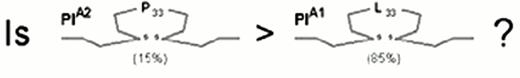Abstract
This review covers new developments and their clinical implications in three areas: platelet antigen polymorphisms, inhibition of platelet glycoprotein IIb-IIIa, and autoimmune thrombocytopenia (ITP).
In Section I, Dr. Kunicki reviews platelet polymorphisms and their clinical implications. A current tabulation of the numerous platelet antigens, both those that are platelet specific and not platelet specific, are summarized. The immunogenic clinical implications of these polymorphisms are considered, including fetal and neonatal alloimmune thrombocytopenia, post transfusion purpura, and refractoriness to platelet transfusion. The functional relationship to hemostasis and thrombosis is also discussed, in particular whether one haplotype of the PIA1/PIA2 (HPA-1a/1b) polymorphism predisposes to myocardial infarction. Finally, novel investigations of polymorphisms will be considered, including hormonal induction of certain polymorphisms.
In Section II, Dr. Michelson reviews the newest generation of platelet inhibitors, those blocking glycoprotein IIB/IIIA, from the point of view of the hematologist who might be consulted about a patient receiving this form of treatment. The current use of available IIb-IIIa inhibitors and those in trial and the accepted and possible future indications for their use are addressed. The mechanism of action and actual and theoretical advantages and disadvantages of each inhibitor are explored. Scenarios that prompt consultation with a hematologist are presented, including management of bleeding, thrombocytopenia, and management of the patient requiring emergency surgery.
In Section III, Dr. Bussel reviews controversies in ITP, looking at both the current state of the art and the potential for the future. Case presentations are used to illustrate the issues in both children and adults. Three primary areas are addressed: 1) the diagnosis of ITP, 2) when and for which patient to recommend splenectomy, and 3) the management of the refractory splenectomized patient who still has a low platelet count and bleeding symptoms.
I. Platelet Antigen Polymorphisms
Thomas J. Kunicki, Ph.D.*
The Scripps Research Institute, 10550 North Torrey Pines Road, Maildrop MEM-150, La Jolla CA 92037
Inherited polymorphisms within platelet membrane glycoprotein genes can alter their antigenicity, regulate their expression levels, and modulate their functional properties. This section will focus on the impact of these polymorphisms on the alloantigenic makeup and the hemostatic and thrombotic function of the platelet.
“Platelet-Nonspecific” Alloantigens: Platelets express a number of alloantigens that are not restricted to the megakaryocyte lineage, including major histocompatibility (MHC) molecules and ABH blood group determinants.
HLA Antigens: HLA antigens are cell-surface glyco-proteins encoded by a series of closely linked genes on chromosome 6.1 Platelets express MHC Class I but not Class II antigens, and anti-HLA Class I antibodies are the major cause of refractoriness to platelet transfusion. Class I antigens are highly polymorphic and further categorized as A, B or C antigens. On platelets, the amount of HLA-A or HLA-B antigen is at least tenfold higher than that of HLA-C antigen,2 making it less necessary to match HLA-C locus antigens for donor-recipient compatibility in platelet transfusions.3 Certain HLA-A or -B antigens also appear to be weakly expressed on platelets of some donors and may not elicit antibodies when mismatched.4
ABH Blood Group Antigens: Platelet membrane glycoproteins that carry ABH blood group antigens include the glycoprotein (GP) Ib complex and the integrins αIIbβ3 (platelet cohesion receptor) and α2β1 (platelet collagen receptor).5 ABH antigens are also expressed by platelet glycolipids.6 If platelets are mismatched for blood group A or B, one will observe a roughly 25% decrease in recovery immediately after transfusion. However, the mismatched platelets that remain in circulation will exhibit a normal survival.7,8 A decreased recovery is most striking when platelets from A1 donors are transfused into incompatible recipients,8 and recoveries are inversely proportional to the isoagglutinin titer of the recipient.8 The otherwise normal survival of platelets that escape initial clearance can best be explained by the high degree of variation in the number of A, B, and H antigens per platelet, as compared to the more homogeneous distribution of HLA antigens.9
Polymorphic Glycoprotein Receptors
The GPIb complex
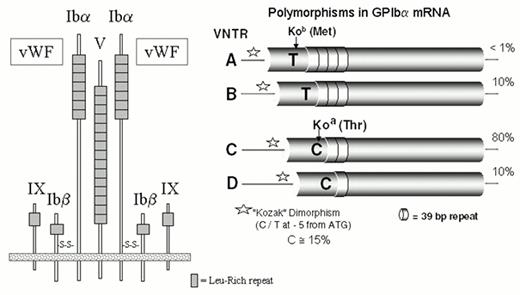
This receptor is composed of four proteins that are the products of distinct genes: GPIbα (143 kDa), GPIbβ (22 kDa), GPIX (20 kDa) and GPV (83 kDa) (Figure 1). The most notable structural similarity between these subunits is the common presence of leucine-rich glycoprotein (LRG) repeats. The GPIb complex is a heptamer composed of one molecule of GPV noncovalently associated with two molecules each of GPIb and GPIX (Figure 1).10 The GPIb complex mediates the initial and transient tethering of platelets to the extracellular matrix under a wide range of flow conditions via the binding of von Willebrand factor (vWF) to the amino-terminal domain of glycoprotein Ibα.11
Five alleles of GPIbα (Table 1) differ by both the single amino acid replacement, Thr/Met, at residue 14512 and four polymorphic size variants (Figure 1). The latter variants, termed A, B, C, and D (ranging from largest to smallest), are generated by differences in the number of tandem 13-amino acid repeats present in the macroglycopeptide portion of the molecule.13 The D isoform of glycoprotein Ibα contains one repeat; the C isoform, two repeats, and so on. The alloantigen system HPA-2 is defined by the Thr/Met dimorphism, and the number of 13-amino acid repeats markedly influences antigenicity. Another unlinked polymorphism, a single nucleotide substitution (T/C) in the immediate 5′ untranslated sequence of GPIbα mRNA (position -5), lies within a typical Kozak sequence and influences the efficiency of mRNA translation14 (Figure 1). The C allele has a gene frequency of about 0.15 in Caucasian populations. In both transfected cells and platelets, the level of the GPIb complex increases directly with the dosage of the C allele (relative ratios: TT 1.0, T/C 1.3, and C/C 1.5).
Platelet membrane glycoprotein alleles.*
| Glycoprotein . | Alleles . | Gene Frequency . | HPA-Determinants . |
|---|---|---|---|
| Integrin Subunit α2 | Allele 2: Glu505, Thr799** | 0.53 | 5a, 12aw |
| Allele 1: Glu505, Thr799 | 0.39 | 5a, 12aw | |
| Allele 3: Lys505, Thr799 | 0.076 | 5b, 12aw | |
| Allele 4: Glu505, Met799 | <0.01 | 5a, 12bw | |
| GPIb α | Thr145, VNTR C isoform | 0.82 (0.539) | 2a |
| Thr145, VNTR D isoform | 0.11 (0.296) | 2a | |
| Met145, VNTR B isoform | 0.07 (0.01) | 2b | |
| Met145, VNTR C isoform | <0.01 (<0.01) | 2b | |
| Met145, VNTR A isoform | <0.01 (0.155) | 2b | |
| GPIb β | Gly15 | 0.99 | 11aw |
| Glu15 | <0.01 | 11bw | |
| Integrin Subunit αIIb | Val837, Ile843 | 0.61 | 3a, 9a |
| Val837, Ser843 | 0.36 | 3b, 9a | |
| Met837, Ser843 | 0.03 | 3b, 9b | |
| Integrin Subunit b3 | Leu33, Leu40, Arg62, Arg143, Pro407, Arg489, Arg636 | 0.85 | 1a, 10a, 4a, 7a, 6a, 8a |
| Pro33, Leu40, Arg62, Arg143, Pro407, Arg489, Arg636 | 0.15 | 1b, 10a, 4a, 7a, 6a, 8a | |
| Pro33, Arg40, Arg62, Arg143, Pro407, Arg489, Arg636 | 0.005 | 1b, 10a, 4a, 7a, 6a, 8a | |
| Leu33, Leu40, Gln62, Arg143, Pro407, Arg489, Arg636 | <0.001 | 1a, 10b, 4a, 7a, 6a, 8a | |
| Leu33, Leu40, Arg62, Gln143, Pro407, Arg489, Arg636 | <0.01 | 1a, 10a, 4b, 7a, 6a, 8a | |
| Leu33, Leu40, Arg62, Arg143, Ala407, Arg489, Arg636 | <0.001 | 1a, 10a, 4a, 7b, 6a, 8a | |
| Leu33, Leu40, Arg62, Arg143, Pro407, Gln489, Arg636 | <0.001 | 1a, 10a, 4a, 7a, 6b, 8a | |
| Leu33, Leu40, Arg62, Arg143, Pro407, Arg489, Cys636 | <0.001 | 1a, 10a, 4a, 7a, 6a, 8b | |
| Gene frequencies are for Caucasian populations or Japanese populations (in parentheses). | |||
| Glycoprotein . | Alleles . | Gene Frequency . | HPA-Determinants . |
|---|---|---|---|
| Integrin Subunit α2 | Allele 2: Glu505, Thr799** | 0.53 | 5a, 12aw |
| Allele 1: Glu505, Thr799 | 0.39 | 5a, 12aw | |
| Allele 3: Lys505, Thr799 | 0.076 | 5b, 12aw | |
| Allele 4: Glu505, Met799 | <0.01 | 5a, 12bw | |
| GPIb α | Thr145, VNTR C isoform | 0.82 (0.539) | 2a |
| Thr145, VNTR D isoform | 0.11 (0.296) | 2a | |
| Met145, VNTR B isoform | 0.07 (0.01) | 2b | |
| Met145, VNTR C isoform | <0.01 (<0.01) | 2b | |
| Met145, VNTR A isoform | <0.01 (0.155) | 2b | |
| GPIb β | Gly15 | 0.99 | 11aw |
| Glu15 | <0.01 | 11bw | |
| Integrin Subunit αIIb | Val837, Ile843 | 0.61 | 3a, 9a |
| Val837, Ser843 | 0.36 | 3b, 9a | |
| Met837, Ser843 | 0.03 | 3b, 9b | |
| Integrin Subunit b3 | Leu33, Leu40, Arg62, Arg143, Pro407, Arg489, Arg636 | 0.85 | 1a, 10a, 4a, 7a, 6a, 8a |
| Pro33, Leu40, Arg62, Arg143, Pro407, Arg489, Arg636 | 0.15 | 1b, 10a, 4a, 7a, 6a, 8a | |
| Pro33, Arg40, Arg62, Arg143, Pro407, Arg489, Arg636 | 0.005 | 1b, 10a, 4a, 7a, 6a, 8a | |
| Leu33, Leu40, Gln62, Arg143, Pro407, Arg489, Arg636 | <0.001 | 1a, 10b, 4a, 7a, 6a, 8a | |
| Leu33, Leu40, Arg62, Gln143, Pro407, Arg489, Arg636 | <0.01 | 1a, 10a, 4b, 7a, 6a, 8a | |
| Leu33, Leu40, Arg62, Arg143, Ala407, Arg489, Arg636 | <0.001 | 1a, 10a, 4a, 7b, 6a, 8a | |
| Leu33, Leu40, Arg62, Arg143, Pro407, Gln489, Arg636 | <0.001 | 1a, 10a, 4a, 7a, 6b, 8a | |
| Leu33, Leu40, Arg62, Arg143, Pro407, Arg489, Cys636 | <0.001 | 1a, 10a, 4a, 7a, 6a, 8b | |
| Gene frequencies are for Caucasian populations or Japanese populations (in parentheses). | |||
Alleles 1 and 2 of integrin α2 are defined by several linked dimorphisms that are silent (see 25 ).
The glycoprotein Ibβ subunit (the “light” chain of glycoprotein Ib) is represented by two allelic variants. A Gly15/Glu15 substitution gives rise to the antigens Iyb (HPA-11aw) and Iya (HPA-11bw), respectively.15
The integrin αIIbβ3
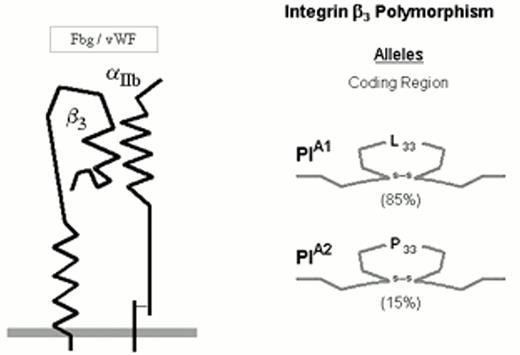
The numerically predominant platelet integrin αIIbβ3 (initially designated glycoprotein IIb-IIIa) mediates platelet aggregation via the binding of adhesive proteins, such as fibrinogen and von Willebrand factor (vWF). The αIIbβ3 heterodimer complex is depicted schematically in Figure 2.
Three allelic variants of the integrin subunit αIIb (Table 1) have been defined which differ at either residue 837 (Val or Met)16 or residue 843 (Ser or Ile).17 Eight different alleles of the β3 can be distinguished (Table 1), which differ at six positions within the coding sequence. These dimorphic residues and their serologic designations include Leu33/Pro33 (HPA-1a/HPA-1b).18 The eighth, rare HPA-1b allele characterized by the presence of Arg40 does not appear to be serologically distinguishable from the HPA-1b allele bearing the more common Leu40.24
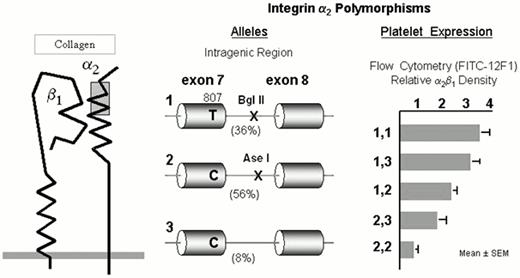
The integrin α2β1
The integrin collagen receptor α2β1 (GPIa-IIa) (Figure 3) plays a fundamental role in adhesion of blood platelets to both fibrillar (e.g., types I or III) or nonfibrillar (e.g., types IV or VI) collagens. The expression of α2β1 on platelets differs markedly among normal subjects and depends upon the inheritance of at least four alleles of the α2 gene.25 The number of α2β1 molecules per platelet varies by an order of magnitude and correlates precisely with quantitative estimates of platelet adhesion to type I or type III collagens.26 The high frequency alleles 1 (high receptor density) and 2 (low receptor density) of the α2 gene bear the residue Lys505 that defines the alloantigen HPA-5b25 , while the HPA-5a determinant Glu505 is restricted to the low frequency allele 3 (low receptor density).
Clinical relevance of alloimmunization to HPA antigens
There are two clinical syndromes in which sensitization to platelet glycoprotein polymorphisms plays a major role; post-transfusion purpura (PTP) and neonatal alloimmune thrombocytopenia (NAIT). In PTP, antigen-negative transfusion recipients are sensitized against antigen-positive donor platelets, and the ensuing immune response enigmatically causes the clearance of both donor and recipient platelets. The antigen most frequently implicated in PTP in Caucasians is HPA-1a.28 Reduction of leukocytes by filtration or ultraviolet B irradiation of platelet concentrates is effective in preventing alloimmunization resulting from platelet transfusion.29 In NAIT, maternal sensitization to paternal antigens carried by fetal platelets leads to thrombocytopenia of the newborn infant. The antigens most frequently implicated in NAIT are HPA-1a (78%) and HPA-5a (19%) in Caucasians30 and HPA-4a (80%) and HPA-3a in Asians (15%).31 Transfusion of washed or concentrated maternal or other antigen-negative platelets is an effective therapy in NAIT.
Platelet Glycoprotein Polymorphisms and Risk for Arterial Thrombosis
Since the platelet glycoprotein receptors are so important to platelet function at both the early adhesion phase (GPIb-IX-V, integrin α2β1 and GPVI) and the later stages of thrombus formation (integrin αIIbβ3), polymorphisms that regulate expression or activity could influence risk for adverse outcomes in any disease that compromises hemostasis.
The integrin αIIbβ3
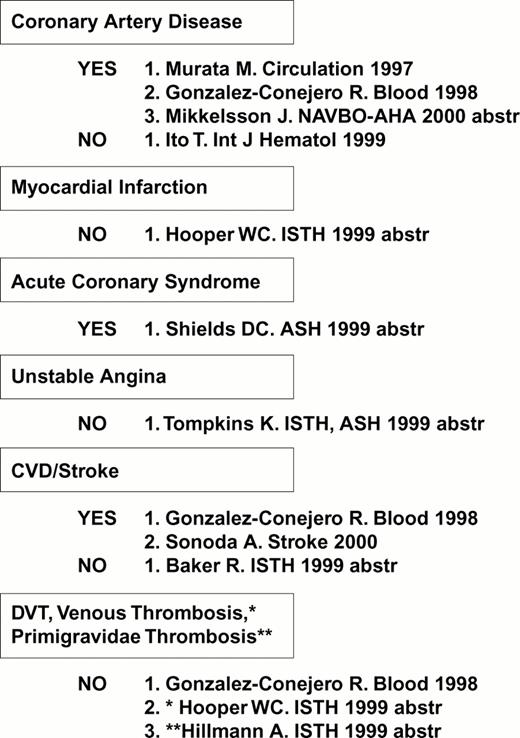
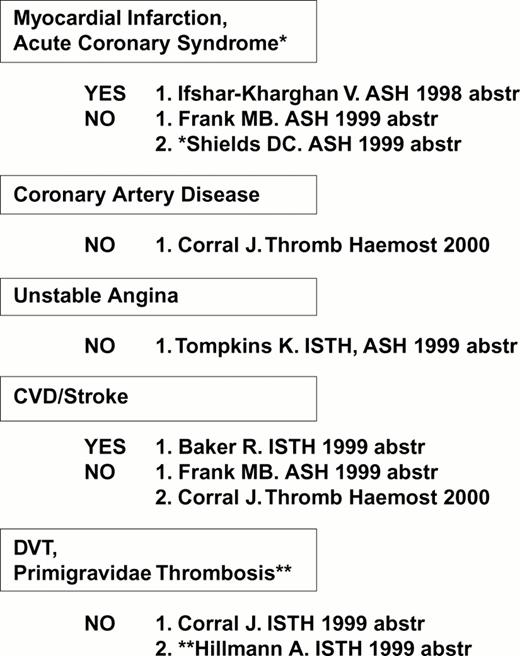
The first receptor to be scrutinized was αIIbβ3. Weiss et al32 reported that the gene frequency of the β3 PlA2 (Pro33) allele was 3.6 times higher among younger patients (< 60 years of age) with myocardial infarction or unstable angina as compared to age-matched controls (odds ratio = 6.2). This observation was rapidly confirmed or refuted by numerous studies, summarized in Figure 4. Other clinical settings where risk might be involved are restenosis after stent33,34 or acute renal allograft rejection.35 On the other hand, no correlation between this platelet-specific marker and risk for venous thrombosis has yet been observed, a finding that has been universal for the platelet glycoprotein dimorphisms that are currently under study.
Expression of the integrin β3 PlA2 allele: A risk factor? Compiled April 2000
After the β3 Pro33 allele had been implicated in risk for thrombosis, the search began for any indication that this allele might confer increased biologic activity upon the integrin αIIbβ3 (Figure 5). Although the differences identified to date have not been dramatic, they are statistically significant and suggest that this allele confers a lower threshold for agonist-induced platelet responses, particularly ADP-induced fibrinogen binding or activation.
The GPIb-IX-V complex
As summarized in Figure 6, limited studies have suggested that an association between the VNTR B allele and risk for arterial thrombosis may exist. The majority of studies fail to find a correlation between the inheritance of the Kozak C genotype (high receptor density) and coronary artery disease (Figure 7).
The integrin α2β1
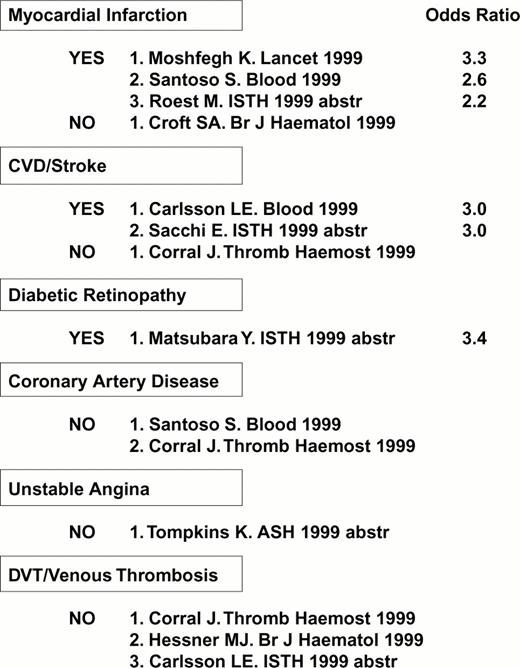
Several studies have uncovered a correlation between the high density α2 allele 1 and risk for myocardial infarction, cerebral vascular disease (stroke) or diabetic retinopathy (Figure 8), consistent with in vitro findings of an increased platelet adhesive function among individuals who express this allele.
Increased platelet α2β1 density (α2 allele 1): A risk factor? Compiled April 2000
Increased platelet α2β1 density (α2 allele 1): A risk factor? Compiled April 2000
Hormonal regulation of receptor expression
Gender differences figure prominently in risk for thromboembolic disease. It has been known for some time that hormone replacement therapy (HRT) in postmenopausal women can predispose to venous thrombosis, but only recently has a potential influence of HRT on platelets in the coronary circulation has been proposed.36,37 Tarantino et al36 observed that, in women, platelet adhesion to type I collagen shows a biphasic periodicity in synchrony with the menstrual cycle and that human megakaryocytes express the estrogen receptor (ER). Furthermore, megakaryocytes respond to testosterone in a regulated manner. This finding implies that platelet collagen receptor function may be modulated by sex hormone levels and would be consistent with the known correlation between ER and α2β1 expression in certain cell lineages. Khetawat et al37 observed that human megakaryocytes contain mRNA specific for the ER β and the androgen receptor (AR), and that the transcripts of both can be located in the platelet cytoplasm. These reports provide compelling evidence that sex hormones may mediate gender differences in platelet function and antigenicity. It remains to be determined to what extent the effects of these hormones occur through genomic or nongenomic pathways and their clinical relevance.
Summary
Platelet glycoprotein polymorphisms have a profound impact on the antigenic makeup of the platelet. Known clinical syndromes dependent on these polymorphisms include platelet transfusion refractoriness, alloimmune thrombocytopenia, and post-transfusion purpura. The importance of platelet glycoprotein polymorphisms in risk for arterial thrombosis is a new area of human genomics that must be carefully evaluated. Nonetheless, there is already substantial evidence that the integrin β3 P1A2 allele, the GPIb Met145 (VNTR A or B) alleles and especially the integrin α2 allele 1 (807T) contribute to the risk for acute myocardial infarction or stroke in younger individuals (≤ 60 years old) and diabetic retinopathy. Future clinical risk assessments need to evaluate the cumulative or synergistic effects of these platelet risk factors together with the defined coagulation protein risk factors.
II. GPIIb-IIIa Antagonists
Alan D. Michelson, M.D.*
Center for Platelet Function Studies, Room 55-846, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester MA 01655
In 1974, Nurden and Caen discovered that Glanzmann's thrombasthenia, an inherited platelet function defect characterized by lack of platelet aggregation to all physiological agonists, was the result of a lack of the platelet glycoprotein IIb-IIIa complex.1 In 1995, based on work of Coller and many others, the first GPIIb-IIIa antagonist (abciximab [ReoPro]), was FDA approved as an anti-platelet agent in patients.2 Eptifibatide (Integrilin) and tirofiban (Aggrastat) were FDA approved in 1998. GPIIb-IIIa antagonists are particularly effective because, irrespective of the platelet agonist(s), they inhibit the final common pathway of platelet aggregation: fibrinogen or von Willebrand factor binding to the GPIIb-IIIa complex.2 Therefore, GPIIb-IIIa antagonists more completely inhibit platelet aggregation than aspirin or clopidogrel, both of which inhibit only one initiating pathway of platelet aggregation (cyclooxygenase and ADP, respectively). In addition, GPIIb-IIIa antagonists have a specific anticoagulant action as evidenced by prolongation of the activated clotting time,3 inhibition of thrombin generation,4 and inhibition of platelet procoagulant activity.5 The mechanisms of this anticoagulant effect include inhibition of prothrombin binding to the GPIIb-IIIa complex with less resultant thrombin generation6 and inhibition of procoagulant platelet-derived microparticle formation.4 This presentation will summarize the available GPIIb-IIIa antagonists, including current indications, doses, side effects, and possible future indications.
Available Drugs
Abciximab, eptifibatide, and tirofiban, the FDA-approved GPIIb-IIIa antagonists (Table 2), are only available in intravenous form. There are significant differences between the drugs. Abciximab is a human-murine chimeric monoclonal antibody fragment. Eptifibatide is a cyclic heptapeptide based upon the Lys-Gly-Asp (KGD) amino acid sequence. Tirofiban is a tyrosine-derivative non-peptide mimetic. Lamifiban, another intravenous non-peptide mimetic, is undergoing phase III trials. Abciximab binds to GPIIb-IIIa with high affinity and a slow dissociation rate (Table 2).7 Although abciximab is rapidly cleared from the plasma (Table 2), it is detectable bound to circulating platelets for more than 2 weeks.8 Eptifibatide, tirofiban, and lamifiban are competitive inhibitors of the GPIIb-IIIa complex with lower affinities, higher dissociation rates, and short (2-2.5 hour) plasma half-lives (Table 2).9,10,11 Platelet aggregation is inhibited by abciximab, eptifibatide, and tirofiban in a dose-dependent manner, with > 80% GPIIb-IIIa receptor occupancy resulting in > 80% inhibition of platelet aggregation.7 After discontinuation of abciximab, platelet aggregation returns towards baseline over the subsequent 12 to 36 hours.7 Platelet aggregation returns to normal more rapidly (30 minutes to 4 hours) after discontinuation of eptifibatide, tirofiban, or lamifiban.9,10 In addition to GPIIb-IIIa (αIIbβ3), abciximab (but not eptifibatide or tirofiban) binds to αVβ3 (the vitronectin receptor) on endothelial and smooth muscle cells and activated αMβ2 (also known as Mac-1 or CD11b/CD18) on leukocytes.12 Tirofiban and eptifibatide induce leukocyte-platelet aggregation, but abciximab does not.13
Intravenous GPIIb-IIIa Antagonists
. | Abciximab . | Eptifibatide . | Tirofiban . | Lamifiban . |
|---|---|---|---|---|
| Brand name | ReoPro | Integrilin | Aggrastat | — |
| Structure | Chimeric antibody | Cyclic heptapeptide | Nonpeptide | Nonpeptide |
| Fab fragment | ||||
| Molecular weight (Daltons) | 47,600 | 832 | 495 | 468 |
| KD (nM) | 5 | 120 | 15 | 9 |
| Plasma half-life | 10-30 min | ∼2.5 h | ∼2 h | ∼2 h |
| Excretion | Unknown | ∼50% renal | 39-69% renal | 90% renal |
| Approved indications* | PCI. Refractory unstable angina when PCI is planned within 24 h. | ACS (unstable. angina and non-Q wave MI) PCI. | ACS (unstable angina and non-Q wave MI). | Not approved — undergoing trials. |
| Approved dose* | For PCI: 0.25 mg/kg bolus, 0.125 μg/kg/min (max 10 μg/min) infusion × 12 h. | For ACS: 180 μg/kg bolus, 2.0 μg/kg/min infusion × 72-96 h (PURSUIT dose). | For ACS: 0.4 μg/kg/min × 30 min, then 0.1 μg/kg/min × 48-108 h. | Not approved — undergoing trials. |
| †For unstable angina: 0.25 mg/kg bolus, 10 μg/min infusion × 18-24 h before PCI, continued 1 h after PCI. | †For PCI: 135 μg/kg bolus, 0.5 μg/kg/min infusion for 20-24 h (IMPACT II dose). | |||
| Abbreviations: ACS, acute coronary syndromes; IMPACT II, Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis-II; KD, dissociation constant; MI, myocardial infarction; PCI, percutaneous coronary intervention; PURSUIT, Platelet IIb/IIIa in Unstable angina: Receptor Suppression Using Integrilin Therapy. | ||||
. | Abciximab . | Eptifibatide . | Tirofiban . | Lamifiban . |
|---|---|---|---|---|
| Brand name | ReoPro | Integrilin | Aggrastat | — |
| Structure | Chimeric antibody | Cyclic heptapeptide | Nonpeptide | Nonpeptide |
| Fab fragment | ||||
| Molecular weight (Daltons) | 47,600 | 832 | 495 | 468 |
| KD (nM) | 5 | 120 | 15 | 9 |
| Plasma half-life | 10-30 min | ∼2.5 h | ∼2 h | ∼2 h |
| Excretion | Unknown | ∼50% renal | 39-69% renal | 90% renal |
| Approved indications* | PCI. Refractory unstable angina when PCI is planned within 24 h. | ACS (unstable. angina and non-Q wave MI) PCI. | ACS (unstable angina and non-Q wave MI). | Not approved — undergoing trials. |
| Approved dose* | For PCI: 0.25 mg/kg bolus, 0.125 μg/kg/min (max 10 μg/min) infusion × 12 h. | For ACS: 180 μg/kg bolus, 2.0 μg/kg/min infusion × 72-96 h (PURSUIT dose). | For ACS: 0.4 μg/kg/min × 30 min, then 0.1 μg/kg/min × 48-108 h. | Not approved — undergoing trials. |
| †For unstable angina: 0.25 mg/kg bolus, 10 μg/min infusion × 18-24 h before PCI, continued 1 h after PCI. | †For PCI: 135 μg/kg bolus, 0.5 μg/kg/min infusion for 20-24 h (IMPACT II dose). | |||
| Abbreviations: ACS, acute coronary syndromes; IMPACT II, Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis-II; KD, dissociation constant; MI, myocardial infarction; PCI, percutaneous coronary intervention; PURSUIT, Platelet IIb/IIIa in Unstable angina: Receptor Suppression Using Integrilin Therapy. | ||||
Approved indications and approved dose according to label of United States Food and Drug Administration (FDA); †FDA approved dose for indication, but may not be optimal (see text). For abciximab in unstable angina, an infusion of 12 h after PCI is recommended. For eptifibatide during PCI, the PURSUIT dose of 180 μg/kg bolus and 2.0 μg/kg/min infusion is recommended. Modified from Lincoff et al. JACC 35, 1103, 2000.
Clinical trials of patients undergoing percutaneous coronary intervention have been interpreted as suggesting a survival advantage in favor of abciximab.14 However, no randomized clinical trial has compared the outcomes of patients receiving different GPIIb-IIIa antagonists. Therefore, it is not currently possible to determine whether apparent differences in efficacy between different GPIIb-IIIa antagonists are related to the above-described differences between the drugs or to differences in the clinical settings tested. The current TARGET trial is directly comparing tirofiban and abciximab in patients undergoing coronary stent implantation.
Current Indications and Doses
Over 33,000 patients have been evaluated in 11 placebo-controlled trials of abciximab, tirofiban, eptifibatide, and lamifiban.15 Current FDA-approved indications are listed in Table 2.
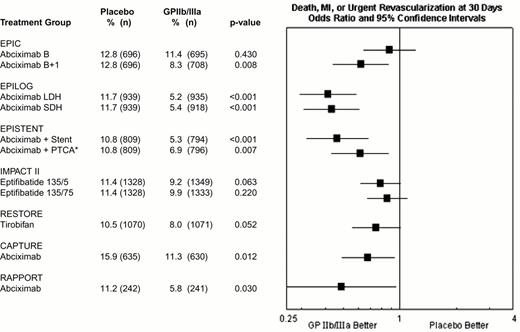
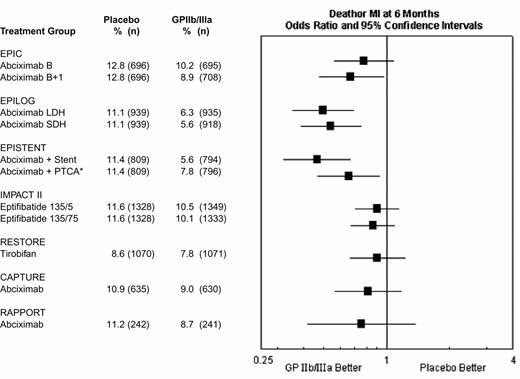
During percutaneous coronary intervention trials, the 30-day risk of death, acute myocardial infarction, or repeat urgent revascularization was reduced from 10.8-15.9% in patients who did not receive a GPIIb-IIIa antagonists to 5.2-11.3% in patients who did receive a GPIIb-IIIa antagonist, representing an absolute reduction of 1.5% to 6.5% (Figure 9). Treatment effect has been achieved early in every group of patients with every modality of revascularization (balloon angioplasty, directional atherectomy, or stenting) and, with abciximab, is maintained at 6 months (Figure 10) and up to 3 years.15 The relatively modest treatment effects in IMPACT II (Figures 9, 10) were at least in part the result of inadequate eptifibatide dosing based upon phase II studies that used sodium citrate as the anticoagulant for ex vivo platelet aggregation studies. This artifactually augmented measured eptifibatide-induced inhibition of platelet aggregation.16
Composite 30-day end point (death, myocardial infarction or urgent repeat revascularization) event rates for the 7 GPIIb-IIIa interventional trials.
This was not the prespecified primary end point of RESTORE or RAPPORT. RESTORE trial end points listed here are for the published post-hoc analysis including only urgent repeat revascularization. RESTORE trial end points listed here differ from those of the other trials in that only patients with successful crossing of the lesion with the guidewire were included in the efficacy analysis of RESTORE, whereas all randomized patients were included in the other studies. RAPPORT trial end points listed here are for secondary end point of death, myocardial infarction or urgent repeat target vessel revascularization. *EPISTENT trial groups compared with reference group of Placebo + Stent; thus, the “placebo control” for the Abciximab + PTCA group underwent stenting rather than PTCA.
Abbreviations: B, bolus; B + I, bolus plus infusion; CAPTURE, C7E3 AntiPlatelet Therapy in Unstable REfractory angina; EPIC, Evaluation of c7E3 for Prevention of Ischemic Complications; EPILOG, Evaluation in PTCA to Improve Long-term Outcome with abciximab GP IIb/IIIa blockade; EPISTENT, Evaluation of Platelet Inhibition in STENTing; IMPACT, Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis; LDH, low-dose, weight-adjusted heparin; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; RAPPORT, ReoPro And Primary PTCA Organization and Randomized Trial; RESTORE, Randomized Efficacy Study of Tirofiban for Outcomes and Restenosis; SDH, standard-dose, weight-adjusted heparin; 135/.5 and 135/.75, eptifibatide doses: 135 μg/kg bolus, followed by infusions of 0.5 or 0.75 μg/kg/min.
Reproduced with permission from Lincoff et al. JACC 35: 1103, 2000.
Composite 30-day end point (death, myocardial infarction or urgent repeat revascularization) event rates for the 7 GPIIb-IIIa interventional trials.
This was not the prespecified primary end point of RESTORE or RAPPORT. RESTORE trial end points listed here are for the published post-hoc analysis including only urgent repeat revascularization. RESTORE trial end points listed here differ from those of the other trials in that only patients with successful crossing of the lesion with the guidewire were included in the efficacy analysis of RESTORE, whereas all randomized patients were included in the other studies. RAPPORT trial end points listed here are for secondary end point of death, myocardial infarction or urgent repeat target vessel revascularization. *EPISTENT trial groups compared with reference group of Placebo + Stent; thus, the “placebo control” for the Abciximab + PTCA group underwent stenting rather than PTCA.
Abbreviations: B, bolus; B + I, bolus plus infusion; CAPTURE, C7E3 AntiPlatelet Therapy in Unstable REfractory angina; EPIC, Evaluation of c7E3 for Prevention of Ischemic Complications; EPILOG, Evaluation in PTCA to Improve Long-term Outcome with abciximab GP IIb/IIIa blockade; EPISTENT, Evaluation of Platelet Inhibition in STENTing; IMPACT, Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis; LDH, low-dose, weight-adjusted heparin; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; RAPPORT, ReoPro And Primary PTCA Organization and Randomized Trial; RESTORE, Randomized Efficacy Study of Tirofiban for Outcomes and Restenosis; SDH, standard-dose, weight-adjusted heparin; 135/.5 and 135/.75, eptifibatide doses: 135 μg/kg bolus, followed by infusions of 0.5 or 0.75 μg/kg/min.
Reproduced with permission from Lincoff et al. JACC 35: 1103, 2000.
Composite 6-month end point of death or myocardial (re-)infarction event rates for the GPIIb-IIIa interventional trials.
*EPISTENT trial groups compared with reference group of Placebo + Stent.
Abbreviations as in Figure 9. Reproduced with permission from Lincoff et al. JACC 2000;35:1103.
Composite 6-month end point of death or myocardial (re-)infarction event rates for the GPIIb-IIIa interventional trials.
*EPISTENT trial groups compared with reference group of Placebo + Stent.
Abbreviations as in Figure 9. Reproduced with permission from Lincoff et al. JACC 2000;35:1103.
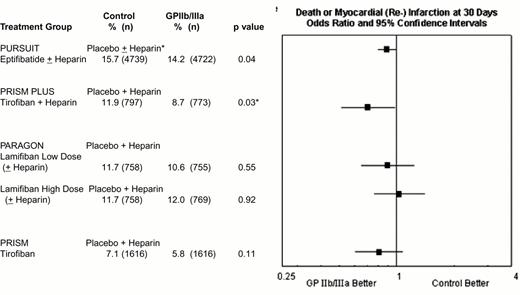
In the acute coronary syndromes without ST segment elevation, absolute 1.5% to 3.2% reductions in 30 day rates of death or myocardial (re-) infarction have been achieved with 2- to 4-day courses of tirofiban or eptifibatide (Figure 11). Abciximab is FDA-approved for medical management of patients with refractory unstable angina who are to undergo planned percutaneous coronary intervention within 24 hours of initiation of abciximab therapy (Table 2), but no data exists in patients with unstable angina who are not scheduled to have a percutaneous intervention.
Composite 30-day composite end point of death or myocardial (re-)infarction in the four GPIIb-IIIa unstable ischemic syndrome trials.
Efficacy data are not provided for the low-dose eptifibatide group PURSUIT and the tirofiban plus placebo group PRISM PLUS treatment arms, which were discontinued before completion of the trials.
*p = 0.03 in PRISM PLUS did not reach the prespecified level of p = 0.025 for a three-group trial.
† Heparin use in PURSUIT was encouraged, but not mandated, and was administered to 89.9% and 89.7% of patients randomized to placebo and eptifibatide, respectively.
Abbreviations: PARAGON, Platelet Iib/IIIa Antagonisms for the Reduction of Acute Coronary Syndrome events in the Global Organization Network; PRISM PLUS, Platelet Receptor Inhibition in the Ischemic Syndrome Management sin Patients Limited by Unstable Signs; PURSUIT, Platelet IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy.
Reproduced with permission from Lincoff et al. JACC. 2000;35:1103.
Composite 30-day composite end point of death or myocardial (re-)infarction in the four GPIIb-IIIa unstable ischemic syndrome trials.
Efficacy data are not provided for the low-dose eptifibatide group PURSUIT and the tirofiban plus placebo group PRISM PLUS treatment arms, which were discontinued before completion of the trials.
*p = 0.03 in PRISM PLUS did not reach the prespecified level of p = 0.025 for a three-group trial.
† Heparin use in PURSUIT was encouraged, but not mandated, and was administered to 89.9% and 89.7% of patients randomized to placebo and eptifibatide, respectively.
Abbreviations: PARAGON, Platelet Iib/IIIa Antagonisms for the Reduction of Acute Coronary Syndrome events in the Global Organization Network; PRISM PLUS, Platelet Receptor Inhibition in the Ischemic Syndrome Management sin Patients Limited by Unstable Signs; PURSUIT, Platelet IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy.
Reproduced with permission from Lincoff et al. JACC. 2000;35:1103.
Approved doses of the GPIIb-IIIa antagonists are listed in Table 2. Variables that may influence the appropriate dose of GPIIb-IIIa antagonists include heparin concentration17 and the PLA2 polymorphism in GPIIIa.18
The costs of eptifibatide and tirofiban are significantly less than abciximab only if their durations of infusion are less than those used in their respective efficacy trials.19 The cost effectiveness of abciximab in reducing mortality during coronary artery stenting is in the range of other accepted medical therapies, i.e. coronary bypass surgery, fibrinolytic treatment with tPA in acute MI, and hemodialysis.20 Patients with acute coronary syndromes who receive a GPIIb-IIIa antagonist also receive aspirin and heparin (or low molecular weight heparin). Patients undergoing percutaneous coronary intervention will also receive aspirin, heparin (or low molecular weight heparin), and, if they are being stented, either clopidogrel or ticlopidine.
Side Effects
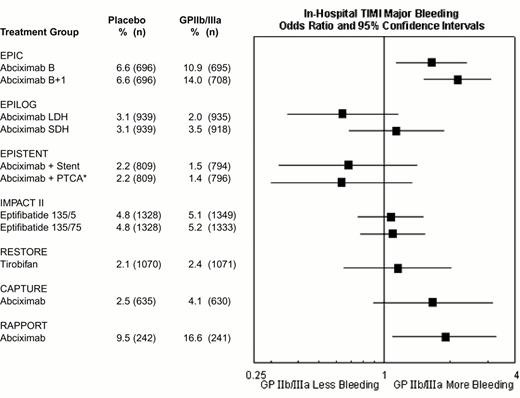
The rates of major hemorrhage in the seven GPIIb-IIIa antagonist interventional trials are shown in Figure 12. In the first trial (EPIC), there was a substantially increased risk of bleeding. However, there was no significant increase in hemorrhagic risk in most subsequent trials of GPIIb-IIIa antagonists, as a result of reduction and weight adjustment of concomitant heparin dosing (typically 70 U/kg initial bolus if baseline ACT < 150, followed by 20 U/kg as required to maintain an ACT of 200-300 seconds) and early removal of vascular sheaths. For example, in the EPILOG trial there was reduced in-hospital Thrombolysis In Myocardial Infarction (TIMI) major bleeding when abciximab was given with low dose heparin rather than standard-dose heparin (Figure 12). The high rates of bleeding in RAPPORT and CAPTURE (Figure 12) were likely related to long vascular access sheath dwell times. The incidence of intracranial hemorrhage was not increased by GPIIb-IIIa antagonist therapy.21 Nevertheless, bleeding may occur in certain patients, such as those who receive GPIIb-IIIa antagonists as part of an unplanned or “bailout” percutaneous coronary intervention after failed full-dose fibrinolytic therapy for acute myocardial infarction.15 Ideally, GPIIb-IIIa treatment in these patients should be delayed until systemic thrombolytic effects have subsided, usually at 15-18 hours22 (although earlier administration at 6-8 hours may be well tolerated23 ). In similar patients receiving full-dose heparin, a shorter time interval and/or protamine can be used. For patients who develop refractory or life-threatening bleeding, the antiplatelet effect of abciximab may be reversed by discontinuation of drug infusion and by platelet transfusion (the latter being more effective after the ∼ 10-30 minutes required for clearance of the drug from plasma. After transfusion, abciximab redistributes from the patient's platelets to the transfused platelets, thereby reducing the mean level of receptor blockade and improving platelet function. Platelet transfusions are rarely necessary with the rapidly reversible agents eptifibatide and tirofiban and might not be effective during the ∼ 2 hours required for clearance of these agents from plasma.15 Monitoring of GPIIb-IIIa antagonist therapy, for example by aggregation24 or receptor occupancy assays,25,26 has not been demonstrated to be clinically helpful in this setting.27
Rates of in-hospital thrombolysis in myocardial infarction (TIMI) major bleeding in the GPIIb-IIIa interventional trials.
*EPISTENT trial groups compared with reference group of Placebo + Stent. Major bleeding defined as intracranial hemorrhage or a fall in hemoglobin by >5 mg/dL.
Abbreviations as in Figure 9.
Reproduced with permission from Lincoff et al. JACC 35:1103, 2000.
Rates of in-hospital thrombolysis in myocardial infarction (TIMI) major bleeding in the GPIIb-IIIa interventional trials.
*EPISTENT trial groups compared with reference group of Placebo + Stent. Major bleeding defined as intracranial hemorrhage or a fall in hemoglobin by >5 mg/dL.
Abbreviations as in Figure 9.
Reproduced with permission from Lincoff et al. JACC 35:1103, 2000.
In the emergent setting of failed percutaneous coronary intervention, coronary artery bypass graft surgery (with its associated administration of heparin) has a high rate of perioperative major bleeding and mortality. In this setting, GPIIb-IIIa antagonist therapy should be immediately discontinued and, ideally, surgery delayed for 12-24 hours after abciximab and 4-6 hours after eptifibatide or tirofiban.23 However, the rapidly reversible agents eptifibatide and tirofiban result in little increase in the risk of bleeding.28,29 With regard to the much less reversible abciximab, platelet transfusion appears to reduce hemorrhagic risk,30,31 and blood loss was only modestly increased among patients who required emergency surgery in the EPILOG and EPISTENT trials.32 Because of the anticoagulant effect of GPIIb-IIIa antagonists,3,4,5 a reduction in heparin dosing during cardiopulmonary bypass is recommended.23 The combination of warfarin and GPIIb-IIIa antagonists should be avoided.22
Thrombocytopenia is an infrequent but well-described complication of GPIIb-IIIa antagonists. The reported risk of profound thrombocytopenia associated with abciximab (0.4-1.1%) is higher than with eptifibatide (0-0.2%), tirofiban (0.1-0.3%), or lamifiban (0-0.1%).15 The mechanism is unknown, but may be the result of ligand-induced binding sites (LIBS) that result in rapid platelet clearance33 and/or pre-existing drug-dependent antibodies in plasma.34 More than one mechanism may exist, because the onset can vary from 1 hour to days after exposure to the GPIIb-IIIa antagonist. Therefore, platelet counts should be measured within the first 2-4 hours after administration of a GPIIb-IIIa antagonist and followed at least daily during therapy. The management of GPIIb-IIIa antagonist-induced thrombocytopenia of major degree is discontinuation of the antagonist and, if necessary, platelet transfusion.
EDTA-induced pseudothrombocytopenia has been reported after GPIIb-IIIa antagonist therapy.35 Therefore, when a low platelet count is reported with GPIIb-IIIa antagonist therapy, a peripheral blood smear needs to be reviewed for platelet agglutination or a platelet count needs to be determined in citrated blood. This will avoid unnecessary discontinuation of the GPIIb-IIIa antagonist as well as platelet transfusions.
Approximately 5% of patients develop an antichimeric antibody within a month of receiving abciximab.36 No anti-drug antibodies have been reported after treatment with eptifibatide, tirofiban, or lamifiban. A prospective abciximab readministration registry of 500 patients found no cases of hypersensitivity or anaphylactic reactions after abciximab readministration, and the reduction in ischemic complications appears to be similar with readministration.37 However, the incidence of thrombocytopenia after readministration was somewhat higher even though the presence of an anti-chimeric antibody was not predictive of lack of clinical effectiveness, thrombocytopenia, or other sequelae.
Possible Future Indications
In the setting of acute myocardial infarction, GPIIb-IIIa antagonists are under investigation as combination therapy with low dose thrombolytic agents and antithrombin agents. GPIIb-IIIa antagonists are also under investigation for the treatment of cerebrovascular thrombotic disease. Abciximab, because of its binding to aVβ3, could in the future prove to be of use as an inhibitor of tumor angiogenesis and/or sickle cell microvascular occlusion.12 Long-term oral GPIIb-IIIa therapy for the secondary prevention of coronary disease is an attractive concept, but no oral GPIIb-IIIa antagonists are currently FDA approved. Preliminary studies have suggested that oral agents can be administered for up to 3 months without a prohibitive increase in major bleeding complications.38,39 However, their efficacy has been disappointing.38,39,40 Analysis of the OPUS-TIMI 16 trial revealed that the excess mortality rate occurred among patients with a creatinine clearance of < 90 ml/min, which suggests that dosing of oral GPIIb-IIIa antagonists may need to be individualized.39 Monitoring of oral GPIIb-IIIa antagonist therapy may therefore be of benefit in standardizing, and possibly improving the efficacy and safety of, oral GPIIb-IIIa antagonist therapy. Although a number of assays, including some point-of-care assays, are available for the monitoring of GPIIb-IIIa antagonist therapy,24,25,26,27 their role in clinical care remains to be established.
Conclusions
Intravenous GPIIb-IIIa antagonists are potent anti-platelet drugs that significantly reduce thrombotic complications in patients undergoing percutaneous coronary interventions and/or with acute coronary syndromes. While the currently FDA-approved intravenous GPIIb-IIIa antagonists are often used interchangeably, randomized clinical trials that directly compare these agents are needed.
III. Immune Thrombocytopenic Purpura
James B. Bussel, M.D.*
Division of Pediatrics, New York Hospital, 525 East 68th Street, Payson 6, New York NY 10021
ITP has been labeled as both “idiopathic” and “immune” thrombocytopenic purpura. This dichotomy has persisted at least in part because of the inability to develop an antiplatelet antibody or other specific test that can reliably distinguish immune from non-immune thrombocytopenia.1,2 The clinical significance of this issue revolves around the difference in treatment that would be likely to be effective to resolve bleeding, or a high risk of bleeding, in affected patients. For example, if a patient had non-immune thrombocytopenia, then probably platelet transfusion or even recombinant human (rhu) factor VIIa would be primary modalities to correct bleeding in addition to supportive measures such as antifibrinolytics (epsilon-aminocaproic acid [amicar], transexamic acid), increasing the hematocrit to augment platelet delivery to the vessel wall, and potentially improving platelet function with DDAVP and cryoprecipitate. In contrast, in patients with ITP, the goal is to substantially and rapidly increase the platelet count by treatments designed to counteract the effects of the anti-platelet antibodies.2 The number and variety of available treatments have dramatically increased in recent years, increasing the possibilities for effective therapy even though there is limited information from controlled trials.
The goal of this presentation is to present several case scenarios to illustrate controversies in the management of patients with “ITP,” here used as “immune” thrombocytopenic purpura. Non-immune thrombocytopenia will be called “NIT.” Much of the information will relate to both adults and children; differences will be highlighted in the course of the discussion. A recurring central theme is that better understanding of the biology of ITP to allow individualization of patient selection for different treatments (including no treatment) is critical. Currently this is largely unavailable and assessment is typically based upon clinical behavior in response to treatments.
Case #1, Part 1
A 73-year-old woman is noted to have bruises and gum bleeding and is found to have a platelet count of 5,000/μl with a Hgb of 12.7 gm/dl, an MCV of 98 fl, and a WBC of 4600/μl with 57% neutrophils. Her physical examination reveals only chronic arthritis and large bruises, scatered petechiae, and one large hemorrhagic bulla on the left buccal mucosa. There is no hepatosplenomegaly or adenopathy. Does she have ITP or NIT?
At this patient's age there is an increased risk of myelodysplasia or other marrow abnormalities, so most clinicians would perform a bone marrow examination as part of their assessment. In a younger patient, this would be less of a concern although the MCV is high normal (or > normal for a child). Even if estimates of marrow platelet production were available (platelet reticulocyte count, glycocalicin level, or plasma thrombopoietin level are candidate tests) and proven to distinguish marrow failure states from those of severe ITP, examination of the marrow would probably still be required.5,6,7,8,9,10 Unfortunately, in subtle cases the distinction between myelodysplasia and “normal” is still very difficult, and available tests, i.e. cytogenetics, are helpful in the minority of cases. The availability of previous CBCs could exclude a long-lasting hematologic disorder. In children with chronic bruising and no previously normal platelet count, the possibility of an inherited (familial) non-immune thrombocytopenia is greater. Specific features that are uncommon in ITP but identified in specific NIT syndromes can be considered, e.g. platelet and neutrophil appearance on smear, pattern of bleeding in relationship to the platelet count, hearing, renal function, and family history of malignancy among others. No study has described what fraction of cases of presumed ITP actually turn out to be NIT but an estimate would be 3-5%. One reason for this is the lack of a gold standard to distinguish the two entities when obvious diagnostic features of one or the other are lacking. In general, familial non-ITP thrombocytopenias usually but not always share the following characteristics: 1) more than one affected family member; 2) no history suggestive of systemic lupus erythematosus (SLE) or other autoimmune tendency in the family; 3) a mild thrombocytopenia, most commonly 40-80,000/μl; and 4) at least adequate platelet function with infrequent significant hemorrhage.
Probably the best diagnostic test is repeated response to treatments that are thought to be largely specific to ITP: intravenous gammaglobulin (IVIG) or intravenous anti-D immunoglobulin. Response to steroids and splenectomy are less ITP specific. Exactly what level of response to which dose is not well defined; however, a platelet increase twice of > 20,000-30,000/μl with the correct temporal sequence and return to baseline in 2-4 weeks is highly consistent with ITP. A separate issue for diagnosis includes consideration of secondary ITP in which the thrombocytopenia has an immune basis but with a specific etiology, i.e. CVI, SLE or chronic lymphocytic leukemia (CLL).
Case #1, Part 2
With the severe thrombocytopenia and the wet purpura, how does one increase the platelet count rapidly in presumed ITP?
The frontline treatments known to achieve a substantial platelet increase are steroids and IVIG. Limited data suggests that higher-dose, parenteral steroids have more rapid effects than conventional oral steroids, i.e. 1 gm of IV methylprednisolone in an adult as compared to 1-2 mg/kg of oral prednisone. Depending upon the setting and the study, IVIG remains the single treatment with the most rapid, consistent platelet increase. In the past, IV anti-D was not recommended as frontline treatment because of the slower platelet response to the standard dose of 50 μg/kg.15 However, newer data suggest that an overnight platelet increase will be achieved in the majority of adults and children using a dose of 75 μg/kg.20
Acute or chronic refractory patients may not respond to any single treatment. In difficult cases, combinations of IVIG and IV methylprednisolone, also including the addition of IV anti-D and/or vincristine, have been acutely effective in 60-80% of very refractory patients. Plasmapheresis may be useful in combination with other treatments as may IV cyclophosphamide but these are not typically required at or near diagnosis. Combinations of agents are likely to work better than single agents and should be considered 1) when the patient appears refractory to one or more single agents; 2) has a history of being difficult to treat, or 3) appears to be at a high risk of bleeding as a consequence of severe thrombocytopenia. If platelet transfusion is urgently required because of ongoing bleeding or trauma, the use of a bolus followed immediately by continuous platelet infusion has been successful in anecdotal cases in conjunction with other ITP therapy, which may help to “protect” the transfused platelets. Frequent platelet counts are necessary initially to determine the rate of platelet infusion required in these rare cases.
Case #2, part 1
A 34-year-old woman has had ITP for 2 months, presenting with a platelet count of 20,000/μl obtained on a yearly checkup with her obstetrician-gynecologist. Previous counts had been normal and easy bruisability was identified in retrospect. She initially responded to prednisone with a platelet count > 100,000/μl but her count has fallen to 35,000/μl with taper of prednisone to 10 mg/day. How should her case be managed?
Conventional wisdom and most clinicians would increase the prednisone dose and schedule splenectomy, and this approach remains the standard of care. Splenectomy is easier for patients when laparoscopy is utilized since it appears equivalently effective to conventional open splenectomy. Unanswered questions include 1) below what level of platelet count that is stable without treatment is splenectomy the preferred alternative, and 2) should all adults go to splenectomy if they fail to stabilize above that level in the first 2-3 months of treatment?
Very few patients have any significant bleeding at platelet counts > 20,000-30,000/μl and therefore it is my opinion that the platelet count should be consistently < 30,000/μl in an asymptomatic, untreated adult prior to planning a splenectomy. General practice, however, has been to use 50,000-60,000/μl as a minimum. Many clinicians believe that hemorrhagic problems will inevitably arise for patients whose platelets are in the range of 30,000-50,000/μl either with a traumatic emergency, the need for urgent surgery, pregnancy, or merely with the passage of time. Studies of deferral of splenectomy in adults are in progress. In children there is a high rate of spontaneous improvement of ITP and the rule of thumb is to wait at least one year from diagnosis if at all possible and longer in a child < 5 years of age. A study is ongoing to explore the potential curative effects of repeated IVIG or dexamethasone in children with early chronic ITP.
Another piece of the puzzle is the long-term outcome of splenectomy for ITP. Review of the literature suggests that the response rate is 60-70%, but long-term follow up is restricted to a median of 1-2 years in essentially all of these studies. Overwhelming post-splenectomy sepsis can occur at any time but should be minimized by repeated immunizations with pneumovax, meningiococcal, and H Flu B vaccines at 5-10 year intervals as well as immediate provision of IV antibiotics for fever.
Returning to case #2, I would recommend a continued slow taper of the prednisone while initiating discussion of the various options. Proceeding to splenectomy is likely to be required since the platelets have decreased to 35,000/μl while still on 10 mg/day of prednisone. Continued use of high dose prednisone would be the only unacceptable approach in this patient. Certain patients, possibly this one, can maintain an adequate platelet count with the use of 10 mg of prednisone every other day. Any plan that involves persistent use of “low-dose” steroids is acceptable but requires specific assessment of toxicity—especially osteoporosis—from the viewpoint of family history (susceptibility), diet, other medical conditions, and activity. Bone density should be monitored in these cases.
Case #2, Part 2
The patient opts not to undergo splenectomy and subsequently becomes pregnant while on prednisone 10 mg every other day. Her count has been approximately 30,000/μl and she has no bleeding symptoms. What special considerations apply?11,12,13
The answer needs to be divided into issues affecting the mother and those that affect the fetus. The mother does not require a higher platelet count during pregnancy. Specifically, if she maintains a platelet count of > 20,000-30,000/μl during pregnancy without bleeding symptoms, no additional treatment would be required throughout most of the pregnancy. If the platelet count fell with the onset of or during pregnancy, then either additional prednisone could be used or it could be supplemented with IVIG. A study is ongoing with IV anti-D to see if it is effective and safe (for the fetus) in the treatment of ITP during pregnancy. Because pregnancy is associated with hyperglycemia, weight gain and fluid retention, hypertension, mood swings, and bone calcium loss even without prednisone, these side effects of too much prednisone become especially significant. However, the time period of prednisone usage is finite so the decision needs to be individualized to the patient. Splenectomy should be avoided if at all possible.
More difficult treatment issues center around delivery. The platelet count should optimally be > 50,000/μl and obstetric anesthesiologists may require platelet counts of 80,000/μl to provide epidural anesthesia. While IVIG can be used to supplement the platelet count, the effect is usually too short-lived unless the delivery is scheduled for a particular date. Often one IVIG infusion combined with 20 mg/day of prednisone will serve to maintain the platelet count at a substantially increased level for a prolonged period (weeks), allowing an optimal unscheduled delivery. Platelet transfusions should probably not be routinely administered but rather be immediately available if there is any increased degree of hemorrhage at the time of delivery.
The platelet count of the fetus at birth is probably the best-studied aspect of pregnancy and ITP. In 8.9-14.7% of cases of known ITP in pregnancy, the neonatal birth platelet count will be < 50,000/μl. It will fall this low later in additional neonates, but this may be less significant than a low count at birth in regard to the risk of intracranial hemorrhage (ICH). The risk of ICH is low even in severely thrombocytopenic neonates; approximately 0.5-1.0% of neonates of mothers with known ITP will suffer an ICH. The risk of antenatal ICH, unlike that seen with alloimmune thrombocytopenia, is also very low so an antenatal treatment program to prevent fetal and neonatal hemorrhage is appropriate in very few cases. The only accepted predictor of the presence or absence of neonatal thrombocytopenia in infants of mothers with ITP is having had a previous sibling while the mother had ITP. Whatever platelet count the previous sibling had, it appears that the subsequent sibling will have a similar count.
Management of the neonate has several cardinal features:
The platelet count can be incorrect on cord blood obtained at birth (usually falsely low) so the blood count should be repeated.
The platelet count can fall dramatically after birth so it should be repeated at 24-48 hours even if normal at birth.
If the neonate is thrombocytopenic at birth (< 50,000/μl), an immediate head ultrasound examination or other cranial imaging study (CT or MRI) is required in all babies because of the risk of ICH and the potential for lessening its impact with vigorous therapy.
A corollary is that treatment depends upon clinical bleeding:
If the neonate has suffered an ICH, then platelet transfusions to instantaneously correct the platelet count while awaiting the effect of IVIG combined with steroids is appropriate
If no ICH or other serious (e.g. gastrointestinal) hemorrhage is ongoing, then IVIG 1 gm/kg combined with steroids i.e. 1 mg q 8h of IV methylprednisolone (total daily dose of approximately 1 mg/kg) is appropriate and platelet transfusion is probably not required.
Thrombocytopenia may persist for longer than expected, so retreatment may be required even if initial treatment is successful. Persistent thrombocytopenia does not necessitate prolonged hospitalization but does mandate outpatient follow-up until the platelet count is stable off treatment for several weeks.
Case #3
A patient is referred who has had ITP for 8 months. She initially received prednisone but the platelet count never exceeded 45,000/μl. After 6 months of increasing and decreasing the dose of prednisone, she underwent splenectomy. Her platelet count increased immediately afterwards but then fell and is currently 14,000/μl on 30 mg/day of prednisone now at 2 months after splenectomy. What is the optimal management?
First, every effort needs to be made to clarify the diagnosis as discussed previously since only prednisone has been used and the platelet increase with it was equivocal. If necessary, IVIG could be given to clarify the diagnosis as well as the responsiveness of the patient; IV anti-D is relatively ineffective after splenectomy. Second there is no consensus and essentially no controlled trials are available to inform the decision in this situation, so the treatment decision is a matter of physician and patient preference.14 ,16,17,18,19 In particular, it may depend upon what the patient can tolerate. For example, if the transaminases are even mildly increased then it is unlikely that danazol can be used and the same may be true for azathioprine. If the patient is elderly she will probably tolerate prolonged steroids and chemotherapy less well, especially the neurotoxicity of vinca alkaloids. If she is intending to have additional children, drugs that impact fertility, e.g. cyclophosphamide, are less desirable. Third, a primary reason for the lack of consensus is that no single agent has an efficacy rate of > 50% (possibly as low as 30%) in this patient population. The efficacy of all treatments is even lower when the goal is “cure” (taking the treatment for a period of time and then stopping the treatment but still maintaining an adequate platelet count) rather than increasing the platelet count while taking the medication. More detailed and extensive commentary on these and other possible treatments has been provided by McMillan.2
Experimental treatments of ITP are undergoing exploration, and this patient could well be a candidate since it is not mandatory that any patient receive all conventional agents prior to beginning experimental agents. This is particularly true for ITP, in which the “available” agents are known not to be highly effective. These experimental and/or novel treatments include: 1) mycophenalate mofetil (cellsept); 2) IL-11 (Neumega); 3) thrombopoietin; 4) bone marrow transplantation; 5) anti-CD40ligand; and 6) anti-CD20 (rituximab; Rituxan). Other agents are also being considered in these patients. In general it appears that mycophenalate mofetil and IL-11 have limited responses. Thrombopoietin has not undergone clinical trial in ITP because of development of antibodies to administered TPO, which crossreacted with native endogenous TPO resulting in chronic thrombocytopenia. Autologous bone marrow transplantation has been best studied by Dunbar et al at the NIH with some preliminary but encouraging results; more follow-up is required to better assess the long-term outcome. Three studies of anti-CD40ligand have been completed with very exciting results in refractory patients. However, thromboembolic toxicity has resulted in at least temporary suspension of clinical trials. Anti-CD20 has been successful in anecdotal cases and in a preliminary trial; a more definitive study is underway.
It is clear that the management of the patient failing splenectomy is highly complex as a result of the paucity of directive information. The option of choosing no treatment is possible but raises the potential that even in a stable patient with a low count, < 10,000-20,000/μl, with minimal bleeding signs and symptoms, an intercurrent infection or other “destabilizing” event can occur and serious hemorrhage (e.g. ICH) can result.
Better ability to assess platelet function may help treatment decisions in ITP as well. Unfortunately, the current state of platelet antibodies does not allow specific diagnosis of immune TP. In the future, study of platelet polymorphisms may be clinically important in ITP. The development of ITP with infusion of antagonists of glycoprotein IIb-IIIa may suggest an etiology of idiopathic ITP via the creation of LIBS and exposure of neoantigens; this is currently under study.