Abstract
This article reviews the experience in hematopoietic stem cell transplantation (HSCT) for non-malignant disease. HSCT has long been applied as treatment of life-threatening congenital immunodeficiency and metabolic diseases. In Section I, Dr. Parkman reviews that experience for severe combined immunodeficiency, Wiscott-Aldrich syndrome, hyper IGM syndrome, Chédiak-Higashi disease and hereditary lymphohistiocytosis. The value of HSCT in genetic metabolic diseases such as osteopetrosis, osteogenesis imperfecta and the storage diseases are reviewed. In Section II, Dr. Walters reviews the experience over the last decade with allogeneic stem cell transplantation in patients with thalassemia major and sickle cell disease. In Section III, Dr. Sullivan reviews the more recent investigations using stem cell transplantation in patients with advanced autoimmune diseases such as systemic sclerosis, systemic lupus erythematosus, multiple sclerosis and juvenile rheumatoid arthritis. The pathogenesis and outcome with conventional care of these patients, the selection criteria and current results for HSCT, and the future directions in clinical research and patient care using this modality are addressed.
I. Hematopoietic Stem Cell Transplantation for Primary Immunodeficiency and Metabolic Diseases
Robertson Parkman, M.D.*
Research Immunology—BMT Division, Children's Hospital of Los Angeles, 4650 Sunset Boulevard, Box #62, Los Angeles CA 90027
Primary Immunodeficiencies
The first successful allogenic hematopoietic stem cell transplants (HSCT) were performed in 1968 in two patients with primary immunodeficiencies (severe combined immunodeficiency [SCID] and Wiskott-Aldrich syndrome [WAS]).1,2 Many of the biological lessons learned from the transplantation of patients with primary immunodeficiencies including the first use of T cell-depleted (TCD) transplants, unrelated donor transplants and gene therapy have now been applied to patients with oncological and hematological diseases.3,4,5 HSCT continues to be the only curative therapy for patients with many primary immunodeficiencies and metabolic diseases, although enzyme replacement therapy is available for some diseases (adenosine deaminase [ADA] deficiency and Gaucher's disease).
HSCT is the treatment of choice for patients with all forms of SCID. The majority of patients transplanted with HSC for SCID are recipients of TCD haploidentical bone marrow since only a minority of patients have histocompatible siblings donors. The overall survival rate of patients receiving histocompatible HSC is 80%, whereas the survival of recipients of TCD haploidentical bone marrow ranges between 60-70% depending upon the patients' clinical condition at the time of transplantation. Donor T lymphocytes are detected in the peripheral blood of recipients of histocompatible bone marrow within two to three weeks of transplantation, while the recipients of TCD transplants require three to four months before donor T lymphocytes are first identified.6,7,8,9 The difference in the time of appearance of donor T lymphocytes reflects the infusion of significant numbers of mature donor T lymphocytes at the time of transplantation in histocompatible HSCT, whereas following TCD HSCT a recapitulation of immunological ontogeny occurs that requires a minimum of three to four months before significant numbers of T lymphocytes derived from the newly engrafted donor HSC are detectable in the peripheral blood. Thus, the recipients of histocompatible HSCT may derive clinical benefits from their HSCT significantly sooner than the recipients of TCD HSCT. Although the presence of donor T lymphocytes are a uniform finding in SCID patients who are successfully transplanted, the presence of donor B lymphocytes is a variable finding. The biological basis for the lack of reproducible donor B lymphopoiesis is still unclear. The clinical consequences of the lack of reproducible donor B lymphopoiesis is that significant numbers of SCID patients require the long-term administration of replacement intravenous immunoglobulin (IVIGG) to compensate for their ongoing lack of normal B cell function although normal antigen-specific T lymphocyte function is present.
The area of greatest controversy in the transplantation of SCID patients is whether pretransplant chemotherapy is required. The recent Duke experience reported that 60 of 77 recipients of TCD HSCT were alive; however, some patients required multiple transplants, and the majority of the patients still required IVIGG administration due to a lack of normal B cell function.10 In contradiction to the Duke experience in which patients do not receive pretransplant chemotherapy, other centers that use myeloablative therapy report recipients who have complete donor lymphohematopoietic engraftment with a shift to donor B lymphopoiesis and no need for long-term IVIGG therapy.6,7,9 The need for multiple transplants is also avoided by the use of pretransplant conditioning, presumably due to the elimination of recipient natural killer cells, which are capable of interfering with donor HSC engraftment. Evaluation of the long-term effects of pretransplant conditioning (cyclophosphamide [CY] with or without busulfan [BU]) will have to be compared to the effects of long-term IVIGG replacement therapy with the knowledge that some patients receiving IVIGG are still at risk of developing viral infections, particularly of the central nervous system (CNS), with viruses that are not pathogenic in the general population.11 Thus, the question of whether pretransplant chemotherapy is clinically indicated is an unresolved issue.
The clinical features that have the greatest impact on the likelihood of a successful HSCT in SCID patients besides the nature of a transplant (histocompatible versus TCD) are the age of the patient at the time of transplantation, the presence of opportunistic infections and the presence of transplacentally acquired maternal graft-versus-host disease (GVHD).6,12 These factors are not independent variables since the older a patient is at the time of transplantation, the more likely it is that the patient will have acquired an opportunistic infection. Patients who have a family history of a previously infected sibling and have been diagnosed prenatally or at time of birth can be transplanted within the first two months of age and do better than children without a previous family history, who are diagnosed only after a series of opportunistic infections, which finally lead to the diagnosis of SCID and their referral to a pediatric transplantation center.
The primary immunodeficiency that is the second most frequently treated by HSCT is WAS. WAS was the first genetic disease involving hematopoiesis to be successfully treated by histocompatible bone marrow transplantation.13 In contradistinction to transplantation for SCID, where no pretransplant conditioning to prevent rejection is required for histocompatible HSCT, the early experience with WAS indicated that a combination of agents capable of immunosuppression and ablation of host HSC were required for successful lymphohematopoietic engraftment. Initially the combination of CY and total body irradiation (TBI) were utilized; however, TBI has now been superseded by BU and CY to permit adequate immunosuppression and ablation of the abnormal recipient HSC without the long-term toxicity of irradiation. One hundred-seventy patients with WAS have been reported to the International Bone Marrow Transplant Registry (IBMTR); 75 received histocompatible sibling bone marrow, 48 received transplants from relatives other than histocompatible siblings, and 67 from unrelated donors. The overall survival rate for the recipients of histocompatible bone marrow was 86%; those of other related donors, 51%; recipients of unrelated bone marrow who were younger than 5 years old, 83%; and recipients of unrelated donors, more than 5 years old, 26%. The great majority of recipients of histocompatible or unrelated marrow were completely cured of both the immunological and hematopoietic components of their disease, indicating that histocompatible or unrelated transplantation at an early age is the treatment of choice for patients diagnosed with WAS. The initial experience with other related donors, primarily TCD haploidentical bone marrow donors, was characterized by a high incidence of non-engraftment, and the use of related bone marrow that is not histocompatible is no longer indicated. The observation that patients more than 5 years old who received unrelated HSCT have a poor survival rate is due in part to the fact that many of these patients have developed other complications of WAS (in particular, autoimmune diseases).14,15 Therefore, patients with WAS who do not have histocompatible donors should be transplanted at a relatively early age to avoid the development of complications that may increase transplant-related mortality and to reduce the probability of developing chronic GVHD. Concerns have been raised as to whether successful HSCT reduces the likelihood of WAS patients developing Epstein-Barr virus (EBV)-associated lymphomas, a significant problem in older non-transplanted WAS patients. To date no successfully transplanted patients have developed EBV lymphomas.
A variety of other immunodeficiencies including CD40 ligand deficiency (hyper IGM syndrome), combined immune deficiency (CID) and even rare cases of common variable agammaglobulinemia (CVID) have been successfully transplanted, particularly with histocompatible donors. Debate presently exists about the use of HSCT for the treatment of Chédiak-Higashi syndrome since long-term follow-up of patients with successful donor HSC engraftment has revealed progression of their CNS abnormalities, suggesting that successful donor lymphohematopoietic engraftment does not modify this manifestation of the Chédiak-Higashi syndrome.
Hereditary lymphohistiocytosis (HLH) has recently been shown to be due to defects in the perforin gene in some patients.16 Although HLH has been considered to be an immunologically mediated disease for many years, it was originally thought to be a clonal abnormality. These new findings indicate that HLH is a primary immunodeficiency and substantiate the previous clinical findings that both histocompatible and unrelated HSCT are curative and can reverse all the clinical features of the disease.17 Debate still exists as to the optimal pretransplant conditioning regimen for patients with HLH, especially those with active CNS involvement at the time of transplantation. The combination of BU and CY is adequate to establish donor lymphohematopoietic engraftment and, therefore, ultimately a cure for the disease. The ongoing question is whether the addition of other agents such as etoposide (VP-16) and/or antithymocyte globulin (ATG) are necessary as part of the conditioning regime to control the underlying active disease.18 Thus, the preparative regimens for patients with HLH may differ between those who have the diagnosis but no overt symptomatology and those who have active clinical disease.
Gene Therapy
For SCID patients without appropriate allogeneic HSC donors, alternative curative strategies involving genetic correction have been pursued for almost 10 years.19,20,21,22 Initial attempts in the early 1990s focused on the treatment of ADA-deficient SCID using T lymphocytes and later HSC transduced with retroviral vectors. The transduced cells were then infused into patients who were also treated with enzyme replacement therapy (PEG-ADA). Although the long-term persistence of the transduced T lymphocytes or the progeny of the transduced HSC (T lymphocytes, granulocytes, B-lymphocytes and NK cells) have been demonstrated by several centers, none of the patients were able to be successfully removed from their enzyme replacement therapy.5 However, ADA gene expression in resting antigen-specific peripheral blood T lymphocytes has been inadequate to sustain protective immunity.
Recently, French investigators have reported the immunological correction of patients with X-linked SCID following the transplantation of autologous bone marrow HSC transduced with a retrovirus containing a normal copy of the human common γ chain gene, the affected locus in X-SCID.22 The patients have been followed more than one year after their transplantation and have normal numbers of immunophenotypic T and B lymphocytes, with in vitro demonstration of antigen-specific T lymphocyte function and specific antibody production in vivo. This immunological correction has occurred in the absence of any other therapeutic intervention demonstrating that normally functioning T and B lymphocytes expressing the transduced gene have persisted for more than a year. Longer-term follow-up will be necessary to demonstrate continued persistence of the transduced T and B lymphocytes and sustained protective immunity. The successful treatment of X-linked SCID by gene therapy gives hope that other molecular forms of SCID may be treated by gene therapy by using autologous HSC, avoiding the need for haploidentical transplantation and its associated problems of chemotherapy, immunodeficiency and GVHD.
Metabolic Diseases
Besides the use of HSCT as curative therapy for the genetically determined primary immunodeficiencies, HSCT has also become the standard treatment for several other genetic metabolic and hematologic diseases. Osteopetrosis patients were first successfully transplanted in 1980.23 At that time the biological origin or lineage derivation of osteoclasts and osteoblasts was not known. The fact that osteopetrosis patients were successfully treated by transplantation suggested that osteoclasts were of donor, and thus hematopoietic, origin. This was later confirmed experimentally. The patients were prepared for transplantation with a combination of CY and BU to obtain adequate immunosuppression and recipient HSC ablation. Following successful lymphohematopoietic engraftment, however, no changes in the patient's clinical status occurred during the first four months following HSCT. Subsequently, there was the onset of bone remodeling, with an increase in marrow space and in serum calcium. These clinical observations indicated that the turnover of osteoclasts was significantly slower than that of the circulating myeloid cells and suggested that significant numbers of donor-derived osteoclasts were not present until four to six months following HSCT. Therefore, one of the dilemmas facing physicians involved in the potential transplantation of patients with osteopetrosis is the fact that unlike immunodeficiencies and hemoglobinopathies where the disease can be “cured” within a month of transplantation, the underlying pathophysiology of osteopetrosis is not modified for the first four to six months after HSCT, during which time disease progression will continue. Thus, the patients' vision and hearing may continue to deteriorate, and it is difficult to predict accurately what level of neurological function will be present when the donor osteoclasts begin to have a clinical impact on the patients' disease. Thus, although parents may be willing to deal with the problems their child has at the time of transplantation, it may be more difficult for them to deal with the problems that develop following transplantation. Thus, the counseling of parents about the appropriateness of HSCT for osteopetrosis is difficult, especially when it concerns children who already have neurological impairment.
More recently a controversial study was published showing that HSCT with bone marrow has been shown to impact the natural history of osteogenesis imperfecta, which is due to abnormal osteoblast function.24 Whereas osteoclasts are derived from HSC, osteoblasts are derived from mesenchymal stem cells (MSC), which are also present in the bone marrow but have not previously been thought to be transferred with HSCT. The patients reported in this study had very low (1%) demonstrable donor-derived osteoblasts but still had evidence of clinical improvement. Thus, the use of MSC transplantation to treat genetic diseases involving mesenchymally derived cells, including osteoblasts, chrondrocytes and particularly myocytes, is an area of clinical exploration at present.
The first inborn error of metabolism to be corrected by histocompatible bone marrow transplantation was Gaucher's disease.25 Gaucher's disease is due to abnormalities in the enzyme glucocerebrosidase. The first patients transplanted had Type I disease, in which patients did not have neurological involvement. Successful donor lymphohematopoietic engraftment following pretransplant conditioning with CY and BU did not result in the resolution of the patients' hepatosplenomegaly and bone marrow involvement until six months following HSCT. Thus, as in the case of osteopetrosis, the turnover of HSC-derived tissue macrophages was delayed as compared to the circulating myeloid elements. The need for allogeneic transplantation in patients with Gaucher's disease has been modified by the availability of commercial glucocerebrosidase enzyme replacement. This therapy has been proven effective but can cost approximately $500,000 a year. Fewer patients have been treated by HSCT with Type III (subacute neurotropic type) Gaucher's disease.26 The follow-up of transplanted patients indicates that successful donor lymphohematopoietic engraftment can result in the stabilization and, in some cases, the improvement of neuropsychological function, suggesting that the turnover of the tissue macrophages within the CNS (microglial cells) can modify the natural history of the disease. Enzyme replacement does not reverse CNS abnormalities; thus, continued exploration of HSCT in these patients is warranted.
The inborn error of metabolism for which the greatest clinical experience with HSCT exists is Hurler's syndrome, a deficiency of α-iduronidase.27,28 Successful donor lymphohematopoietic engraftment (usually after preparation with BU and CY) results in all circulating lymphohematopoietic elements being of donor origin and an increase in α-iduronidase to levels found in the donor, either homozygous or heterozygous. Following successful donor engraftment there is resolution of many of the systemic symptoms of the disease, including hepatosplenomegaly and a reduction but not an elimination of the corneal clouding. However, successful donor lymphohematopoietic engraftment does not result in the improvement of chondrocyte and osteoblast function since these cells are not in general derived from transplanted HSC. Because of the poor blood supply to these cells, little of the donor-derived enzyme present in the plasma (which is taken up by the recipient hepatocytes) is taken up by chondrocytes and osteoblasts, resulting in no improvement of the underlying disease at these sites. The area of greater concern has been the effect of successful engraftment on the patients' CNS function. Longitudinal evaluation of patients has indicated that successful donor lymphohematopoietic engraftment can result in disease stabilization and in some cases improvement of neuropsychological function starting six months following transplantation.29 Thus, as in the case of osteopetrosis and Gaucher's disease, there is a delay in the replacement of abnormal recipient macrophages (microglial cells) by donor-derived cells that may take almost six months following HSCT. Patients who have significant defects in neuropsychological function prior to transplantation (DQ < 70) rarely show improvement following HSCT. As in the cases of osteopetrosis, disease progression can occur in spite of successful donor engraftment; therefore, uncertainty exists as to what the ultimate level of CNS function of successfully transplanted patients will be.
In contradistinction to the improvement that has been observed with Hurler's syndrome, clinical benefits in patients with Hunter's syndrome (iduronate sulfatase deficiency) have rarely been observed. In spite of successful donor lymphohematopoietic engraftment, little change in the clinical symptomatology of their patients has occurred. The lack of efficacy of HSCT in Hunter's syndrome is due to the fact the affected somatic cells do not take up the enzyme present in the plasma (iduronate sulfatase). Therefore, although donor-derived enzyme is present, it is not transported to the sites of substrate accumulation; therefore, no impact on the clinical symptomatology of the disease occurs.
The other mucopolysaccharidoses (MPS) for which HSCT has been attempted includes Maroteaux-Lamy syndrome (MPS VI), San Filippo syndrome (MPS III) and Morquio syndrome (MPS IV). Patients with Maroteaux-Lamy syndrome have no CNS abnormalities, and successful donor engraftment results in the improvement of many but not all of the clinical symptomatology.30 The clinical symptoms that are not affected by successful donor lymphohematopoietic engraftment include the skeletal abnormalities, because enzyme in the plasma cannot gain access to the sites of substrate accumulation. HSCT for Morquio syndrome and San Filippo disease have been without reproducible clinical benefit and, therefore, are not advocated by most transplant centers.
The other group of metabolic diseases that has been treated by HSCT are the sphingolipidoses including adrenoleukodystrophy (ALD), metachromatic leuko dystrophy (MLD) and globoid leukodystrophy (GLD). ALD is due to peroxisomal enzymatic deficiencies, which lead to an inability to degrade long chain fatty acids. In this X-linked disease there is no consistent familial pattern of disease expression complicating decisions about the appropriate timing of HSCT. Non-transplant therapies have been unsuccessful in modifying or stabilizing the natural history of the disease. HSCT can in some cases escalate disease progression in the peri-transplant period. Many centers, therefore, closely follow patients with the established diagnosis of ALD by both nuclear magnetic resonance (MRI) and neuropsychological testing and are prepared to initiate HSCT at the first sign of disease progression. The results of histocompatible HSCT have been heartening, with demonstrated improvement in neuropsychological testing and the reversal of pre-existing MRI abnormalities in some patients if successful HSCT is done early in the patient's clinical course.31 Unrelated HSCT shows fewer positive results, with an increased incidence of GVHD and a suggestion that GVHD accelerates disease progression. HSCT has had the greatest clinical impact when a histocompatible donor is available and the patient is transplanted early in the disease course.
Metachromatic leukodystrophy was the first CNS storage disease to be successfully transplanted.32 Following the establishment of donor lymphohematopoietic engraftment, CNS function stabilized but peripheral neurological abnormalities progressed, suggesting that more donor enzyme was present within the CNS than was available to peripheral nerves. Overall the response of patients with MLD to successful donor lymphohematopoietic engraftment has been variable, and improvement has not been as reproducible as in the case of patients with ALD. Patients with infantile GLD have had no clinical improvement following donor lymphohematopoietic engraftment; therefore, most centers will no longer transplant patients with Krabbe's disease. Some patients with the juvenile and adult forms of GLD have had disease stabilization following HSCT.
Summary
HSCT is an established therapy for patients with both primary immunodeficiencies and metabolic diseases. The best results are achieved when histocompatible donors are available, although the use of alternative donors, either parents or matched unrelated donors, can result in clinically significant results. Patients with appropriate diseases should be transplanted as soon as possible to reduce the long-term effects of the primary disease and transplant-related toxicities.
II. Hematopoietic Cell Transplantation for β-Thalassemia Major and Sickle Cell Anemia: Current Results and Future Directions
Mark C. Walters, M.D.*
Children's Hospital of Oakland, 747 52nd Street, Oakland CA 94609-1859
Hereditary anemias caused by β-globin mutations that give rise to β-thalassemia major and sickle cell anemia account for the most prevalent form of human genetic diseases worldwide. The genotypes of these disorders are very well understood and were among the first to be characterized by their molecular genetic alterations.1,2,3 However, for the purpose of discussions about the role of HSCT as a treatment for β-thalassemia major and sickle cell anemia, the erythroid progenitor cell can be considered a target of ablative transplant preparation. With subsequent rescue of erythropoiesis by donor cells, the clinical sequelae of ineffective erythropoiesis and hemolysis might also be eliminated. Thus, it is perhaps not surprising that HSCT offers curative potential for these disorders, which derive their cellular origin from the pluripotent hematopoietic stem cell.4,5 The challenge of HSCT, however, has to do with understanding to whom and when to offer this therapy.6,7 These deliberations are perhaps simplified for patients who have β-thalassemia major, where even though there are many different mutations that reduce or abolish β-globin production, patients who inherit them express a phenotype that does not vary from individual to individual (one notable exception being patients with E/β° thalassemia). In contrast, in sickle cell anemia a single, common point mutation is associated with a broad spectrum of clinical phenotypes, and reliable genetic or epigenetic indicators of severe disease that might direct more severely affected individuals to HSCT are not yet available.8 In addition, alternative supportive therapies that offer significant amelioration of clinical symptoms are available for both disorders.9,10 For these reasons, the role of HSCT for β-thalassemia major and sickle cell anemia is evolving and will undoubtedly undergo further revision as new methods of allografting and preventing or treating transplant-related complications are developed. This summary will review the current status of HSCT for these disorders, and project likely areas of future investigation.
Outcome after BMT for β-Thalassemia Major: Impact of Risk Status
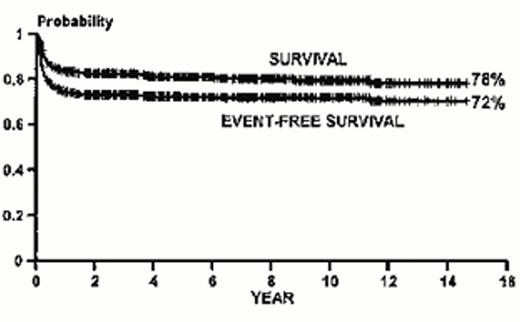
Since the first report of successful bone marrow transplantation for β-thalassemia major in Seattle in 1982, nearly 900 patients in Pesaro Italy and more than 1,500 worldwide have received this treatment.4,11 Initially, it was reasoned that the risk of transplantation might be increased by the presence of iron overload, and the risk of graft rejection increased by exposure to minor histocompatibility locus antigens expressed on leukocytes present in transfused blood products. Therefore, younger patients were initially targeted for enrollment with the expectation of a high probability for survival. These predictions were realized in the experience from Pesaro.12 Young patients with β-thalassemia major received matched sibling allografts after preparation for transplantation with a myeloablative combination of BU and CY. An overall event-free survival probability of 72% among all patients between 1 and 35 years of age was observed (Figure 1); however, the best results occurred in the youngest, least transfused patients.13,14 In these good-risk, Lucarelli class I patients, the event-free survival was 91%; alternatively, among poor-risk patients with iron overload and liver disease from inadequate chelation, the event-free survival was approximately 50%.15 The toxicity of the myeloablative preparation was particularly high in young adult patients with β-thalassemia major. Among those who were older than 17 years of age the event-free survival after matched sibling transplants was 62%, and the probability of dying was 35%.16 These data strongly suggest that the optimal timing of transplantation is in the very young patient who has a HLA-identical sibling donor. On the other hand, this conclusion is challenged by the observation that these individuals are also very well served by standard medical therapy, where individuals who receive regular transfusions and are compliant with iron chelation therapy have excellent cardiac disease-free survival.17,18 Ideally, it would seem most logical to reserve transplantation for individuals who have failed transfusion therapy and whose risk of dying of complications from iron overload has become high. Unfortunately, these same patients currently experience poor outcome after allogeneic bone marrow transplantation. Thus, a new approach to transplantation that might reduce its toxicity in this setting would be very useful.19
Kaplan-Meier probabilities of survival and event-free survival for 826 patients with thalassemia major who received marrow allografts between December 1981 and April 1997. (Reprinted with permission from the New York Academy of Sciences.)
Kaplan-Meier probabilities of survival and event-free survival for 826 patients with thalassemia major who received marrow allografts between December 1981 and April 1997. (Reprinted with permission from the New York Academy of Sciences.)
Consequences of Iron Overload and Other Late Complications of HSCT
An advantage to developing less toxic pretransplant conditioning for transplantation is the prediction that late adverse effects of transplantation might also be reduced. Among children with β-thalassemia major, the contribution to neuroendocrine dysfunction by myeloablative doses of BU is complicated by the co-variable of iron overload, which together tend to amplify the risk of delays in growth and development.20,21,22 Gonadal toxicity following high-dose therapy with alkylators has been commonly observed.23 Of 26 females with β-thalassemia major aged 3.6 to 14.5 years at time of transplantation, 24 had elevated gonadotropin levels 1-9 years after transplantation and 12 who had no or arrested pubertal development eventually received hormonal replacement therapy.21 There was preservation of testicular function in some ex-thalassemic males after transplantation, where four of 12 Tanner stage 1 and nine of 12 Tanner stage 2 to 5 boys had elevated gonadotropin levels, suggesting that some patients will pass through puberty despite gonadal dysfunction. Among those who have stable engraftment of donor cells, the toxic effect of total body iron overload on the pituitary-gonadal axis might be mitigated by efforts that promote iron unloading. It has been observed that iron stores remain elevated after transplantation, perhaps contributing to progression of liver disease in those patients who had advanced stage characteristics before transplantation.13,24 However, regular phlebotomy after transplantation has proved efficacious in lowering the hepatic iron concentration with parallel improvements in liver inflammation scores in these patients.25 It is not yet clear whether stabilization or improvement in hepatic fibrosis will occur after transplantation, especially in those with advanced disease before transplantation or with hepatitis C infection.
Persistence of Donor-Host Mixed Hematopoietic Chimerism after HSCT
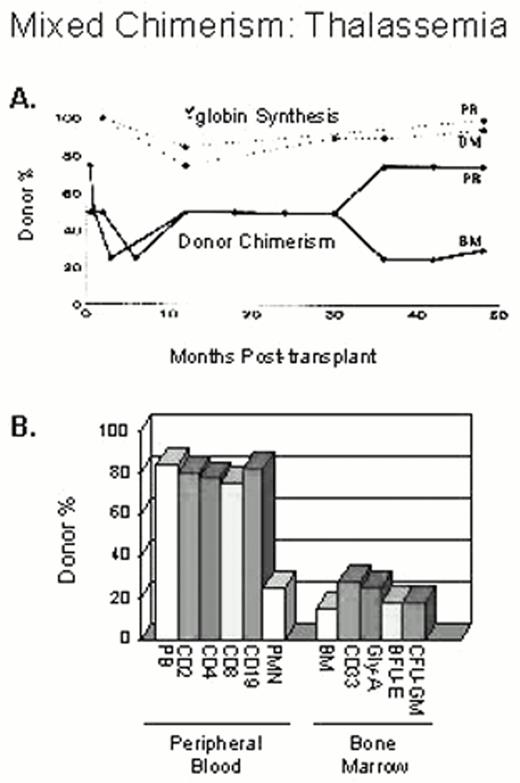
Mixed hematopoietic chimerism after transplantation has been observed in individuals treated for β-thalassemia major.26,27,28 Approximately 30% of patients demonstrated mixed donor-host hematopoietic chimerism 2 months after transplantation and 8% had stable mixed chimerism that persisted from 2 to 11 years after transplantation. The frequency of stable mixed chimerism was highest among patients who received reduced CY dosing for pre-transplant immunosuppression.29 When the level of persistent host hematopoiesis exceeded 25% at 2 months after transplantation, virtually all these patients experienced graft rejection with return of β-thalassemia major. However, when donor cells accounted for fewer than 10% of the total, 27% of these individuals eventually developed mixed chimerism that was stable. Patients who had as few as 10 to 20% donor cells in the bone marrow experienced marked enrichment of donor cells in the peripheral blood where β-globin chain synthesis reached levels of 80-95% of normal30 (Figure 2). A similar observation was made in five patients reported from the multicenter study of bone marrow transplantation for sickle cell disease.30,31,32 One patient had 10% donor cells in the bone marrow but remained independent of RBC transfusions, with a stable sickle hemoglobin fraction of 20-30% more than 5 years after transplantation.
Donor-recipient origin of different peripheral blood and marrow cell populations at 4 years after transplantation.
The percentage of donor chimerism in the blood and marrow nucleated cells is shown in A. β-globin chain synthesis by marrow and reticulocytes was greater than 90% at 4 years after transplant. Although there is an enrichment for donor cells in the mature red blood cell compartment as demonstrated by the high rate of β-globin synthesis, the percentage of donor cells in the immature red blood cell compartment (BFU-E and glycophorin-A positive cells) was approximately 20% (in B).
Donor-recipient origin of different peripheral blood and marrow cell populations at 4 years after transplantation.
The percentage of donor chimerism in the blood and marrow nucleated cells is shown in A. β-globin chain synthesis by marrow and reticulocytes was greater than 90% at 4 years after transplant. Although there is an enrichment for donor cells in the mature red blood cell compartment as demonstrated by the high rate of β-globin synthesis, the percentage of donor cells in the immature red blood cell compartment (BFU-E and glycophorin-A positive cells) was approximately 20% (in B).
Mini-Allografts for β-Thalassemia Major?
These observations have raised the possibility that less toxic conditioning for transplantation may still permit engraftment of donor cells and establish stable mixed chimerism.33,34 To do so, however, will require a better understanding of how to establish immunologic tolerance in a multiply transfused host. In general, graft rejection has occurred more frequently after HSCT for hemoglobinopathies than in malignant disorders, even following preparation with myeloablative doses of chemotherapy. Thus, the chief risk of using reduced intensity preparation for β-thalassemia is recurrent disease. This view is supported by observations in Lucarelli class III risk group patients in whom graft rejection was more frequent after the dose intensity of transplant conditioning was reduced.15 However, the possibility that donor-host tolerance might be induced among the most extensively transfused patients was suggested by the observation that patients who received more than 100 red blood cell transfusions before transplantation experienced a lower probability of graft rejection/recurrent thalassemia than those patients exposed to fewer than 100 transfusions (24% vs. 53%, respectively). Moreover, lowering the dose of CY in the group of young adults who had predominantly class III characteristics did not appreciably increase the risk of graft rejection/recurrent thalassemia, where the principal cause of failure was death.16 Of interest, all but one of the adult patients had received more than 100 transfusions. These data support the idea that myeloablative conditioning before transplantation might not be required for engraftment of donor cells. In addition, it is likely that a reduction in pretransplant dose intensity will translate into improved survival.
Unfortunately, preclinical models of non-myeloablative allografting do not necessarily lend themselves well to the thalassemia clinical model, where pre-sensitization to minor histocompatibility antigens from transfusion exposures and a proliferative marrow present barriers to engraftment of donor cells.35 Insights into the mechanism of tolerance have been suggested by studies of the T cell repertoire from a small number of thalassemic patients who had persistent chimerism after transplantation.36 These patients had a skewing of the normal Gaussian distribution of T cell populations, due to expansion of selected oligoclonal populations of T lymphocytes rather than a contraction of the lymphocytic diversity and impaired immunocompetence. Of interest, normal responses to recall and unrelated third-party alloantigens by coexistent donor and host lymphocytes were observed. Presumably, the expansion of these selected T cell clones relates to the establishment and/or maintenance of the chimeric state, and it is possible that studies to elucidate the identity and function of these cell populations will provide further insight about how tolerance evolves in these patients.
Indications and Outcome after HSCT for Sickle Cell Anemia
While the worldwide experience of HSCT for sickle cell anemia currently encompasses approximately 175 cases, far fewer sickle cell patients have undergone this therapy compared to β-thalassemia major (Table 1).37 Because the outcome after HSCT for sickle cell anemia mirrors the experience for thalassemia, there are undoubtedly reasons not related to outcome that might explain why so few have been performed. The primary barrier to HSCT for sickle cell anemia is the lack of a suitable stem cell donor; thus, efforts to develop the safe utilization of alternative sources of stem cells and to overcome the histocompatibility barrier should take on primary importance for expanding this potentially curative treatment.38,39 The other important reasons why physicians and their patients might be reluctant to pursue HSCT are probably related to the variable individual and temporal expression of disease severity, and the development of pharmacological alternatives such as hydroxyurea that are reasonably safe, effective, and readily available to nearly all patients.10,40,41
Hematopoietic stem cell transplantation for sickle cell disease.
| Location . | Belgium43 . | Pesaro69 . | France42,70 . | Multicenter31,46,49 . | Other US/Europe5,43 ,71,72,73,74,75,76 . | Total . | |
|---|---|---|---|---|---|---|---|
| Regimen | BU,CY (30) | BU,CY (17) | BU,CY (12) | BU,CY,ATG | BU,CY,ATG (13) | ||
| BU,CY,TLI (6) | BU,CY,TL1 (1) | CY/TBI (3) | |||||
| BU,CY,ATG (14) | BU,CY,ATG (21) | ||||||
| Number of patients | 36 | 14 | 19 | 34¶ | 59 | 16 | 175¶ |
| Median age (range) | 8.6 (1.7-23) | 2.0 (0.9-15) | 7 (4-38) | 8.6 (2.3-17.2) | 9.9 (3.3-15.9) | NA | |
| Clinical Status: | |||||||
| Asymptomatic | 0 | 14 | 3 | 0 | 0 | NS | |
| Stroke/CNS | 6 | 0 | 1 | 16 | 31 | 2 | |
| ACS | 20 | 0 | NS | 15 | 20 | 1 | |
| VOC | 36 | 0 | 15 | 18 | 8 | 1 | |
| Other/unknown | — | — | — | — | — | 11 | |
| MM donor | — | — | 1 | — | — | ||
| Survival (%) | 34 (94) | 14 (100) | 14 (74) | 31 (91) | 55 (94) | 13 (81) | 159 (91) |
| Deaths | 2 | 0 | 5 | 3¶ | 4 | 3 | 16¶ |
| Graft rejection/recurrent SCD (%) | 4 | 1 | 1 | 4 | 5 | 1 | 16 (9) |
| Stable mixed chimerism | 6** | — | 5 | 1 | 10 | — | 11% |
| Disease-free survival (%) | 30 (83)* | 13 (93) | 13 (68) | 27 (79)* | 50 (85) | 12 (80)* | 143 (82) |
| aGVHD | # 15## | 5 | 4 | 6 (>grade II) | 11 (Grade I-III) | 2 | 25% |
| cGVHD | 8 | 2 | 2 | 2 | 5 | 1 | 12% |
| Seizures | 18 | 1 | 7/26 | 13 | 1 | 25% | |
| Abbreviations: ACS, acute chest syndrome; aGVHD, acute graft-versus-host disease; ATG, antithymocyte globulin; BU, busulfan; cGVHD, chronic graft-versus-host disease; CNS, central nervous system; CY, cyclophosphamide; MM, mismatched; SCD, sickle cell disease; TLI, total lymphoid irradiation; TBI, total body irradiation, VOC, vasoocclusive crisis; NS, not stated; NA, not available; HSCT, hematopoietic stem cell transplantation | |||||||
| Location . | Belgium43 . | Pesaro69 . | France42,70 . | Multicenter31,46,49 . | Other US/Europe5,43 ,71,72,73,74,75,76 . | Total . | |
|---|---|---|---|---|---|---|---|
| Regimen | BU,CY (30) | BU,CY (17) | BU,CY (12) | BU,CY,ATG | BU,CY,ATG (13) | ||
| BU,CY,TLI (6) | BU,CY,TL1 (1) | CY/TBI (3) | |||||
| BU,CY,ATG (14) | BU,CY,ATG (21) | ||||||
| Number of patients | 36 | 14 | 19 | 34¶ | 59 | 16 | 175¶ |
| Median age (range) | 8.6 (1.7-23) | 2.0 (0.9-15) | 7 (4-38) | 8.6 (2.3-17.2) | 9.9 (3.3-15.9) | NA | |
| Clinical Status: | |||||||
| Asymptomatic | 0 | 14 | 3 | 0 | 0 | NS | |
| Stroke/CNS | 6 | 0 | 1 | 16 | 31 | 2 | |
| ACS | 20 | 0 | NS | 15 | 20 | 1 | |
| VOC | 36 | 0 | 15 | 18 | 8 | 1 | |
| Other/unknown | — | — | — | — | — | 11 | |
| MM donor | — | — | 1 | — | — | ||
| Survival (%) | 34 (94) | 14 (100) | 14 (74) | 31 (91) | 55 (94) | 13 (81) | 159 (91) |
| Deaths | 2 | 0 | 5 | 3¶ | 4 | 3 | 16¶ |
| Graft rejection/recurrent SCD (%) | 4 | 1 | 1 | 4 | 5 | 1 | 16 (9) |
| Stable mixed chimerism | 6** | — | 5 | 1 | 10 | — | 11% |
| Disease-free survival (%) | 30 (83)* | 13 (93) | 13 (68) | 27 (79)* | 50 (85) | 12 (80)* | 143 (82) |
| aGVHD | # 15## | 5 | 4 | 6 (>grade II) | 11 (Grade I-III) | 2 | 25% |
| cGVHD | 8 | 2 | 2 | 2 | 5 | 1 | 12% |
| Seizures | 18 | 1 | 7/26 | 13 | 1 | 25% | |
| Abbreviations: ACS, acute chest syndrome; aGVHD, acute graft-versus-host disease; ATG, antithymocyte globulin; BU, busulfan; cGVHD, chronic graft-versus-host disease; CNS, central nervous system; CY, cyclophosphamide; MM, mismatched; SCD, sickle cell disease; TLI, total lymphoid irradiation; TBI, total body irradiation, VOC, vasoocclusive crisis; NS, not stated; NA, not available; HSCT, hematopoietic stem cell transplantation | |||||||
Three patients were reported twice in the two French series42,70 ; one of them died after HSCT and was also counted twice among the deaths. These three patients are counted only once in the totals.
1 patient was disease-free after a second HSCT
3 had > 30% recipient cells
1 patient had Morquio's disease and 2 had acute leukemia concomitantly.
Only 1 had > grade II GVHD
To date, most investigational protocols have required a significant clinical complication of sickle cell anemia (such as stroke or recurrent painful episodes) as a prerequisite for HSCT.31,42,43 As the risks and benefits of HSCT for sickle cell anemia become more defined and the natural history of sickle cell anemia more completely understood, there is renewed interest in viewing HSCT as a proactive rather than reactive intervention.44 To do so, of course, would be facilitated by having predictors of a morbid outcome to identify the subset of patients at risk for early mortality. Recent progress by the Cooperative Study of Sickle Cell Disease in developing clinical predictors of severe disease has been reported, but these are more likely to be useful for excluding `healthy' children from risky interventions rather than to prospectively identify high-risk patients in a reliable way.45 If, on the other hand, one takes the view that on average, sickle cell anemia is seldom, if ever, a benign disorder, universal application of early HSCT when a sibling donor exists might be a logical response. This approach takes on added merit if and when the toxicity of HSCT is reduced. Thus, future studies of HSCT for sickle cell anemia will very likely focus on novel methods to minimize its toxicity and broaden hematopoietic stem cell sources.
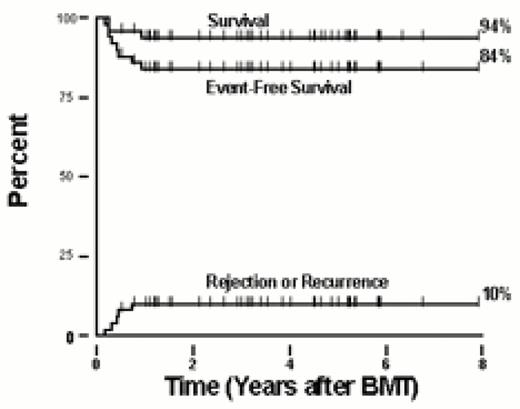
The outcome after HSCT for sickle cell anemia is summarized in Table 1. Most of the patients were younger than 17 years of age, and all but one received matched sibling marrow allografts. The first was performed in 1984, although most patients received treatment in the last decade. All patients treated for the indication of having sickle cell anemia received pre-transplant preparation with myeloablative doses of BU and CY. Many patients also received anti-T lymphocyte antibody infusions before transplant, and a smaller number were administered total lymphoid irradiation as pretransplant immunosuppression. BU pharmacokinetics was evaluated in some patients and, since 1994, in all the North American patients enrolled in the collaborative multicenter trial.46 Prophylaxis for GVHD varied among institutions, but most commonly consisted of a combination of methotrexate and cyclosporine. The complications from sickle cell anemia experienced by the patients before HSCT are listed in Table 1. Most had vasoocclusive complications such as stroke and recurrent episodes of acute chest syndrome and/or pain before HSCT. Overall, 91% of the patients survived, and 82% survived free of sickle cell anemia after HSCT. Graft rejection or recurrent sickle cell disease occurred in 9% of the patients. Neurologic complications occurred in 25% of patients after HSCT; seizures were the most frequent event, although long-term sequelae from these were not observed.47,48 Stable donor-host hematopoietic chimerism was reported in 11% of patients. In the Belgian cohort, there was a trend toward improved survival and disease-free survival among patients who received allografting before they developed clinical complications from sickle cell anemia.43 The Kaplan-Meier probabilities of survival and event-free survival among 50 children enrolled in the multicenter collaborative trial with up to 8 years follow-up are depicted in Figure 3.
Outcome after HSCT for sickle cell anemia. The Kaplan-Meier probabilities of survival, event-free survival, and a cumulative incidence curve for graft rejection/recurrent sickle cell disease are shown. Fifty patients who had at least 6 months follow-up received matched sibling marrow allografts between September 1991 and October, 1999.
Outcome after HSCT for sickle cell anemia. The Kaplan-Meier probabilities of survival, event-free survival, and a cumulative incidence curve for graft rejection/recurrent sickle cell disease are shown. Fifty patients who had at least 6 months follow-up received matched sibling marrow allografts between September 1991 and October, 1999.
Impact of HSCT on Sickle Cell Disease Expression and Other Late Effects
Among patients who had stable engraftment of donor cells after HSCT, none experienced subsequent clinical vasoocclusive events.43,49 Evaluation of the effects of transplantation on sickle cell-related organ damage is ongoing but suggests stabilization and, in some cases, improvement in pre-existing sickle vasculopathy. Several groups have reported recovery of splenic function, stabilization of CNS disease and bony abnormalities after transplantation.42,50,51 In addition, in most patients pulmonary disease remained stable as evaluated by pulmonary function testing, and improved linear growth was observed. Preliminary results suggest that most patients have excellent quality of life after HSCT.
Cytotoxic conditioning and allogeneic HSCT can cause long-term adverse effects. Chronic GVHD was reported in 20 of 174 patients after HSCT for sickle cell anemia and resulted or contributed to the death of eight patients. A decreased rate of growth velocity was associated with chronic GVHD.43 Gonadal function is often adversely affected by myeloablative doses of BU; thus, ovarian dysfunction after HSCT is common, especially among post-pubertal women. Of six evaluable prepubertal girls transplanted in Belgium, five had primary amenorrhea with elevated serum luteinizing hormone (LH) and follicle stimulating hormone (FSH), and two post-pubertal females developed secondary amenorrhea. Similar findings were observed in females enrolled in the multicenter trial.49 Testicular function may be less often adversely affected by therapy, and none of four males who were more than 13 years of age from the multicenter trial had elevated serum LH/FSH levels. However, among six boys from the Belgian centers who developed normally, four had decreased testosterone and elevated FSH levels. In addition, there are concerns about the long-term risk of malignancy after HSCT, although occurrence of cancer after HSCT for thalassemia was uncommon, affecting fewer than 1%.52,53 The administration of intensive immunosuppression in one patient with chronic GVHD after HSCT for sickle cell anemia was associated with the development of myelodysplastic syndrome and myeloid leukemia.43
Non-Myeloablative Allografts for Sickle Cell Anemia?
The topic of nonmyeloablative preparation for HSCT is also applicable to sickle cell anemia, where a significant ameliorative effect of partial donor chimerism was observed after HSCT just as it was for β-thalassemia major. Ongoing clinical studies have demonstrated that it is possible to establish engraftment after nonmyeloablative preparation in the majority of patients with hematological malignancies or solid tumors who received peripheral blood stem cell (PBSC) grafts from HLA-identical siblings.34,35 ,54,55,56,57 The challenge of overcoming sensitization caused by frequent transfusions in sickle cell anemia might be addressed by increasing the intensity of immunosuppression before HSCT or the total stem cell dose. This notion is supported by an observation of successful grafting for β-thalassemia major when an intensive but non-myeloablative regimen of BU, fludarabine, and ATG was employed.54 As in thalassemia major, it is perhaps most appealing to consider this approach in older, heavily transfused patients who currently are not suitable candidates for conventional allografting procedures. However, the development of graft rejection after HSCT may present a difficult problem in older patients. In a large animal preclinical model, Storb and colleagues demonstrated that engraftment could be established in transfusion-naïve animals following non-myeloablative preparation that caused minimal myelosuppression.35,58 This experience supports the view that optimal candidates might be young patients who have experienced significant complications but not yet exposed to frequent RBC transfusions that increase the risk of graft rejection. A national collaborative trial has been initiated to test this hypothesis.
Use of Alternative Donors
While the curative potential of matched sibling transplants for clinically significant hemoglobinopathies has been established, only 30 to 35% of patients will have such a donor, with even lower rates among sickle cell disease patients. Thus, the development of alternative stem cell donations offers an obvious, albeit unrealized, method for expanding the availability of HSCT. The outcome after HSCT from alternative donors for 29 patients with β-thalassemia major was recently reported.59 These patients received phenotypically matched allografts (N = 6) or haploidentical grafts mismatched for one (N = 15), two (N = 5), or three (N = 3) HLA antigens. Unfortunately, histoincompatibility between donor-host pairs was associated with decreased engraftment of donor cells, and the probability of graft failure or rejection was 55%. In addition, patients had a higher risk of developing chronic GVHD (37.5%), which contributed to an overall event-free survival of 21% for these patients. There was no apparent impact of the degree of HLA disparity on survival in this small group of patients. There have been no controlled trials of transplantation from volunteer unrelated donors (URD) for sickle cell anemia or β-thalassemia major. However, sporadic case reports that account for as many as 10 thalassemic patients suggest an event-free survival of approximately 50% after URD HSCT.60,61,62 This limited experience must be expanded in well-controlled clinical trials that tackle the difficulties of GVHD and graft rejection before any clear recommendation can be made about the suitability of mismatched related or URD transplants. These should be restricted to patients without suitable sibling donors, who have experienced a failure or significant complication of supportive medical therapy.
Several large patient series have demonstrated that umbilical cord blood (UCB) stem cells are a suitable source of hematopoietic cells for transplantation.63,64,65 Reports suggesting a lower rate of acute and chronic GVHD after HLA-matched and mismatched transplants raise the possibility of considering mismatched sibling donor or URD donors for sickle cell disease and β-thalassemia major. However, the current difficulties with UCB transplantation for these disorders are highlighted by the European experience.42 ,66,67,68 Eleven patients with β-thalassemia major and three with sickle cell disease received HLA-identical sibling UCB transplants (one donor for a thalassemia patient was mismatched for a single HLA locus). Among thalassemic patients who were 1 to 8 years of age, eight had Lucarelli class I-II characteristics and four had class II. Patients were prepared for transplantation with a combination of BU and CY in six patients, BU, CY and ATG in four, and in five patients, thiotepa as a third drug. Patients received cyclosporine alone or in combination with methotrexate for GVHD prophylaxis, and nine of 12 thalassemic patients received granulocyte-colony stimulating factor after transplantation. While all patients are surviving, five patients with β-thalassemia major and one with sickle cell disease experienced recurrence of the underlying hemoglobinopathy after transplantation. Of interest, among five patients who received thiotepa as part of cytoreductive preparation for HSCT, only one had recurrent thalassemia. In this small group, the event-free survival after UCB transplantation was 49% with a follow-up of 3 to 38 months. Thus, all patients survived after UCB transplantation, and the incidence of GVHD was low. Recurrent disease was the primary reason for failure, suggesting that the best strategy for improving outcome is to modify the immunosuppression given before and after HSCT to overcome immunologic rejection by host cells. Well-designed, controlled clinical trials will be necessary to develop this alternate stem cell source, especially for adult patients.
Summary
Currently, HSCT is the only therapy for sickle cell anemia and β-thalassemia major that has curative potential. For those who have an HLA-identical sibling donor, the overall probability of event-free survival is approximately 75% to 85%. The results of HSCT are optimal when performed in young patients; adults with these disorders have an increased risk of significant transplant-related complications. If, by reducing the intensity of conditioning it is still possible to ensure engraftment of donor cells, it is likely that HSCT would become more widely available. The obvious first candidates for a modified transplant would be those who have high-risk features such as older patients, but the reduction of transplant-related toxicity might also facilitate treatment of younger patients before they develop disease-related complications. Preliminary results of HSCT from alternate sources of stem cells suggest that selected individuals might benefit from these transplants; however, additional studies must be performed to more completely define the risks and benefits. If methods for the prevention and treatment of GVHD were improved, for example by utilizing high resolution HLA-typing methodology to select suitable URD, it is possible that HSCT from unrelated stem cell donations could significantly broaden the role of transplantation for these genetic disorders.
III. Hematopoietic Stem Cell Transplantation for Autoimmune Disease
Keith M. Sullivan, M.D.*
Medical Oncology and Transplantation, Duke University Medical Center, Box 3476 DUMC, Durham NC 27710
Autoimmune diseases afflict numerous patients causing considerable morbidity, cost of care and mortality from the disease or its treatment with immunosuppressive agents. The diagnostic criteria for rheumatologic and non-rheumatologic autoimmune diseases have been well defined,1 and these disorders have long held interest for the hematologist. This interest has recently intensified with evidence that some autoimmune diseases may originate as stem cell disorders and that HSCT may offer effective treatment.2,3,4,5,6
Basis for Transplantation
Animal models of autoimmune disease
Preclinical models of autoimmunity can be either systemic or organ specific and may arise either spontaneously (genetic model) or from antigenic induction (acquired model).7 In genetic models, allogeneic HSCT can both prevent the development of disease and reverse associated organ damage.8,9 In antigen-induced models, myeloablative conditioning and autologous or syngeneic HSCT can correct the disorder.10,11,12 Examples of animal models of autoimmune disease demonstrating prevention or correction after HSCT include adjuvant arthritis, antiphospholipid syndrome, autoimmune arthritis, autoimmune nephritis, diabetes mellitus, myasthenia gravis, relapsing experimental autoimmune encephalomyelitis, and systemic lupus erythematosus (SLE).
Coincident human autoimmune disease and HSCT
The potential role of stem cell transplantation for human autoimmune disease has been illuminated by a few individuals with autoimmune disease who developed a life-threatening hematologic disorder (aplastic anemia, leukemia, or lymphoma) and were treated with myeloablative conditioning and allogeneic HSCT. With rare exception,13 allografts have led to long-term remission of autoimmunity.14,15,16,17 Autologous transplants have also induced remissions, but relapses of autoimmune disease have been reported, especially in individuals receiving reinfusion of unmanipulated stem cells.18 Examples of control of human autoimmune disease by HSCT in these coincident disease settings include Crohn's disease, dermatitis herpetiformis, insulin-dependent diabetes mellitus, lupus erythematosus, vasculitis, psoriasis, rheumatoid arthritis (RA) and multiple sclerosis (MS).
Autoimmune Diseases
Pathogenesis of autoimmunity
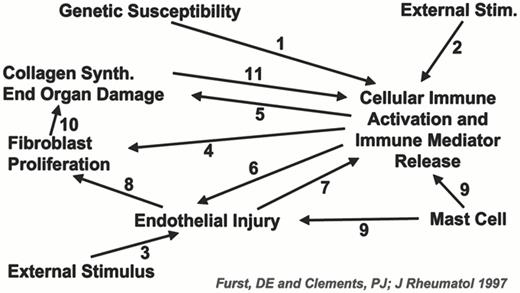
Loss of tolerance to self-antigens is likely due to both genetic and environmental factors.19 Genetic predisposition is inferred on the basis of HLA and familial associations. In this context, environmental factors may activate cellular immune responses. In the case of systemic sclerosis (SSc), this may lead to epithelial damage, fibroblast activation and collagen production.20 Figure 4 presents a hypothetical depiction of this cascade. More recent research postulates that non-host cells (i.e. donor/host microchimerism) may lead to fetal-antimaternal graft-versus-host responses.21 Alternative mechanisms for microchimerism include blood product transfusions or engraftment of maternal origin cells in the child.
Proposed pathogenesis of systemic sclerosis involving 11 potential pathways.20
Proposed pathogenesis of systemic sclerosis involving 11 potential pathways.20
Autoantibody production and immune complex deposition are characteristics of SLE. Immunopathogenesis of this heterogeneous disease is likely governed by multiple genetic loci triggered by environmental events. Cellular breakdown exposes nuclear and cryptic self antigens, resulting in autoantibody formation, expansion of autoreactive T cells, release of cytokines and inflammatory responses.22
Prevalence and outcome of autoimmune disease
The prevalence of autoimmune disease in Western countries has been reported to range from 3% to 7% of the population.23 Many patients respond to immunosuppressive therapy, but a significant subset fail conventional treatment and suffer considerable morbidity.
RA is the most common rheumatologic autoimmune disease. In patients with widespread joint involvement, RA can be both maiming and mortal.24 Juvenile rheumatoid arthritis (JRA) is the most frequent autoimmune disease of childhood. In more than one-third of patients, the disease may remain active for decades and extend well into the adult years.25 Methotrexate and anti-tumor necrosis factor (TNF) therapy are often efficacious, but resistant or recurrent disease is not uncommon.26,27
SLE has an annual incidence of 50-70 cases/million population and a prevalence of 500/million.28 Mortality at 10 years after diagnosis is approximately 30%.29 Although clinical manifestations are protean, certain features in children and adults identify individuals with increased risk of morbidity and mortality.30,31,32,33
MS is an immune-mediated demyelinating disease of Northern Europeans and their descendants. Approximately two-thirds of patients with MS have a relapsing, remitting course. Life expectancy is normal, and patients appear to benefit from treatment with interferon β and Copaxone, a basic synthetic copolymer.34,35 With treatment the number of attacks of MS can be reduced by 30-50%. However, individuals with progressive MS suffer from loss of functional activity and early mortality.
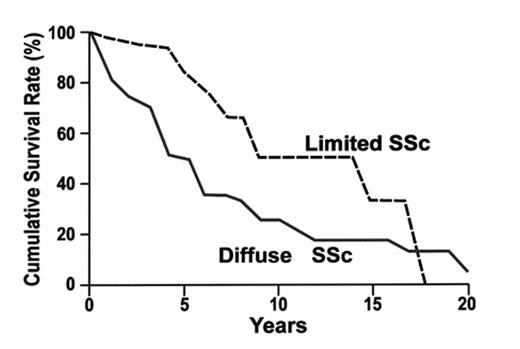
Ssc (scleroderma) is an uncommon autoimmune disease with an annual incidence of 1 to 20/million population.36 Females are more commonly afflicted, especially in early adult life with female:male ratios varying between 3:1 and 8:1. Cutaneous disease is commonly classified as limited or diffuse based on whether there is dermal involvement proximal (diffuse) or distal (limited) to the elbows and knees. Individuals with diffuse disease have increased mortality with 5-year survival of approximately 50%.37 Figure 5 depicts this life expectancy. The Rodnan skin score is used to assess dermal thickening and several studies have reported increased mortality in patients with increased skin scores.38
Overall survival in systemic sclerosis (Giordano M, et al. J Rheumatol. 1986;13: 911.)
Overall survival in systemic sclerosis (Giordano M, et al. J Rheumatol. 1986;13: 911.)
Protocol Development
Consensus conferences held in Seattle in 19953 and Basel in 199639 brought together experts in rheumatology and bone marrow transplantation to explore issues of protocol development for applying HSCT in systemic autoimmune diseases.39,40,41 With safety as the first goal, pilot studies of myeloablative conditioning regimens concentrated on autologous rather than allogeneic transplantation.
If autoimmune diseases are genetic disorder of HSC, allografting of stem cells from a normal sibling donor may have theoretic appeal. However, if environmental triggers are no longer operative or resetting immunoregulatory control after lymphoablative conditioning is successful, then safety concerns favor initial trials of autologous transplantation.
Transplant regimen
The choice of mobilization and pretransplant preparative regimen remains an arbitrary decision; however, the goal is to avoid a proliferation of different approaches. Regimens for stem cell mobilization follow historical preference using G-CSF alone or combined with CY at 4 gm/m2.42 In general, groups in Europe have mobilized with CY and growth factors while those in the United States have used growth factor alone. Recommendations for pretransplant preparative conditioning are based on historical regimens (e.g. CY 200 mg/kg ± ATG) as used in aplastic anemia and BEAM (carmustine, etoposide, cytosine arabinoside, melphalan) as used in the first trial in MS and widely used in lymphoma treatment or on animal models of autoimmune disease (e.g, CY and TBI for SSc and JRA).12,43,44,45
For autologous transplantation, US investigators have chosen conditioning with CY, TBI, and ATG to insure complete ablation of host lymphohemopoiesis. Recovery is afforded by reinfusion by peripheral blood stem cell (PBSC) selected by CD34+-separation technology. In contrast, some researchers have employed an intensive but not myeloablative regimen of CY 200 mg/kg without stem cell rescue.46 Apparent complete remissions have been obtained in half the patients but relapses of autoimmune diseases have been observed.
Animal data underscore the importance of removing mature T cells from the graft before myeloablative autologous transplant for autoimmune disorders. T cell depletion has been performed in many of the clinical reports to date, and the absence of purging may be responsible for early recurrence in some patients.18
Non-myeloablative allogeneic transplants (so-called mini-allografts) that promote stable donor/host mixed chimerism are being developed for patients with hematologic malignancies.47,48 If donor chimerism is successful and cells are durably engrafted without excess toxicity, application to autoimmune diseases can proceed in clinical trials.
Hematopoietic cell transplantation cannot be considered standard therapy for autoimmune diseases. Accordingly, protocols should follow the principles of involving new therapy, including formal written informed consent approved by ethics or review boards. Safety is a paramount concern and information should be promptly and fully available to the scientific community.
Patient selection
Patients selected for HSCT should be those experiencing considerable morbidity from autoimmune disease who, despite a high likelihood of progressive disease, do not yet have irreversible organ impairment that would preclude safe transplantation. Examples include patients with SSc, severe SLE, immune thrombocytopenic purpura (ITP), and JRA who require sustained high doses of immunosuppressive therapy to control disease manifestations. In some circumstances the prognosis of the disease is more clearly defined by specific laboratory or clinical criteria. In SSc, early involvement of proximal skin and internal organs predicts for significantly shortened survival without convincing evidence that conventional therapy is effective.
Autologous peripheral blood stem cells are recommended for the initial trials on the basis of their safety profile. If available, a monozygotic twin is ideal. Current protocol selection criteria include 1) patients with early SSc and significant dermal and internal organ involvement; 2) individuals with life-threatening SLE and inadequate response to 3 or more months of cytotoxic and high dose corticosteroid therapy; 3) patients with JRA with active disease despite aggressive therapy and evidence of unresponsiveness or unacceptable toxicity; 4) individuals with MS (primary progressive, relapsing remitting with multiple attacks in the prior two years or secondary progressive) with extended disability status scores (EDSS) of 5.5-8.0; and 5) patients with severe life-threatening ITP unresponsive to splenectomy and IVIGG.30,38,49,50,51
Toxicities of HSCT
Procedure-related mortality may be higher in patients with autoimmunity compared to that reported for patients undergoing transplantation for adjuvant treatment of breast carcinoma. Initial experience appears similar to the mortality rates observed after autologous transplantation for lymphoma beyond first remission. However, patients with disabling chronic autoimmune diseases may be willing to accept a rather substantial risk of mortality in exchange for the chance for cure.52
Preliminary data indicate that myeloablative regimens can be safely given before autologous PBSCT for autoimmune disease.53 One-year transplant-related mortality is approximately 9%.54 These risks need to be balanced against the morbidity and mortality associated with prolonged administration of conventional immunosuppressive therapy for the autoimmune disease.55,56
Disease response
Follow-up of autologous HSCT for autoimmune disease is shorter than the follow-up for allogeneic transplants in patients with autoimmune disease. Reappearance of autoimmune disease after autologous transplantation could be related to 1) reinfusion of autoreactive cells in the stem cell product; 2) failure to eliminate autoreactive cells in the host after high-dose immunosuppressive therapy; or 3) rechallenge from the autoantigen(s). Gene-marking studies of the stem cell innoculum are being planned to more fully detail the nature of recurrences.57
It is important to consider the heterogeneity of disease activity patterns for various autoimmune disorders.15 As shown in Figure 6, there may be considerable differences across the diseases. In addition, sufficient follow-up should be allowed to observe patients off all immuno-suppressive agents.58 Criteria for response and recurrence have not been uniformly determined, especially for patients treated in Europe, where a common transplant protocol and response criteria have not been applied.
Activity patterns of autoimmune diseases.15
Abbreviations for Figure 6 and Table 2. EBMT, European Blood and Marrow Transplant Group; JCA, juvenile chronic arthritis (JRA; juvenile rheumatoid arthritis); MS, multiple sclerosis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; TRM, transplant-related mortality; IDDM, insulin-dependent diabetes mellitus
Activity patterns of autoimmune diseases.15
Abbreviations for Figure 6 and Table 2. EBMT, European Blood and Marrow Transplant Group; JCA, juvenile chronic arthritis (JRA; juvenile rheumatoid arthritis); MS, multiple sclerosis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; TRM, transplant-related mortality; IDDM, insulin-dependent diabetes mellitus
Transplant Results
European Blood and Marrow Transplant Group
Table 2 presents a recent summary of transplant registry results from 60 teams in 20 countries reporting to the European Blood and Marrow Transplant Group (EBMT) (A. Tyndall and A. Gratwohl, personal communication). To date, MS and SSc are the most commonly transplanted autoimmune diseases. As previously noted, there are no uniform criteria in EBMT for patient selection, mobilization, purging or preparative regimens. Accordingly, assessment of response and recurrence rates are complicated.
Autologous stem cell transplantation for autoimmune disease (EBMT 3/31/00).
. | MS . | SSc . | RA . | JCA . | SLE . |
|---|---|---|---|---|---|
| Missing follow-up data | 7 | 4 | - | - | 1 |
| No transplant | 1 | 5 | 1 | - | - |
| TRM | 7 | 5 | 1 | 5 | 2 |
| Too early | 3 | 1 | 1 | - | 2 |
| Worse | 12 | 2 | 6 | 1 | 1 |
| Better then worse | 27 | 7 | 13 | 6 | 3 |
| Stable disease | 4 | 3 | 2 | 1 | - |
| Better | 24 | 20 | 14 | 15 | 10 |
| Evaluable transplants | 77 | 38 | 37 | 28 | 18 |
. | MS . | SSc . | RA . | JCA . | SLE . |
|---|---|---|---|---|---|
| Missing follow-up data | 7 | 4 | - | - | 1 |
| No transplant | 1 | 5 | 1 | - | - |
| TRM | 7 | 5 | 1 | 5 | 2 |
| Too early | 3 | 1 | 1 | - | 2 |
| Worse | 12 | 2 | 6 | 1 | 1 |
| Better then worse | 27 | 7 | 13 | 6 | 3 |
| Stable disease | 4 | 3 | 2 | 1 | - |
| Better | 24 | 20 | 14 | 15 | 10 |
| Evaluable transplants | 77 | 38 | 37 | 28 | 18 |
Multiple sclerosis
In a recent series of patients treated in Greece, 24 individuals with MS were conditioned with BEAM chemotherapy and ATG. Fifteen received unmanipulated autologous PBSC while nine received CD 34+-selected cells. The probability of progression-free survival at 3 years was 92% for patients with secondary progressive disease and 39% for patients with primary progressive MS.49
Twenty patients with MS have been treated on the US National Collaborative Study (15 with secondary progressive, four with primary progressive and one with relapsing remitting MS). All received preparative conditioning with TBI (800 cGy), CY (120 mg/kg) and ATG (90 mg/kg) and CD 34+-selected autologous peripheral blood stem cells. One patient had a flare of MS during G-CSF (16 μg/kg/day) mobilization. Importantly, no new or enhancing cerebral MRI lesions have been observed in the others.59 Similar apparent stabilization of disease has been reported in four patients with secondary progressive and two patients with primary progressive MS prepared with CY (120 mg/kg), TBI (1200 cGy), and methylprednisolone.53
Systemic sclerosis
Eighteen patients with diffuse SSc were treated with TBI (800 cGy), CY (120 mg/kg) and ATG (90 mg/kg) on the US National Collaborative Study. Significant improvements in the Rodnan skin scores and health assessment HAQ scores were observed. Fatal pulmonary insufficiency developed in two of the initial eight patients. With institution of lung shielding to 200 cGy transmission, none of the next 10 patients developed respiratory compromise.60 Significant improvement was noted in skin scores at 1 and 2 years post-transplant. Similar sustained improvement beyond 2 years has been reported in a child with SSc.61
Recently the European and American results of autologous HCST for SSc have been analyzed.62 Forty-one patients were identified (37 with diffuse and four with limited disease) and 37 were transplanted. With median follow-up of 12 (range 3-55) months, an improvement in skin scores of greater than 25% following transplantation occurred in 69% of evaluable patients. Although follow-up is still limited, this dramatic initial improvement is of considerable interest.
Systemic lupus erythematosis
Of all the autoimmune disorders, SLE is perhaps the most responsive to immunosuppressive treatment. Accordingly, survival has improved over the last three decades and thus it is not surprising that the patients referred for an autologous transplantation have been heavily treated with prolonged courses of immunosuppressive agents. In a recently published series from Northwestern University, seven patients with advanced SLE uncontrolled with conventional doses of CY were prepared for autologous transplant with high dose CY (200 mg/kg), methylprednisolone (1 gm), and ATG (90 mg/kg). Pulmonary edema and fluid retention were frequent peritransplant complications. Improvement was observed in laboratory abnormalities, clinical symptoms and SLEDAI scores.63
Rheumatoid arthritis
A number of case reports have appeared in the last two years describing transplant results in RA patients. Complete remission of joint and systemic symptoms in a patient with treatment refractory disease occurred after high-dose CY, ATG and syngeneic PBSCT.64 A dose escalation study compared four patients with RA prepared for transplant with CY (100 mg/kg) with four patients given CY (200mg/kg) before unmodified autologous PBSCT. Responses were transient (2-3 months) with the lower dose, but substantial improvement was sustained beyond 17 months in patients receiving the higher dose regimen.65 When these eight patients were combined with an additional eight individuals described in two other studies,66,67 it was noted that a substantial fraction of the patients did not sustain the improvement in symptoms.68 This trend is confirmed from the registry report (Table 2).
Juvenile chronic arthritis
The largest series of autologous HSCT for refractory JRA has been compiled by Dutch investigators. Reported in 1999, four children were shown to have a marked decrease in joint swelling, pain, and stiffness.51 Of 13 patients now followed, there were three deaths associated with macrophage activation syndrome (a life-threatening condition characterized by fever, hepatosplenomegaly, hypofibrinoginemia, cytopenia, and the presence in the bone marrow of hemophagocytosis by activated macrophages. This syndrome occurs predominantly in children with JRA and is thought to be triggered by infections or drugs). Eight patients remain in complete remission and are no longer on drug therapy (N. Wulffraat, personal communication).
Future Directions
To date nearly 400 autologous and standard allogeneic transplants for autoimmune disease have been performed worldwide. Initial questions of safety, feasibility and early efficacy have been partially answered. However, given the considerable diversity in diseases, selection criteria, transplant regimens and response criteria, more questions and challenges have arisen. Systematic long-term follow-up of current patients is essential to track the durability of remission and any associated late complications of the transplant procedure.69 Importantly, data are now maturing from the US National Collaborative Study of pilot protocols with uniform transplant regimens, which will allow design of the next generation of clinical trials.59,60 Such a standardized collaborative approach has been of value in defining the toxicities, efficacy and late effects of HSCT for sickle cell disease and has allowed rapid dissemination of results of all treated patients.70 The design of future randomized clinical trials in autoimmune disease will offer challenges in patient accrual, funding, and definition of laboratory and clinical outcomes. It will also offer a unique opportunity to explore the immunobiology of autoimmune disorders before and after lymphohematopoietic reconstitution. In this manner, hematologists, rheumatologists and neurologists will contribute across disciplines to further basic and applied research in these fascinating but devastating diseases.