of co-stimulation available and the availability of help in the form of specific cytokines. Following primary infection, many virus-specific memory cells appear to be long-lived non-dividing cells that may survive in a partially activated state sufficient to maintain surface expression of some but not all activation markers; other memory cells may periodically undergo cell division as long-lived clones.
For the re-stimulation of murine virus-specific memory CTL precursors (CTLp) into effector cells in vitro, costimulation by molecules of the B7 family on APC is essential. However, CD28 expression is not required either for generation of murine memory CTL or for their restimulation into effector CTL in vitro. It is therefore likely that co-stimulatory molecules on the T cell other than CD28 can participate in the restimulation of CD28-memory cells into effector CTL.
Brief Overview of CMV Immunopathology
The CMV viral life cycle provides insight into the most effective time frame for targeting a vaccine to maximally disrupt virus production and spread. Following CMV entry into the host cell and uncoating, the viral genome is expressed sequentially, giving rise to production of immediate early (0-2 hour), early (< 24 hour) and late (> 24 hour) viral proteins.16 CMVpp65 and pp150, two viral structural matrix or tegument proteins, are produced during the early/late phase of gene expression, which would seemingly make them poor candidates as vaccine targets. However, mature CMVpp65 protein is transferred into cells with the CMV virion at the onset of infection, even before the initiation of viral gene expression.17,18 As a result of its predominance within infected cells, it may serve as the principal target of a mature CD8+ CTL response against CMV, without the requirement for de novo viral gene expression.19 These properties indicate why CMVpp65 is so useful in CMV diagnosis and suggest that it is potentially a very good target for immune intervention.
Cellular Immune Response to CMV
HLA-restricted CTL against CMVpp65 have been previously described. CTL have been raised from asymptomatic donors by stimulating their peripheral blood lymphocytes (PBL) in vitro using autologous fibroblasts infected with CMV laboratory strains20,21,22,23,24,25 or using EBV-transformed B cell lines infected with recombinant viruses26 or loaded with peptides.27 Initial investigations focused on the immediate early protein (IE1 or pp72) as an important target for cellular immune responses, by analogy with its importance in the murine CMV model.28,30 However, limiting dilution analysis (LDA) of peripheral blood mononuclear cells (PBMC) from asymptomatic volunteers indicated CTL were present at high frequency against CMVpp65, to a lesser degree against CMVpp150, and CMV-IE, with very few to CMVgB.22,24,31 It had been proposed that the relatively low frequency of CMV-IE-specific CTL was the result of interference by CMVpp65 in the antigen presentation of CMV-IE.19,31 However, recent data have suggested CMV-IE-specific CTL are present at higher frequency than previously thought in CMV-seropositive donors.32,33
CMV-Specific CD8+ CTL Are Large Oligoclonal Expansions
To quantify the clone size of individual virus-specific CTL clones in vivo, Sissons and colleagues generated multiple independent CTL clones specific for defined peptides of CMV or HIV and sequenced the hypervariable VDJ region of their TCR β chain. In a given patient, most of the CTL clones specific for a defined viral peptide used identical TCR nucleotide sequences. From these sequences, clonotype-specific oligonucleotide probes were designed to quantify the clone size of individual CTL clones directly ex vivo and independently of function. The size of an individual CTL clone could be very large (between 1-3% of all peripheral blood CD8+ T cells) and was stable over 3-6 months.34 The high frequency of CTL for a given CMV protein is thus accounted for by one or a few greatly expanded clones, responding to one or a few peptides within that protein. These expanded virus-specific clones may account for the oligoclonal T cell expansions previously noted in human peripheral blood, but whose function was unknown.
CMV-Specific CD8+ CTL and Markers of Memory and Activation
To understand how virus-specific memory CTL are maintained in vivo, Sissons and colleagues analyzed their phenotypic markers related to different states of activation. Quiescent human CD8+ T cells express the high molecular weight isoform CD45RA, whereas highly activated CD8+ T cells express the low molecular weight isoform CD45RO. These investigators purified subpopulations of CD45RO+ or CD45RA+ CD8+ T cells from PBMC of healthy CMV carriers and in functional assays found high frequencies of CMVpp65-specific memory CTLp in both subpopulations. Quantitation of the proportion of individual CMV-specific CTL clones in each subpopulation confirmed that most of the cells of each defined clone in PBMC were in the quiescent CD45RA+ population. In acute primary CMV infection, using longitudinal clonotype analysis of an expanded CMV-specific CTL clone that was initially CD45RO+, they showed for the first time direct evidence of clonal reversion to CD45RA+ CD45RO- during the transition to long-term memory.35
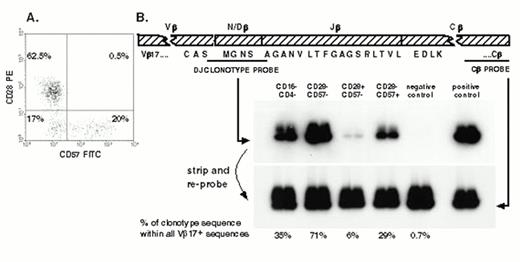
In healthy adults 20-30% of CD8+ T cells are CD28-, most of which are CD45RA+; the proportion of CD28- CD8+ T cells increases in advancing HIV infection. Sissons and colleagues investigated for the first time the relation between CD28 expression and CMV-specific CTL memory. In healthy CMV carriers, the clone size of individual CMV-specific clones was consistently much greater in the CD28- subpopulation than in the CD28+ subpopulation. In asymptomatic HIV-infected patients, large memory CTL clones specific for either HIV or CMV were predominantly CD28- and were distributed among CD57+ and CD57- cells36,37 (see Figure 1). In these patients, strong functional CMV-specific and HIV-specific CTL responses in LDA were observed when CD28- memory cells were stimulated with autologous PBMC pulsed with specific peptides; this indicates CD28- T cells are not terminally differentiated as previously suggested in the literature.
(A) The distribution of cells expressing CD57 and CD28 within the CD8+ T cell population from a healthy CMV seropositive adult. (B) From the TCR β chain of a Vβ17+ pp65 peptide 69-specific CTL clone of this donor, we designed an oligonucleotide probe based on the nucleotide sequence of the hypervariable region and the ends of the adjoining V and J regions.
mRNA was extracted from sorted PBMC, and cDNA was synthesised and amplified using a Vβ17-specific primer and a Cβ-specific primer. A positive control sample of cDNA of the original CTL clone and a negative control sample from pooled PBMC of four healthy donors was also amplified at the same time by using the same primers. PCR products were blotted onto a filter, which was first probed with the labelled clonotypic probe. By stripping the filter and reprobing with a conserved constant region-specific probe that detects all TCRs, it was possible to calculate the relative abundance of clonotype sequence compared to the total sequence within Vβ17.
(A) The distribution of cells expressing CD57 and CD28 within the CD8+ T cell population from a healthy CMV seropositive adult. (B) From the TCR β chain of a Vβ17+ pp65 peptide 69-specific CTL clone of this donor, we designed an oligonucleotide probe based on the nucleotide sequence of the hypervariable region and the ends of the adjoining V and J regions.
mRNA was extracted from sorted PBMC, and cDNA was synthesised and amplified using a Vβ17-specific primer and a Cβ-specific primer. A positive control sample of cDNA of the original CTL clone and a negative control sample from pooled PBMC of four healthy donors was also amplified at the same time by using the same primers. PCR products were blotted onto a filter, which was first probed with the labelled clonotypic probe. By stripping the filter and reprobing with a conserved constant region-specific probe that detects all TCRs, it was possible to calculate the relative abundance of clonotype sequence compared to the total sequence within Vβ17.
Evolution of the CMV-Specific CD8+ CTL Response from Primary to Persistent Infection
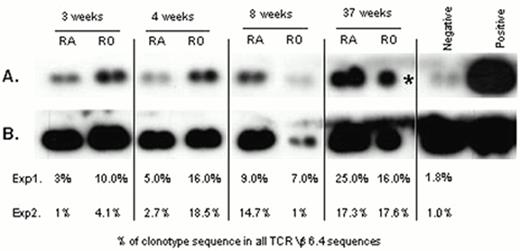
Sissons and colleagues have also studied the CD8+ CTL response to CMV in adults with primary CMV infection. During primary CMV infection fresh ex vivo CMVpp65-specific cytotoxicity could be demonstrated and was associated with a large increase in CD45 RO+ CD8+ T cells in PBMC. The distribution of a specific CMVpp65 CTL clone between subpopulations of T cells bearing different memory and activation markers was studied longitudinally. Three weeks after the onset of symptoms there was little of the clonotype within the CD45 RAhigh cell population compared with the CD45R0high population. By 8 weeks, there were similar levels of the clonotype in both populations, and by 37 weeks the clonotype was distributed between CD45RAhigh and CD45ROhigh in a pattern seen in long-term virus carriers, with higher absolute numbers of the clone in the CD45RAhigh population (see Figure 2). This shows, possibly for the first time, that an individual clone generated during primary infection can revert from CD45 RO to CD45 RAhigh in vivo.35
Quantitation of an individual CMV peptide-specific CTL clone within CD45RAhigh and CD45ROhigh CD8+ T-cell subpopulations derived from PBMC of a donor during and after primary CMV infection.
(A) Duplicate TCR Vβ6.4 PCR products were amplified from each of the T cell populations taken at 3, 4, 8 and 37 weeks after onset of symptoms. PCR product derived from the biological pp65 peptide 56-specific clone (positive control) and pooled PCR product derived from PBMC of four CMV seronegative donors (negative control) were probed with a radio-labelled clonotypic probe. (B) The filter was stripped of bound clonotypic probe and re-probed with a radiolabelled probe specific to the TCR constant region. For each sub-population, the clonotype sequence as a % of all TCR Vβ6.4+ sequences is shown for 2 independent amplifications and probing experiments, % in bold are derived from the gel shown (* duplicate PCR sample failed).
Quantitation of an individual CMV peptide-specific CTL clone within CD45RAhigh and CD45ROhigh CD8+ T-cell subpopulations derived from PBMC of a donor during and after primary CMV infection.
(A) Duplicate TCR Vβ6.4 PCR products were amplified from each of the T cell populations taken at 3, 4, 8 and 37 weeks after onset of symptoms. PCR product derived from the biological pp65 peptide 56-specific clone (positive control) and pooled PCR product derived from PBMC of four CMV seronegative donors (negative control) were probed with a radio-labelled clonotypic probe. (B) The filter was stripped of bound clonotypic probe and re-probed with a radiolabelled probe specific to the TCR constant region. For each sub-population, the clonotype sequence as a % of all TCR Vβ6.4+ sequences is shown for 2 independent amplifications and probing experiments, % in bold are derived from the gel shown (* duplicate PCR sample failed).
The CD45 isoforms designated RA and RO have previously been proposed as single markers for naive and memory cells respectively. Taken together these observations indicate that this is not so and that they instead reflect different states of T cell activation, CD45ROhigh memory cells having more recently been activated by antigen than CD45RAhigh memory cells. True naïve T cells seem likely to reside within the CD45RA+ CD28+ population.
Maintenance of Memory—Antigen and Activation
Much work in experimental models of persistent virus infection has centered on whether or not antigen is required for the maintenance of a high frequency of virus specific CTL. Although the evidence is somewhat conflicting, the balance of opinion suggests that there is no absolute requirement for antigen, although there is a requirement for expression of class I MHC molecules in such models. This latter observation suggests high CTLp frequencies might be maintained by cross stimulation with peptides derived from homologous sequences in other molecules. In the case of CMV, viral antigen clearly does persist and presumably subclinical reactivation of virus provides an ongoing stimulus that maintains CTL frequencies. Nevertheless, it is clear that a large proportion of the CMV-specific CTL (those in the CD45RAhigh population) are in a state of low activation, suggesting they have not recently encountered antigen. Little analogous detailed information yet exists for other human persistent virus infections, so it remains to be seen whether the observations presented here are particular to CMV or a paradigm for other persistent viruses.
Implications for therapy and vaccines.
Work from Riddell and colleagues in Seattle has already shown the feasibility of adoptively transferring CMV-specific CTL clones in BMT recipients; this work is detailed elsewhere in this review. Clearly, reconstitution by the adoptive transfer of a CTL clone may be analogous to the normal CTL response if this is comprised of a few expanded clones. However, it will be interesting to determine whether donor CTL clones derived directly from the transplant reconstitute in the recipient to provide the equivalent proportion of the CTL response that they did in the donor—which may give insights into the homeostatic control of the CTL response.
The fact that the bulk of the normal CTL response to CMV appears to be focused on a few proteins suggests these proteins could be caididates for vaccines. In this context it is interesting to note that recent work shows that purified dense bodies from CMV, composed of tegument proteins and devoid of DNA, induce a CTL response in the mouse almost equivalent to that produced by intact CMVpp65.38
III. Cellular Immunotherapy of CMV
Stanley Riddell, M.D.*
Fred Hutchinson Cancer Research Center, D3100, 1100 Fairview Ave N, Seattle WA 98029
Immunologic Defects Associated with CMV Disease in HCT Recipients
Post-transplant CMV immunity
Due to the persistence of CMV, it is essential that the virus-specific immune responses that were elicited to contain primary infection be maintained to control reactivation from latency. Hematopoietic stem cell transplant recipients have a temporary but severe immunodeficiency and frequently develop progressive CMV infection with visceral involvement.39,40 Patients who undergo HCT after receiving a myeloablative conditioning regimen experience a variable period of severe immunodeficiency characterized by quantitative and qualitative defects in natural killer (NK) cells, B cells, γδ T cells, and CD4+ and CD8+ αβ T cells.41,42,43,44 Many factors may influence the severity and duration of the post-transplant immunodeficiency. These include the T cell content of the stem cell inoculum, the degree of histocompatibility between the donor and recipient, the administration of immunosuppressive drugs, the development of GVHD, and the age and thymic function of the recipient.41,42,43,44,45,46,47,48 Due to the variability and complexity of the immunodeficiency associated with HCT, a major challenge has been to identify the specific defects that are associated with CMV disease and to design immunotherapeutic strategies that can correct these defects.
NK cells and B cells
NK cells provide important contributions to recovery from herpes virus infections in animal models,49 and individuals with a congenital deficiency of NK cells have developed severe herpes virus infections.50 However, NK cells recover relatively early after HCT and before the peak incidence of CMV disease,41 suggesting that defects in NK cells alone are unlikely to account for the development of CMV disease.
The B cell deficiency after HCT may be long lasting, and a deficiency in antibodies to CMV has been associated with CMV disease in HCT recipients.51 Efforts to overcome deficient B cell function by the passive transfer of immunoglobulin preparations containing CMV-specific antibodies have not had a major impact on CMV disease in HCT recipients. A modest beneficial effect has been reported in some studies, but convincing evidence for a significant protective effect of intravenous immunoglobulin in HCT recipients in lacking.52,53 However, treatment of patients with established CMV pneumonia with intravenous immunoglobulin in conjunction with antiviral drug therapy appears to have a beneficial effect.54
γδ and αβ T cells
γδ T cells have not been generally considered to be critical effector cells in virus infection, and due to the low frequency of these cells in the blood, few studies have evaluated recovery of this subset in relation to the development of CMV diseease. A recent study in renal transplant recipients has identified a dramatic expansion of a subset of γδ T cells in the blood coincident with CMV reactivation and provided evidence that these T cells were reacting with antigens expressed by infected cells.55 These provocative findings suggest a more detailed analysis of the potential antiviral properties of this subset of T cells in both immunocompetent and immunodeficient hosts is warranted.
Experiments in animal models have identified a crucial role for MHC-restricted αβ T cells in immunity to viruses including the murine homologue of cytomegalovirus, MCMV.56 These results prompted a careful analysis of the potential contribution of deficiencies of αβ T cells in patients with progressive CMV infection. The initial studies to assess T cell immunity to CMV used in vitro culture techniques to monitor the recovery of CD8+ class I MHC-restricted and CD4+ class II MHC-restricted CMV-specific T cells after HCT. Several studies have now demonstrated that deficiencies of both CD8+ and CD4+ CMV-specific T cell responses are associated with the development of CMV disease in immunocompromised transplant patients.57,58,59 Recently, rapid and sensitive flow cytometric assays have been developed to quantitate antigen-specific T cells in the blood. These assays rely on detecting antigen-reactive T cells by staining with monoclonal antibodies specific for cytokines induced by antigen stimulation or with soluble MHC/peptide tetramers, and have the advantage of being suitable for rapid and repeated monitoring of CMV-specific immunity.60,61,62 Preliminary studies evaluating CMV-specific T cell immunity in immunodeficient patients with these techniques have confirmed the association between deficiencies of CMV-specific αβ T cells and the occurrence of CMV disease.
Adoptive Transfer of CMV-Specific T Cell Clones for Restoring CMV-Specific Immunity
Rationale
The protective function of CMV-specific αβ T cells inferred from the studies analyzing T cell recovery could potentially be definitively established by adoptive transfer of these cells early after transplant. The rationale for developing this approach as a potential therapy for CMV is supported by studies in a model of MCMV infection. MCMV is similar in biologic properties and genomic organization to human CMV. Primary infection is controlled by the immune response in healthy mice but causes a progressive infection involving the lungs and bone marrow in immunodeficient mice. In the MCMV model, the adoptive transfer of syngeneic polyclonal CD4+ and CD8+ MCMV-specific T cells or CD8+ MCMV-specific cytotoxic T cells alone was sufficient to protect irradiated mice from fatal MCMV infection.63,64,65,66
Selection of T Cells for adoptive transfer studies in humans
In contrast to the adoptive transfer studies for MCMV infection, which were all performed using syngeneic T cells, the initial studies of T cell therapy in humans have been performed in allogeneic HCT recipients who are discordant at minor histocompatibility loci. In this setting, it was necessary to derive the CMV-specific T cells to be used in therapy from the allogeneic HCT donor since host T cells could be targets of donor alloreactive T cells and may not persist in vivo. However, the use of polyclonal populations of donor T cells that might contain contaminating alloreactive T cells in addition to CMV-specific T cells could have potentially caused GVHD. To avoid this complication, T cells with reactivity for CMV antigens and lacking recognition of recipient cells were cloned from cultures of donor T cells for use in therapy. The necessity to use T cell clones increased the technical complexity of the approach but ensured that toxicity and/or efficacy of the therapy could only be attributed to the CMV-specific T cells administered and not to other contaminating effector populations.
To assess the potential complications of adoptive immunotherapy for CMV, the initial studies involved transferring only a single subset of αβ T cells. Based on the data in the MCMV model in which CD8+ CTL alone were effective, the safety, immunologic and virologic effects in humans of administering CD8+ CMV-specific T cell clones alone were evaluated first. A subsequent study has evaluated the transfer of both CD8+ CTL and CD4+ TH clones (see below).
Specificity of CD8+ CTL
Preliminary studies of the specificity of CD8+ CTL in healthy immunocompetent CMV-seropositive individuals demonstrated that the majority of the CD8+ CTL response was specific for structural virion proteins, which were processed and presented by CMV-infected cells without the requirement for viral gene expression.17 Subsequent experiments using recombinant vaccinia viruses encoding selected virion proteins identified the tegument proteins pp65 and pp150 as significant target antigens for CMV-specific CTL.21,24,67 Recent studies using MHC tetramers or intracellular cytokine staining to quantitate CTL specific for CMVpp65 or CMVpp150 in normal individuals have demonstrated that 1 to 5% of the CD8+ T cells in the peripheral blood are specific for these antigens. Based on these observations, CMVpp65 has generally been considered to be the immunodominant target of the CD8+ CTL response.
CD8+ CTL reactive with the nonstructural major immediate early (IE) protein and with the viral glycoprotein B have also been identified using vaccinia recombinant viruses.29,31 However, in contrast to CTL specific for tegument proteins, which are capable of lysing infected cells immediately after viral entry and throughout all stages of the viral replicative cycle, CMV-IE- and CMVgB-specific CTL fail to efficiently lyse permissive fibroblasts infected with the laboratory strain AD169 CMV in vitro.31
The inability of infected cells to present newly synthesized viral proteins such as CMV-IE-1 and CMVgB to T cells is largely due to the expression of four viral genes in the unique short region of the genome. US2, US3, US6, and US11 act in a coordinate fashion at IE, early (E) and late (L) times of infection to interfere with the formation and egress of peptide loaded class I MHC molecules.68 Remarkably, the downregulation of class I MHC seems to interfere little with recognition of CMV-infected cells by CMVpp65- or CMVpp150-specific CTL.69 Recognition of CMV-infected cells very early after viral entry by pp65-specific CTL may be explained by the successful formation and transport of MHC/peptide complexes before the expression of the viral genes that downregulate class I MHC. However, the ability of these CTL to recognize infected cells efficiently at later times when class I levels are reduced was unexpected. Recent studies have demonstrated that this reflects a host counterevasion strategy. A cellular gene that is induced by CMV infection binds to a receptor on T cells that serves to facilitate T cell activation even when the target cell expresses only a low peptide/MHC density (V. Groh et al. In preparation.). Thus, CD8+ CTL specific for structural virion proteins such as CMVpp65 or CMVpp150 are able to lyse infected cells at all stages of the replicative cycle and may be essential for eliminating virus-infected cells in vivo.
Based on the in vitro studies of target recognition and on the high frequency of CTL specific for structural virion proteins in healthy hosts, all of the cell transfer studies described below have utilized CTL specific for virion structural proteins. However, there may be potential limitations of only restoring this CTL response since cells reactivating CMV from latency would not be immediately recognized by the immune system. The specificity of CMV-specific CTL that accumulate at local sites of reactivation in vivo has recently been examined, and the preliminary results suggest a much broader repertoire of CTL, including those specific for nonstructural proteins that may be engaged in host control of CMV infection (H. Mutimer and S.R. Riddell, personal communication). Thus, the efficacy of adoptive immunotherapy for controlling CMV reactivation may potentially be improved by broadening the specificity of the transferred CTL.
Specificity of CD4+ TH
The magnitude of CD4+ CMV-specific TH responses in normal immunocompetent individuals has been assessed by using intracellular cytokine staining following stimulation with crude extracts of CMV proteins prepared from infected cells.60 In normal individuals, up to 3% of the CD4+ T cells in the blood are specific for CMV suggesting that, as for CD8+ CTL, relatively high levels of CMV-specific CD4+ T cells may be needed to contain CMV infection. CD4+ TH responses to the CMVpp65, CMV-IE, and CMVgB proteins have been detected, but a detailed analysis of the relative immunodominance of responses to individual proteins has not yet been performed. CMV infects class II+ antigen-presenting cells and has an evasion strategy to escape CD4+ TH recognition. In addition to its effects on class I MHC, US2 causes the degradation of the class II DRa and DMa chains in CMV-infected cells and reduces recognition by CD4+ TH.70 The impact of this viral evasion strategy on the repertoire of responding CD4+ T cells requires additional study.
Clinical Trials of Cellular Therapy with CMV-Specific T Cell Clones in Allogeneic HCT Recipients
Safety assessment
A phase I study evaluating the adoptive transfer of donor derived CD8+ CMV-specific CTL clones as prophylaxis for CMV disease in 14 allogeneic HCT recipients has been completed.71,72 A phase II study in which both CD8+ and CD4+ CMV-specific T cell clones were administered has now enrolled over 30 patients. In the phase I study of CD8+ CTL, the T cells were administered in escalating doses of 3.3 × 107/m2, 1 × 108/m2, 3.3 × 108/m2, and 1 × 109/m2, respectively each week for 4 weeks beginning 28-35 days after transplant. No serious toxicity was seen with any of the 56 T cell infusions and therapy was given in the outpatient department. Minor toxicities that may have been related to the T cell infusions were seen in two patients and included a transient fever in one patient and chills during and after the infusions in another patient.72
The phase II study in which up to three infusions of CD8+ CTL clones and two infusions of CD4+ TH clones have been administered to each patient has confirmed the safety of CD8+ CTL and demonstrated that CD4+ TH can also be administered without toxicity. It should be emphasized that these adoptive transfer studies were performed in patients who did not have visceral CMV disease. It is conceivable that transferring CMV-specific T cells after the development of CMV disease could cause toxicity analogous to that observed in animal models. For example, T cells specific for respiratory syncytial virus (RSV) were safe and effective if given as prophylaxis but augmented lung injury if administered to mice with established RSV pneumonia.73
Immunologic monitoring and persistence of adoptively transferred CMV-specific T cells
To assess the efficacy of T cell infusions for reconstituting T cell immunity to CMV, patients on the phase I study had CMV-specific CTL reactivity in peripheral blood assessed before, during and every 2-4 weeks after the infusions. Before therapy, three of the 14 patients had reconstituted CD8+ CTL responses while 11 had absent CTL responses. After the 4-week treatment period, all 11 patients exhibited CTL responses equivalent to those in the donor.72 To distinguish the contribution of infused CTL from recovery of endogenous T cells in the patients, the unique sequence of the rearranged T cell receptor Vβ gene expressed in infused CTL clones was used as a marker. CMV-specific T cell clones were isolated from three patients after therapy was completed, and the T cell receptor Vβ gene was sequenced for comparison with the sequence of the Vβ gene in infused clones. All of the CTL clones isolated at intervals up to 12 weeks after completion of therapy expressed a T cell receptor Vβ gene, which was identical in sequence to that expressed by the infused clones.72 The transfer of CD4+ CMV-specific TH clones was also effective in restoring CMV-specific TH responses in the blood, and detailed studies of the persistence of individual clones is in progress.
Factors affecting the long-term persistence of transferred CMV-specific T cells
In the recipients of adoptively transferred CD8+ CMV-specific CTL on the phase I study, cytolytic responses equivalent to those in the immunocompetent marrow donor were achieved in all patients immediately after the fourth infusion but declined over the ensuing several weeks in the subset of patients who failed to recover endogenous CD4+ CMV-specific TH responses.72 This observation was similar to findings in mice rendered deficient in CD4+ T cells by gene knockout technology where virus-specific CTL may fail to persist or become dysfunctional.74 Although the clinical results are consistent with a requirement for CD4+ CMV-specific TH for persistence of CTL, this subset of patients also had graft versus host disease as a consequence of the BMT and required treatment with both cyclosporine and prednisone. Thus, it was not possible in this study to determine if a deficiency of CD4+ TH or the more intensive immunosuppressive drug therapy was responsible for the decline in transferred CTL. Preliminary results from the second study in which both CD8+ CTL and CD4+ TH were administered suggest that correcting deficient CD4+ TH function does augment CTL responses in patients who were not receiving therapy for GVHD. However, in patients who were receiving prednisone for GVHD, the persistence of transferred CD4+ TH was diminished, and a sustained effect on CTL was not observed.
Virologic monitoring of SCT patients receiving immunotherapy with CD8+ CMV-specific CTL
The 14 patients enrolled in the phase I study of adoptive immunotherapy as prophylaxis for CMV infection were followed for virus reactivation by weekly cultures of the blood, urine and throat. A positive culture for CMV from the throat was obtained in one patient before therapy and became negative after the first T cell infusion. Two patients had a positive urine culture during T cell therapy. No patient had evidence of CMV viremia and none of the 14 patients developed CMV disease.72 The larger phase II study now in progress will provide additional insight into the antiviral activity of this therapy.
IV. Viral Vaccine Strategies against CMV
Don J. Diamond, Ph.D.,* with assistance from C. La Rosa, M. Villacres, S. Lacey, S. Markel, and J. Sun
Laboratory of Vaccine Research, City of Hope National Medical Center, 1500 E Duarte Road, Fox South Bldg., Duarte CA 91010
Overview of CMV Vaccines
The extensive literature on CMV-related vaccines is more than can be described here in detail, but note is made of five general types of vaccines: attenuated live virus vaccines, recombinant live virus vaccines, DNA vaccines, whole protein vaccines, and peptide vaccines. Plotkin and colleagues developed an attenuated CMV, referred to as the “Towne” strain, in the 1970s and evaluated this vaccine in both renal transplant recipients75,76,77,78 and women in child-bearing years.79,80 Although this vaccine was shown to be immunogenic, the use of a live virus in the transplant population presents a potential risk. To circumvent this, poxviruses with limited potential for replication in humans (avipox) have been proposed as vehicles for expression of recombinant proteins.81,82,83
A vaccination strategy circumventing the use of viral vectors, e.g. non-viral approaches, may be advantageous in the setting of patients with reduced immune capability. Examples of non-viral approaches, which have been proposed to obtain the goal of long-term immunity, use full-length proteins that are introduced into a cell in a manner that will cause them to be processed as endogenous proteins. A recombinant form of CMVgB, a major target of the neutralizing antibody (nAb) response against CMV, was evaluated after combining with a powerful adjuvant referred to as MF59 or with standard alum.84,85,86 Results of a trial of 46 seronegative adults showed that the MF59 formulation is superior to alum and stimulated nAb for at least 12 months. Whether nAb against CMVgB will be sufficient to prevent recurrence or primary disease is still an open question.87
Other approaches that show promise in animal models include introduction of DNA vectors encoding the immunogenic protein as a means to elicit CTL.88,89,90 Refinement of DNA vaccine technology, including the use of minimal cytotoxic epitopes as immunogens replacing whole proteins91,92 and of modified proteins that permit increased accessibility to the proteasome or lysosome, may result in more efficient vaccines.93,94,95
An innovative approach to delivery of whole proteins is the use of polysaccharide nanoparticles.96,97 Several in vitro studies have examined the ability of these particles (Biovectors) to stimulate CMV-specific TH cells and CTL. IE-specific TH cells were effectively stimulated when bacterially produced protein was combined with Biovectors. Use of a recombinant fusion protein of CMV-IE and CMVpp65 (CMV-IE/pp65), produced by baculovirus in insect cells and complexed to Biovectors, resulted in strong CMVpp65-specific cytotoxicity (personal communication J. Lulé, C. Davrinche [INSERM], and D. Betbeder [Biovector Therapeutics Inc.]). This delivery method has favorable characteristics because it is non-viral, and human clinical trials will determine the extent of its capacity to elicit CMV-specific immunity.
Peptide Vaccine Strategies Against CMV
Rationale
The standardized use of a vaccine approach for allogeneic BMT is practical only if a less expensive and uncomplicated alternative for preventing CMV infection is available to facilitate immune reconstitution after HCT. As the phase I/II clinical trials at the Fred Hutchinson Cancer Center have indicated98 and as shown by others99,100,101 the control of viral infections requires TH activity for protective immunity. Providing TH in the form of a specific CD4+ TH-binding epitope that is incorporated into peptide vaccines seems to augment CD8 immunity and prolong the durability of the immune response. The clinical approaches to prevention of CMV by Diamond and colleagues are directed at augmenting TH cells, utilizing powerful TH epitopes derived from tetanus and the synthetic PADRE epitope.23,102
Definition of T cell epitopes using physico-chemical methods
A common theme in modern viral immunology has been the elucidation of peptide fragments referred to as minimal cytotoxic epitopes (MCE) from immunogenic viral proteins.103 Recent physical studies have shown that MHC Class I molecules have a preference for binding to peptides that conform to a given sequence motif. The amino acid residues at specific positions of a peptide motif are conserved in order for a peptide to bind to MHC molecules with high affinity.104,105 The average length of these peptides is between 8-11 amino acids as determined by either mass spectrometry or Edman degradation.106,107 Synthetic versions of these peptides bind to MHC molecules and sensitize targets to lysis by CD8+ CTL,106 since they require no further proteolytic processing to be active as CTL epitopes. They can also sensitize the TAP (transporter for antigen presentation)-deficient cell line, T2, for CTL-mediated lysis.108
Derivation of CMVpp65 CTL epitopes
Several groups have used the approach of deriving CTL clones against CMV as a means of mapping CTL epitopes from the immunogenic tegument proteins, pp65 and pp15023,24,25 or IE.32,109 The CD8+ CTL were initially shown to recognize CMV-infected fibroblasts, or endogenously processed full-length CMVpp65. By using an epitope mapping approach to localize portions of the protein necessary for CTL recognition in an MHC-restricted manner, several CTL epitopes have been identified that function efficiently at sensitizing CMV-infected fibroblasts to be recognized by CMV-specific CTL (Table 1). The combined frequency of HLA alleles that are associated with usage of these epitopes forms the basis of the hypothesis that it will be possible to vaccinate a large proportion of CMV-seropositive individuals with a small number of peptides. Because the sequence variability of proteins such as CMVpp65 and CMVpp150 is low between natural isolates of CMV, it is possible that a CTL epitope(s) can be defined which will be applicable to all CMV isolates and infected individuals in the context of the most frequently expressed HLA alleles.
Distribution of HLA alleles for known CMV CTL epitopes.
| . | . | . | . | HLA Antigen Frequency (%) . | |||
|---|---|---|---|---|---|---|---|
| HLA A or B Allele . | CMV Protein . | CTL HLA Restriction Characterized . | Sequence of CTL Epitope Defined . | CA . | BL . | CH . | HS . |
| HLA A*0101 | pp65 | Yes | Yes | 28.6 | 10.1 | 9.2 | 10.1 |
| HLA A*0201 | pp65 | Yes | Yes | 45.8 | 30.3 | 54.9 | 43.0 |
| HLA A*0301 | pp150 | Yes | Yes | 20.6 | 16.3 | 7.1 | 14.8 |
| HLA A*1101/2 | pp65 | Yes | Yes | 9.9 | 3.8 | 33.1 | 7.3 |
| HLA A*2401/2 | pp65 | Yes | Yes | 16.8 | 8.8 | 32.9 | 26.7 |
| HLA A*6801 | pp65 | Yes | Yes | 8.8 | 20.8 | 0.8 | 20.8 |
| HLA A*6801 | pp150 | Yes | Yes | 8.8 | 20.8 | 0.8 | 20.8 |
| HLA B*0702 | pp65 | Yes | Yes | 17.7 | 15.5 | 6.9 | 11.8 |
| HLA B*3502 | pp65 | Yes | Yes | 3.6 | 3.5 | 2.3 | 6.6 |
| HLA B*3801/2 | pp65 | Yes | Yes | 7.6 | 0.2 | 3.4 | 3.6 |
| HLA B*62 | pp150 | Yes | Yes | 10.3 | 2.6 | 14.3 | 3.0 |
| CTL epitopes were defined as minimal cytotoxic epitopes (MCE) as described in the text. Frequencies of HLA alleles in ethnic populations defined as CA (Caucasian), BL (black), CH (Chinese), and HS (Hispanic) are from the 11th International Histocompatibility Workshop.144 Two CTL epitopes in CMVpp65 restricted by HLA B*0702 and HLA A*6801 have been defined. | |||||||
| . | . | . | . | HLA Antigen Frequency (%) . | |||
|---|---|---|---|---|---|---|---|
| HLA A or B Allele . | CMV Protein . | CTL HLA Restriction Characterized . | Sequence of CTL Epitope Defined . | CA . | BL . | CH . | HS . |
| HLA A*0101 | pp65 | Yes | Yes | 28.6 | 10.1 | 9.2 | 10.1 |
| HLA A*0201 | pp65 | Yes | Yes | 45.8 | 30.3 | 54.9 | 43.0 |
| HLA A*0301 | pp150 | Yes | Yes | 20.6 | 16.3 | 7.1 | 14.8 |
| HLA A*1101/2 | pp65 | Yes | Yes | 9.9 | 3.8 | 33.1 | 7.3 |
| HLA A*2401/2 | pp65 | Yes | Yes | 16.8 | 8.8 | 32.9 | 26.7 |
| HLA A*6801 | pp65 | Yes | Yes | 8.8 | 20.8 | 0.8 | 20.8 |
| HLA A*6801 | pp150 | Yes | Yes | 8.8 | 20.8 | 0.8 | 20.8 |
| HLA B*0702 | pp65 | Yes | Yes | 17.7 | 15.5 | 6.9 | 11.8 |
| HLA B*3502 | pp65 | Yes | Yes | 3.6 | 3.5 | 2.3 | 6.6 |
| HLA B*3801/2 | pp65 | Yes | Yes | 7.6 | 0.2 | 3.4 | 3.6 |
| HLA B*62 | pp150 | Yes | Yes | 10.3 | 2.6 | 14.3 | 3.0 |
| CTL epitopes were defined as minimal cytotoxic epitopes (MCE) as described in the text. Frequencies of HLA alleles in ethnic populations defined as CA (Caucasian), BL (black), CH (Chinese), and HS (Hispanic) are from the 11th International Histocompatibility Workshop.144 Two CTL epitopes in CMVpp65 restricted by HLA B*0702 and HLA A*6801 have been defined. | |||||||
Derivation of CMVpp65 and HLA A*0201-restricted CTL and definition of MCE
Methodology that is similar in concept to published protocols was used to derive CD8+ clones from HLA A*0201-typed CMV-seropositive individuals.21,72,110 Representative clones were tested in a chromium release assay (CRA) against CMV proteins pp28, pp65, pp150, and IE expressed from vaccinia (Vac) viruses used to infect autologous or HLA-mismatched EBVLCL (Epstein-Barr virus transformed lymphocyte cell line), with wild type vaccinia (wtVac) as a control. A clone 3-3F4, which had significant reactivity against autologous but not mismatched EBVLCL infected with pp65Vac, was used to define the MCE by screening a series of truncations from the CMVpp65 protein in vaccinia. The region between amino acids (aa) 477-561 was shown to sensitize APC for lysis.23 The sequence of CMVpp65 was scanned for HLA A*0201-binding motif peptides by using a computer algorithm developed for that purpose.111 One nonamer peptide, at aa 495-503, sensitized target cells for lysis as efficiently as infection with pp65Vac, with no activity on HLA-mismatched cells.
Definition of CTL epitopes restricted by alleles other than HLA A*0201
The composition of a vaccine to stimulate immunity against CMV, based on the use of CTL epitopes, is dependent on the gene frequency of unique HLA alleles in the general population. Statistical studies have shown that a large proportion of many populations express at least one of a small number of HLA-A or B alleles, which suggests that identifying a limited number of CTL epitopes from CMV would allow the formulation of a universal vaccine strategy.25,112,113 This is especially relevant, because several studies suggest that the human immune response against CMV is limited to a few proteins, with the predominant response against pp65, and more recently to CMV-IE.24,32,33 CMV epitopes that have been characterized to date, as shown in Table 1, suggest that a large percentage of the U.S. population (∼90% for Caucasians) would be eligible for a vaccine strategy utilizing CMVpp65- and CMVpp150-specific epitopes.25
To elucidate these epitopes, Diamond and colleagues used stimulated PBLs from HLA-typed individuals who were seropositive for CMV, in a technique in which the free peptide epitope (100 mM) is added at a high concentration to PBMC in microwell cultures. The peptide-sensitized and irradiated PBMC are then mixed with CD4+, CD16+ and CD56+ lymphocyte-depleted PBL for a period of 14 days. This depletion of non-specific cytotoxicity resulting from NK and CD4+ T cells is necessary for the detection of a measurable difference in cytotoxicity against CMV or peptide-loaded targets. This approach has been used with CTL epitopes specific for CMVpp65 and restricted by HLA A*0201, A*1101, A*2402, A*6801, and B*0702.25,114 In transgenic mic that express HLA A*0201, for each epitope, five or more CMV-seropositive healthy animals have been shown to respond in the context of the restricting HLA allele (see Figure 3). The demonstration in at least ten randomly chosen human volunteers who respond in vitro to a single epitope (CMVpp65495-503) suggests the potential for a universal response to that epitope from all individuals who express the same restricting class I allele. Studies from several laboratories using similar methodology now document that the CMVpp65495-503 peptide is consistently recognized by HLA A*0201 individuals24 (La Rosa et al, unpublished results).
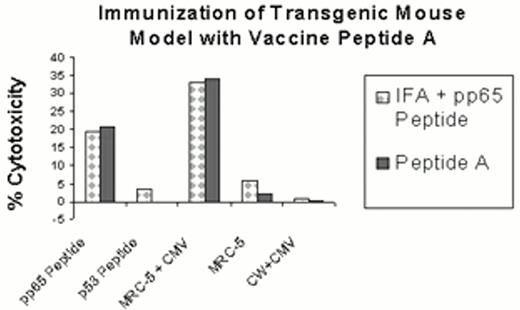
Mouse model of CMV lipopeptide vaccination.
Peptide A (see Table 2) without adjuvant or the CMVpp65495-503 epitope, emulsified with the synthetic PADRE TH epitope and incomplete Freund's adjuvant (IFA) were each injected s.c. into two separate transgenic HLA A2.1 mouse littermates. After twelve days, all mice were sacrificed, the spleens were removed, and a cell suspension was made. The immune spleen cells were restimulated twice in vitro at 7d intervals with peptide-pulsed syngeneic lipopolysaccharide-stimulated (LPS) blasts as described.23,118 After 14d, the restimulated spleen cells were tested for recognition of the immunizing CTL epitope on T2 cells (CMVpp65 peptide) or endogenously presented epitope on CMV-infected HLA A*0201-expressing fibroblasts (MRC-5+CMV) in a chromium release assay (CRA). As controls, an irrelevant peptide-sensitized T2 cell (p53 peptide), uninfected MRC-5 fibroblasts, and HLA-mismatched fibroblasts infected with CMV (CW+CMV) were also tested in the CRA. Data shown is representative of four mice immunized with either peptide, and spleen cells analyzed for CMV-specific immune response.
Mouse model of CMV lipopeptide vaccination.
Peptide A (see Table 2) without adjuvant or the CMVpp65495-503 epitope, emulsified with the synthetic PADRE TH epitope and incomplete Freund's adjuvant (IFA) were each injected s.c. into two separate transgenic HLA A2.1 mouse littermates. After twelve days, all mice were sacrificed, the spleens were removed, and a cell suspension was made. The immune spleen cells were restimulated twice in vitro at 7d intervals with peptide-pulsed syngeneic lipopolysaccharide-stimulated (LPS) blasts as described.23,118 After 14d, the restimulated spleen cells were tested for recognition of the immunizing CTL epitope on T2 cells (CMVpp65 peptide) or endogenously presented epitope on CMV-infected HLA A*0201-expressing fibroblasts (MRC-5+CMV) in a chromium release assay (CRA). As controls, an irrelevant peptide-sensitized T2 cell (p53 peptide), uninfected MRC-5 fibroblasts, and HLA-mismatched fibroblasts infected with CMV (CW+CMV) were also tested in the CRA. Data shown is representative of four mice immunized with either peptide, and spleen cells analyzed for CMV-specific immune response.
Lipopeptide Strategies.
The MCE may serve as a potent vaccine because of its property of direct binding to MHC Class I molecules without the need for further cellular processing. However, it is known that in order to stimulate a CTL response, the MCE must be suspended in a strong adjuvant.115,116,117 Several reports have shown that co-immunization of an MCE with a TH epitope into mice, with both suspended in strong adjuvant, such as incomplete Freund's adjuvant, efficiently stimulates primary CTL responses.23,118,119,120 However, the preferred clinical strategy is to use less harsh reagents as adjuvants, such as alum, MF59,121 or saponin containing adjuvants such as QS-21.122 A refinement of the peptide approach is to use lipidated peptides as part of a vaccine against infectious diseases. Studies originating in 1989 in the laboratory of Rammensee have shown the utility of lipidated CTL epitopes at eliciting CTL against influenza in several different mouse models.123,124,125 Several groups have also explored vaccinating macaques or mice in the absence of adjuvant, and they have obtained good immune responses using lipidated CTL epitopes from HIV.126,127 In addition, safety studies have shown that the lipid moiety does not cause skin inflammation and is safer than many alternatives.128,129 Recently, a clinical trial evaluated the strategy of using monolipidated peptides containing several CTL and TH epitopes in combination to stimulate HIV-specific immunity.130 The most advanced use of a lipidated peptide vaccine was developed by the group at EpImmune, Inc. (La Jolla, CA), in clinical studies directed towards developing a vaccine against hepatitis B virus (HBV).131,132 In their most recent clinical report, as much as 15 mg/dose of lapidated vaccine was administered to chronically infected HBV patients with no apparent toxicity.133 These studies highlight the usefulness of the lipidated peptide approach in conferring immunity to a pathogen in a safe and effective manner.
Proposed lipidated peptides as vaccines for BMT recipients
Previous studies of lipidated CMVpp65 epitopes in a mouse model demonstrated the conferral of primary immunity against CMVpp65. CTL that recognize CMVpp65 were also capable of recognizing and lysing CMV infected human fibroblasts.23 These CMV-specific lipidated peptides are scheduled for evaluation in a clinical setting in which their capacity to limit disease will be determined (see below). The approach of Diamond and colleagues in protecting BMT recipients against CMV infection will be to immunize their donors several weeks prior to HCT transfer and to evaluate whether boosting immunity in the recipients by vaccination will provide additional protection. Since the period of maximal immuno-incompetence is relatively short after BMT, it provides an excellent setting to test whether a CMV vaccine would confer protective immunity, until normal immunocompetence is re-established in the recipient.134,135
Immunization of transgenic mouse models of human MHC Class I- and Class II-dependent immune responses
The results from in vitro stimulation of human PBL using the CMVpp65495-503 peptide suggest that it may function in vivo to stimulate CTLp (CTL precursors) as a memory response from individuals with prior CMV exposure.23,136 To test whether the peptide could stimulate de novo CTLp without prior virus exposure, a transgenic mouse model, the HLA A*0201 mouse, was evaluated.137 The CMVpp65495-503 epitope, but not the p53149-157 HLA A*0201-restricted control, was recognized in a CRA assay.102,118 Murine splenic effectors also effectively recognized endogenously processed CMVpp65 because they kill human EBVLCL infected with pp65Vac- and CMV-infected fibroblasts in an HLA A*0201-restricted manner.23
In a second model, transgenic (Tg) mice were generated that expressed both HLA class 1 (A*0201) and class II (DRB1*0101) DRI molecules.102 Peptide mixtures consisting of a CMV-derived HLA A*0201-restricted CTL peptide epitope (pp65495-503), and tetanus toxin (TT)-derived and promiscuously MHC-binding TH epitopes, were assessed for their capacity to induce in vivo a virus-specific CTL response.138,139,140,141 The peptide mixtures were well tolerated and highly immunogenic, and a single injection resulted in a vigorous CMV-specific CTL response. A significant enhancement in CTL responses was observed in HLA-DR1/HLA-A2.1 Tg mice compared to HLA-A2.1 Tg mice (data not shown). These results demonstrate the efficacy of a CMV vaccine constructed with the CMVpp65495-503 CTL epitope combined with one or more universal TH epitopes from tetanus toxoid protein (Table 2). Finally, the use of HLA-A2.1 and HLA-DR1 double Tg mice constitute a versatile model system (in lieu of immunizing humans) for the study of both HLA class I- and class II-restricted T cell responses.
Primary structure of CMV vaccine peptides.
| Lipid Molecule(s) . | Adaptor Sequence . | TH Type . | TH Epitope Sequence . | Linker Sequence . | CMV CTL Epitope Sequence pp65495-503 . | Carboxyl Terminus . |
|---|---|---|---|---|---|---|
| A. 2 Palmitic Acids | -K-S-S- | PADRE | -A-K-X*-V-A-A-W-T-L-K-A-A-A- | NONE | -N-L-V-P-M-V-A-T-V- | OH |
| B. 2 Palmitic Acids | -K-S-S- | Tetanus | -Q-Y-I-K-A-N-S-K-F-I-G-I-T-E- | -A-A-A- | -N-L-V-P-M-V-A-T-V- | OH |
| The sequences of two vaccines, peptides A and B, are shown. These peptides are proposed for testing in the setting of HCT to prevent CMV disease by active vaccination of donors. The derivation and use of the PADRE TH peptide can be found in ref. # 120, and the tetanus peptide is discussed in ref. # 132 and # 131. | ||||||
| Lipid Molecule(s) . | Adaptor Sequence . | TH Type . | TH Epitope Sequence . | Linker Sequence . | CMV CTL Epitope Sequence pp65495-503 . | Carboxyl Terminus . |
|---|---|---|---|---|---|---|
| A. 2 Palmitic Acids | -K-S-S- | PADRE | -A-K-X*-V-A-A-W-T-L-K-A-A-A- | NONE | -N-L-V-P-M-V-A-T-V- | OH |
| B. 2 Palmitic Acids | -K-S-S- | Tetanus | -Q-Y-I-K-A-N-S-K-F-I-G-I-T-E- | -A-A-A- | -N-L-V-P-M-V-A-T-V- | OH |
| The sequences of two vaccines, peptides A and B, are shown. These peptides are proposed for testing in the setting of HCT to prevent CMV disease by active vaccination of donors. The derivation and use of the PADRE TH peptide can be found in ref. # 120, and the tetanus peptide is discussed in ref. # 132 and # 131. | ||||||
Immunization of transgenic HLA A2/Kb mice with lipidated chimeric peptide vaccines
Studies with a vaccine molecule whose structure is shown in Table 2 (A) have been initiated. This molecule incorporates the CMVpp65 HLA A*0201 epitope, the PADRE TH epitope, and is di-lipidated with palmitic acid on both the α- and ϵ- amino group of the amino terminal lysine of the adaptor sequence. The PADRE epitope was derived by synthetic alteration of optimal peptide binding motifs for human HLA class II DRb1 and b2 alleles.139 It binds with high affinity to at least 90% of the known human HLA class II alleles, and with medium affinity to the remainder. Significantly, it is also a potent stimulator of murine MHC class II responses; therefore, it functions equally well as a stimulator of human and murine proliferative responses.
HLA A2/Kb mice were immunized with 100 nmoles of Peptide A in DMSO without adjuvant. The HLA A2/Kb transgene used in these studies encodes a chimeric molecule in which the a3 domain of the HLA-A*0201 heavy chain was replaced with the corresponding domain from the murine H-2 Kb heavy chain.102 After two weeks, the spleens were harvested as described previously,23 and the splenic effectors showed a 25-fold increase in specificity for the immunizing epitope over control p53 peptide-sensitized targets, with potent cytotoxicity at E:T=20 (Figure 3, Peptide A). The same effectors showed CTL function against CMV-infected A*0201 (20-fold difference compared to uninfected fibroblasts, Figure 3, Peptide A). Other investigators have shown that similar lipidated peptides stimulate durable immune responses and do not cause adverse symptoms in mice for periods as long as one year.131, 142, 143 A recent publication143 confirms the results of Diamond and colleagues that lipopeptides possessing carboxylic acids smaller than 16 carbon chain (palmitic acid) are not immunogenic and do not have vaccine function. These results suggest that vaccination utilizing the CMVpp65495-503 peptide could be effective clinically in controlling CMV virus infection in at-risk groups such as BMT recipients.