Abstract
This review focuses on the different treatment options available for the treatment of Hodgkin's disease, with an emphasis on the importance of the long-term sequelae of these therapies.
In Section I, Dr. Linch reviews the current status of Hodgkin's disease treatment. Survival rates have improved over the last three decades due both to better initial therapies and associated supportive care and to the success of salvage therapy. Unlike most other malignancies, a similar survival endpoint can be achieved by different means, e.g., intensive initial therapy resulting in a low relapse rate or less intensive initial therapy and more reliance on salvage therapy. Overall survival has thus become a difficult end-point for clinical trials of primary therapy, and the value of disease-free survival as an end-point can also be questioned. Quality-of-life issues are to the fore of clinical decision and include the psychological trauma of relapse and fertility status. Patient choice is increasingly important. The high level of success in treating Hodgkin's disease also means that attention must be focused on the very long term results and in this context the occurrence of second malignancies is a major issue.
In Section II, Dr. Gosden with Dr. Tulandi and Dr. Tan review the risks of infertility following radio-therapy and chemotherapy and address the actions that can be taken to overcome this problem, particularly for females and prepubertal boys and girls. Particular attention is paid to the recent developments in ovarian cryopreservation and harvesting immature germ cells.
In Section III, Dr. Hancock gives a comprehensive update of the incidence of secondary acute leukemia, non-Hodgkin's lymphoma and solid tumors in a large population of patients treated for Hodgkin's disease. The roles of radiotherapy, chemotherapy and combined modality treatment as risk factors contributing to the development of these secondary malignancies are reviewed. The importance of efforts to prevent late-occurring solid tumors such as lung cancer through smoking cessation programs and early detection by screening for cancers of the breast, thyroid and skin are emphasized.
I. Choice of Treatment Intensity in Newly Diagnosed Hodgkin's Disease
D. C. Linch, FRCP, FRCPath*
Department of Haematology, University College London, 98 Chenies Mews, London WC1E 6HX, UK
In the recently published WHO classification of hematological malignancies, the sub-classification of Hodgkin's disease is broadly similar to the previous Rye classification (Table 1).1 Lymphocyte-predominant Hodgkin's is clearly a distinct entity from other forms of the disease as shown by immunophenotype analysis (Table 2). A very high proportion of patients present with Stage IA disease and a minimalist approach to treatment may be appropriate.2 Lymphocyte-depleted Hodgkin's disease is now rarely diagnosed in some centers; patients who were once labelled with this entity are now mainly classified as Grade II nodular sclerosis disease or as anaplastic large cell lymphomas. With the possible exception of localized lymphocyte-predominant disease, the remaining types of Hodgkin's disease are treated in a similar way depending upon stage.
WHO Classification of Hodgkin's disease.
|
|
The phenotype of neoplastic cells in classical and nodular lymphocyte-predominant Hodgkin's disease.
. | Classical HL RS cells . | Atypical Lymphocytic and Histiocytic Cells (LPHD) . |
|---|---|---|
| CD20 | Occasionally positive | Usually positive |
| Other B cell antigens | Usually negative | Usually positive |
| J chain | Negative | Positive |
| CD30 | Positive | Negative |
| CD15 | Usually positive | Negative |
| EBV genome | Often positive | Infrequently positive |
| Abbreviations: HL, Hodgkin's lymphoma; RS, Reed-Sternberg cells; LPHD, lymphocyte-predominant Hodgkin's lymphoma; EBV, Epstein-Barr virus | ||
. | Classical HL RS cells . | Atypical Lymphocytic and Histiocytic Cells (LPHD) . |
|---|---|---|
| CD20 | Occasionally positive | Usually positive |
| Other B cell antigens | Usually negative | Usually positive |
| J chain | Negative | Positive |
| CD30 | Positive | Negative |
| CD15 | Usually positive | Negative |
| EBV genome | Often positive | Infrequently positive |
| Abbreviations: HL, Hodgkin's lymphoma; RS, Reed-Sternberg cells; LPHD, lymphocyte-predominant Hodgkin's lymphoma; EBV, Epstein-Barr virus | ||
Staging
The major purposes of staging are to determine prognosis and to ascertain which patients can be treated with radiotherapy. In many centers, patients with stage IA and IIA disease are treated with radiotherapy alone and patients with stage IIB, IIIB and IV disease receive chemotherapy. There has been variable practice in stage IB (rare) and IIIA disease. Staging laparotomy with splenectomy introduced at Stanford in the late 1960s has ceased to be widely used, in part because of improved imaging techniques, but largely because of the realization that although surgically staged patients had an improved disease-free survival, the overall survival was not significantly improved. This conclusion was arrived at in a matched pairs analysis of the British National Lymphoma Investigation (BNLI) database3 and in modeling of the results from the Princess Margaret Hospital in Toronto.4 In the European Organization for Research and Treatment of Cancer (EORTC) H2 trial a randomization to staging laparotomy or splenic irradiation was carried out and revealed only a marginal advantage in relapse-free survival for the laparotomy arm and no survival advantage.5 The later EORTC H6 study randomized good risk CS I and II patients to laparotomy or not with more extensive irradiation in the non-laparotomized patients, and although there was a significant improvement in freedom from relapse in the laparotomy arm there was no difference in survival.6 This reflects the success of chemotherapy salvage in patients who have failed radiotherapy.
Treatment of Localized Disease
Extent of radiation fields
For those patients in whom radiotherapy alone is employed, the extent of the radiation field is critical in determining the relapse rate but probably not the overall survival (Table 3). Specht and colleagues in the International Hodgkin's Disease Collaborative Group carried out a meta-analysis of eight randomized trials involving 1,974 patients who received more or less extended radiation fields.13 The use of more extensive radiation fields was associated with the reduction in the actuarial relapse rate at 10 years from 43% to 31% (P < 0.00001), but the overall survival at 10 years was 77% regardless of the extent of the radiation field.
Selected series of patients with localized Hodgkin's disease treated with different radiation fields.
| Radiation Field . | Center (ref) . | Patients . | No. of Patients . | DFS . | Survival . | @ Years* . |
|---|---|---|---|---|---|---|
| Involved Field | MD Anderson (7) | PS I and II (A and B) | 87 | 54% | — | 10 |
| CS I and II (A and B) | 133 | 35% | — | 10 | ||
| BNLI (3) | PS I and IIA | 107 | 54% | 81% | 10 | |
| Mantle Field | BNLI (3) | PS I and IIA | 212 | 57% | 79% | 10 |
| Melbourne (8) | CS I and II (A and B) | 64 | 46% | 89% | 5 | |
| PS I and II (A and B) | 66 | 67% | 90% | 5 | ||
| EORTC (5) | CS I and II (A and B) | 152 | 38% | 60% | 12 | |
| Toronto (4) | CS I and IIA | 121 | ∼62% | 10 | ||
| Extended Mantle | Boston (9) | PS I and IIA | 108 | 74% | 89% | 5 |
| Chicago (10) | PS I and IIA | 92 | 77% | 71% | 10 | |
| EORTC (5) | CS I and II (A and B) | 108 | 63% | 80% | 7 | |
| STNI/TNI | Stanford (11) | PS I and II (A and B) | 109 | 77% | 84% | 10 |
| Denmark (12) | PS I and II (A and B) | 133 | 90% | 90% | 7 | |
| Abbreviations: DFS, disease-free survival; PS, pathological stage (laparotomy-staged); CS, clinical stage; BNLI, British National Lymphoma Investigation; EORTC, European Organization for Research and Treatment of Cancer; STNI, subtotal nodal irradiation; TNI, total nodal irradiation | ||||||
| Radiation Field . | Center (ref) . | Patients . | No. of Patients . | DFS . | Survival . | @ Years* . |
|---|---|---|---|---|---|---|
| Involved Field | MD Anderson (7) | PS I and II (A and B) | 87 | 54% | — | 10 |
| CS I and II (A and B) | 133 | 35% | — | 10 | ||
| BNLI (3) | PS I and IIA | 107 | 54% | 81% | 10 | |
| Mantle Field | BNLI (3) | PS I and IIA | 212 | 57% | 79% | 10 |
| Melbourne (8) | CS I and II (A and B) | 64 | 46% | 89% | 5 | |
| PS I and II (A and B) | 66 | 67% | 90% | 5 | ||
| EORTC (5) | CS I and II (A and B) | 152 | 38% | 60% | 12 | |
| Toronto (4) | CS I and IIA | 121 | ∼62% | 10 | ||
| Extended Mantle | Boston (9) | PS I and IIA | 108 | 74% | 89% | 5 |
| Chicago (10) | PS I and IIA | 92 | 77% | 71% | 10 | |
| EORTC (5) | CS I and II (A and B) | 108 | 63% | 80% | 7 | |
| STNI/TNI | Stanford (11) | PS I and II (A and B) | 109 | 77% | 84% | 10 |
| Denmark (12) | PS I and II (A and B) | 133 | 90% | 90% | 7 | |
| Abbreviations: DFS, disease-free survival; PS, pathological stage (laparotomy-staged); CS, clinical stage; BNLI, British National Lymphoma Investigation; EORTC, European Organization for Research and Treatment of Cancer; STNI, subtotal nodal irradiation; TNI, total nodal irradiation | ||||||
Time point for DFS and overall survival
Combined modality therapy
Many centers have explored the use of combination chemotherapy and radiotherapy to treat localized disease and this undoubtedly reduces the relapse rate compared to the use of radiotherapy alone. In the EORTC H1 trial the administration of weekly vincristine for 2 years after the completion of regional radiotherapy for CS I and II (A and B) disease markedly improved relapse-free survival (61% vs 38% at 12 years; p < 0.001) and there was a trend to improved overall survival (70% vs 60%; p = 0.08).5 Most trials have not, however, demonstrated a survival advantage. The International Hodgkin's Disease Collaborative Group performed an analysis of 13 trials involving the randomization of combined modality treatment against radiotherapy alone. The addition of chemotherapy halved the 10-year risk of failure from 32.7% to 15.8% (p = 0.00001), but the improvement in survival was insignificant (79.4% to 76.5%).13
A trial from a French co-operative group of combined modality therapy in CS I, IIA and IIIA disease suggested that in the context of combined modality treatment, more limited radiation fields were fully efficacious and that three cycles of MOPP (nitrogen mustard, vincristine, prednisone, procarbazine) were as effective as six cycles.14 A number of other studies have addressed the use of fewer cycles of chemotherapy or the use of less intensive chemotherapy regimens with the aim of reducing the undoubted toxicity of combined modality therapy. At Stanford subtotal nodal irradiation (STNI) was compared with involved field (IF) radiation plus VBM (vinblastine, bleomycin, methotrexate) chemotherapy (non-sterilizing and low leukemogenicity) in laparotomy-staged patients with localized disease.15 There was a highly significant improvement in disease-free survival (95% vs 70%) but again no survival difference. A further study using 6 weeks of VBM in a different schedule, however, revealed considerable pulmonary toxicity attributed to the bleomycin given before the mediastinal radiation.16 In Manchester a month's course of VAPECB (vincristine, doxorubicin, prednisolone, etoposide, cyclophosphamide and bleomycin), another non-sterilizing regimen was added to radiation therapy and compared with radiation alone in a randomized phase II study.17
The progression-free survival at 4 years was 90% with the combined modality, compared to only 64% with radiation alone.
Chemotherapy alone for localized Hodgkin's disease
At the National Cancer Institute patients with PS IB, IIA, IIB and IIA1 disease were randomized to STNI or MOPP alone.18 The projected 10-year disease-free survival of patients randomized to receive radiation was 60% compared to 86% in those who received MOPP (p = 0.009). There was also a borderline significant survival benefit for the MOPP recipients but this was mainly seen in patients with bulky mediastinal disease. In contrast to these excellent results with MOPP an Italian study in PS I and II disease found similar rates of freedom from progression but reduced survival in the MOPP recipients due to a frequent failure of salvage therapy after a relapse from MOPP therapy.19
Summary
Improved disease-free survival can be achieved by using more extensive radiation fields or adding chemotherapy to radiotherapy. The value of chemotherapy alone is not clear. The reduction in relapse rates is not, however, accompanied by a significant increase in overall survival, and decisions about optimal therapy must be based on a consideration of acceptable relapse rates and short- and long-term toxicity.
Treatment of Advanced Hodgkin's Disease
Combination chemotherapy regimens
The introduction of MOPP by DeVita and colleagues in the 1960s20,21 represented a major advance in the treatment of advanced Hodgkin's disease. Many modifications of this regimen were developed (e.g. Ch1VPP [chlorambucil, vinblastine, procarbazine, prednisone]) to reduce acute toxicity and this was probably achieved without any loss of anti-tumor activity.22 The development of ABVD (doxorubincin, bleomycin, vinblastine, dacarbazine) by Bonadonna and colleagues a decade later,23 represents the next most important step in Hodgkin's therapy and is usually regarded as the modern therapeutic `gold standard.' It probably has more anti-tumor activity than MOPP23,24 and is less sterilizing and less leukemogenic. The ABVD regimen was combined early on with MOPP in an alternating schedule,23 and since then many alternating or hybrid schedules have been designed. Overall, these alternating and hybrid regimens have proven to be superior to MOPP but not to ABVD (25 and Table 4). Nonetheless, there has appeared to be a steady improvement in survival over the last three decades,3 due as much to improvements in supportive care and the ability to administrate full-dose therapy as to the development of new regimens.
Selected randomized trials in advanced Hodgkin's disease.
| Study Group (Ref) . | Patients . | Regimens . | No. of Patients . | Survival Results . | ||
|---|---|---|---|---|---|---|
. | . | . | . | FFS . | OS . | @ Years** . |
| CALGB (24) | Stages IIIA2 IIIB and IV or RT relapses | MOPP | 123 | 50% | 66% | 5 |
| MOPP alt ABVD | 23 | 65% * | 75% | 5 | ||
| ABVD | 115 | 61% * | 73% | 5 | ||
| CALGB 8952 (26) | Stage III or IV or RT relapses | ABVD | ∼428 | 65% | 87% | 3 |
| MOPP/ABV hybrid | ∼428 | 67% | 85% | 3 | ||
| INT MILAN (27) | Stage IB, IIA (bulky) | MOPP alt ABVD | 211 | 67% | 74% | 10 |
| III and IV | MOPP/ABV hybrid | 204 | 69% | 72% | 10 | |
| ECOG, CALGB, SWOG (28) | Stage IIIA2 IIIB and IV or RT relapses | MOPP/ABVD sequential | 344 | 54% | 71% | 8 |
| MOPP/ABV hybrid | 347 | 64%* | 79%* | 8 | ||
| NCI-C (29) | Stage IIIB or IV or RT relapses | MOPP alt ABVD | 148 | 67% | 83% | 5 |
| MOPP/ABV hybrid | 153 | 71% | 81% | 5 | ||
| Abbreviations: CALGB, Cancer and Acute Leukemia Group B; ECOG, Eastern Cooperative Oncology Group; SWOG, Southwest Oncology Group; NIC-C, National Cancer Institute of Canada; INT, Instituto Nazionale Tumori; FFS, failure-free survival; OS, overall survival; alt, alternating with; see text for translation of regimen acronyms | ||||||
| Study Group (Ref) . | Patients . | Regimens . | No. of Patients . | Survival Results . | ||
|---|---|---|---|---|---|---|
. | . | . | . | FFS . | OS . | @ Years** . |
| CALGB (24) | Stages IIIA2 IIIB and IV or RT relapses | MOPP | 123 | 50% | 66% | 5 |
| MOPP alt ABVD | 23 | 65% * | 75% | 5 | ||
| ABVD | 115 | 61% * | 73% | 5 | ||
| CALGB 8952 (26) | Stage III or IV or RT relapses | ABVD | ∼428 | 65% | 87% | 3 |
| MOPP/ABV hybrid | ∼428 | 67% | 85% | 3 | ||
| INT MILAN (27) | Stage IB, IIA (bulky) | MOPP alt ABVD | 211 | 67% | 74% | 10 |
| III and IV | MOPP/ABV hybrid | 204 | 69% | 72% | 10 | |
| ECOG, CALGB, SWOG (28) | Stage IIIA2 IIIB and IV or RT relapses | MOPP/ABVD sequential | 344 | 54% | 71% | 8 |
| MOPP/ABV hybrid | 347 | 64%* | 79%* | 8 | ||
| NCI-C (29) | Stage IIIB or IV or RT relapses | MOPP alt ABVD | 148 | 67% | 83% | 5 |
| MOPP/ABV hybrid | 153 | 71% | 81% | 5 | ||
| Abbreviations: CALGB, Cancer and Acute Leukemia Group B; ECOG, Eastern Cooperative Oncology Group; SWOG, Southwest Oncology Group; NIC-C, National Cancer Institute of Canada; INT, Instituto Nazionale Tumori; FFS, failure-free survival; OS, overall survival; alt, alternating with; see text for translation of regimen acronyms | ||||||
Significant difference
Time point for FFS and OS
Consolidation radiotherapy
It is a common practice to give consolidation radiotherapy to sites of bulk disease in patients achieving a complete remission with chemotherapy, but the evidence is not compelling. In the Groupe d'Etude des Lymphomes de l'Adulte (GELA) H89 trial, patients in CR or good PR after six cycles of chemotherapy were randomized to receive two more cycles of the same chemotherapy or STNI.30 There was no advantage to radiotherapy consolidation when a doxorubicin-containing regimen had been used. A meta-analysis by the International Database on Hodgkin's Disease Overview Study Group concluded that, providing an adequate number of chemotherapy cycles were administered, the addition of radiotherapy did not improve tumor control and was associated with a reduced survival probably due to long-term toxicity.31
Escalated therapies
Recently two regimens have been reported to have an improved disease-free survival, namely Stanford V32,33 and escalated BEACOPP34 (Table 5). Stanford V is only a moderately intensive chemotherapy regimen but includes extensive radiotherapy consolidation in most patients, whereas the escalated BEACOPP is a very dose-intensive regimen requiring growth factor support. The overall survival in these regimens is impressive but in a recent BNLI and CLG trial, although the failure-free survival at 3 years in the non-escalated alternating ChlVPP/PABLOE (ChlVPP + prednisolone, doxorubicin, bleomycin, vincristine and etoposide) arm was only 77%, the overall survival at 3 years was 91%.35 A very large trial with long-term follow-up is likely to be required to demonstrate that any other regimen results in better overall survival than this.
Preliminary results of recent regimens for advanced Hodgkin's disease.
| Regimen (Ref) . | No. of Patients . | FFS . | OS . | @ Years* . |
|---|---|---|---|---|
| Stanford V (32) | 94, single center | 89% | 93% | 6 |
| Stanford V (33) | 47, multicenter | 85% | 96% | 6 |
| BEACOPP (34) (escalated) | 403, multicenter | 88% | 91% | 3 |
| Abbreviations: FFS, failure-free survival; OS, overall survival | ||||
| Regimen (Ref) . | No. of Patients . | FFS . | OS . | @ Years* . |
|---|---|---|---|---|
| Stanford V (32) | 94, single center | 89% | 93% | 6 |
| Stanford V (33) | 47, multicenter | 85% | 96% | 6 |
| BEACOPP (34) (escalated) | 403, multicenter | 88% | 91% | 3 |
| Abbreviations: FFS, failure-free survival; OS, overall survival | ||||
Time point for FFS and OS
A further extension of dose-intensification is the use of high-dose therapy in first remission as reported by Carella and colleagues.36 This approach is clearly only appropriate in highly selected patients because most patients will already be cured when they achieve complete remission. Selection is also not straightforward, because most of the poor risk features predict for a reduced complete remission rate and their impact is reduced, albeit not eliminated, once remission is attained.37,38
Summary
The situation in advanced Hodgkin's disease may now be similar to that in localized disease, insofar as more intensive therapies may reduce the treatment failure rate but may not significantly improve overall survival.
The Future
It will be increasingly difficult to demonstrate that new regimens improve overall survival, and multinational collaboration will be required. Most trials are likely to use event-free survival as their end-point, but reliance on this parameter alone must be seriously questioned. Follow-up of trials for extended periods will be required to allow full assessment of long-term side effects, and quality-of-life studies will be necessary. These kinds of studies are exceedingly complex because they need to take into account any salvage therapies as well as the primary therapy. Extended radiation fields such as STNI in localized Hodgkin's disease may result in increased morbidity and possibly more secondary malignancies than a mantle field but they will result in a lower proportion of patients being exposed to full-dose chemotherapy. The more intensive regimens for advanced disease will undoubtedly have greater immediate morbidity and cost and possibly more long-term sequelae, but they will result in fewer patients receiving very high dose therapy with hemopoietic stem cell rescue.
Increasingly patients will be involved in the choice of their therapy, which will be a challenge to the design of clinical trials. Patient attitudes are culturally dependent and may not always correspond to those of their physicians, further complicating treatment choice and trial recruitment.
The early side effects of importance to patients include nausea and vomiting, fatigue and hair loss. Sexual dysfunction and infertility are major problems to some patients, and the next section of this review addresses the issue of fertility. Social and psychological dysfunction following the diagnosis and management of a malignant disease can be profound and the psychological trauma of relapse should not be underestimated, particularly if the patient is not fully prepared for this eventuality. Other long-term sequelae of treatment include cardiac disease and the development of secondary malignancies. The latter topic is dealt with in the final section.
II. Strategies for Fertility Conservation
Roger G. Gosden, Ph.D., D.Sc.,*Togas Tulandi, and Seang-Lin Tan
Division of Reproductive Biology, Dept. of Obstetrics and Gynecology, McGill University, Women's Pavilion (F3-38), Royal Victoria Hospital, 687 Pine Ave W, Montreal, Quebec H3A 1A1, Canada
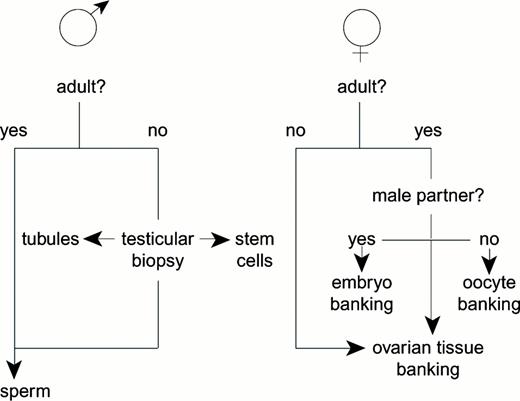
Infertility, premature ovarian failure and hypo-estrogenism are acknowledged side effects of high-dose chemotherapy and abdominal irradiation in patients undergoing curative treatment for malignant diseases. While semen cryopreservation for young men is a routine safeguard for genetic parenting, females have fewer options for fertility conservation and prepubertal boys and girls have none. New strategies, which promise to help more patients, including minors, are now emerging (Table 6). The focus is now on immature germ cells—spermatogonial stem cells, primordial follicles and germinal vesicle oocytes—which can be harvested with less delay, without hormonal priming and at any day of the menstrual cycle.
Theoretical and applied methods of safeguarding future fertility in patients undergoing high-dose cytotoxic treatment.
| Procedure . | Male . | Female . |
|---|---|---|
| Ligand-receptor mediated | GnRHa | GnRHa, steroids |
| Inhibition of apoptosis | - | Bax |
| Surgical | - | Ovarian transposition |
| Cryopreservation | Sperm, spermatogonia | Embryos, oocytes, primordial follicles |
| Abbreviation: GnRHa, gonadotropin-releasing hormone agonists | ||
| Procedure . | Male . | Female . |
|---|---|---|
| Ligand-receptor mediated | GnRHa | GnRHa, steroids |
| Inhibition of apoptosis | - | Bax |
| Surgical | - | Ovarian transposition |
| Cryopreservation | Sperm, spermatogonia | Embryos, oocytes, primordial follicles |
| Abbreviation: GnRHa, gonadotropin-releasing hormone agonists | ||
The Cytotoxic Basis of Infertility
There is ample evidence that ionizing radiation and certain chemotherapeutic drugs, such as alkylating agents and platinum compounds, harm the fertility potential of both males and females.1 While effects on the reproductive system are manifold, the gonads are particularly vulnerable. Destruction of germ cells manifests in males as infertility, usually with undiminished virilization.2 Sterility in females is accompanied by lower serum estrogen levels and the onset of climacteric symptoms because the endocrine and germ cells are united in the developmental units, the follicles.
Cyclophosphamide is one of the most common chemotherapeutic and immunosuppressive agents for neoplastic and autoimmune diseases, and is an example of an agent causing premature reproductive failure. It destroys primordial follicles in the ovary, and this effect can be sufficiently extensive to trigger premature, irreversible ovarian failure.3,4,5,6 Whether there is any genetic risk for future children is still debatable. As yet, follow-up studies of the children of cancer survivors have not revealed an excess risk of congenital abnormalities. On the other hand, it would be surprising if the risk to the germ cells was negligible, and animal studies have indicated that reproductive wastage and genetic damage can be transmitted to offspring, even via the male partner.7,8,9,10,11 Powerful new reproductive technologies, such as intracytoplasmic sperm injection (ICSI), raise potential concerns. They provide a chance of genetic parenthood for male cancer patients who received greater cytotoxic damage and were rendered almost completely azoospermic compared with patients in older studies who spontaneously recovered fertility.
In Vivo Protection of Fertility
There is both clinical and experimental evidence that suppressing gonadotropic stimulation to produce a hypogonadal state can mitigate the sterilizing effects of chemotherapy and radiotherapy. Greater resistance of the ovary to cytotoxic injury before puberty seems to provide prima facie evidence for the hormonal hypothesis, although the advantage of youth is now thought to be mainly due to a larger follicle store rather than a subdued response. Reduced follicle stimulating hormone (FSH) and/or luteinizing hormone (LH) stimulation inhibits entry of spermatogonial stem cells into the expanding and differentiating population, which, it has been supposed, reduces the number of vulnerable cells in mitosis. There is no exact parallel in the ovary, however, because 1) hypogonadotropism does not inhibit follicle recruitment from the primordial pool,12 and 2) primordial follicles are more sensitive to ionizing radiation (and perhaps alkylating agents) than subsequent stages.13 The theory therefore appears to be more robust for males than females. Nevertheless, there is limited clinical and experimental evidence that follicle inhibition, produced either by steroidal contraception14 or gonadotropin-releasing hormone (GnRH) agonists to downregulate the pituitary gonadotrophs, is chemo- and/or radio-protective of the gonad.15 On the other hand, conflicting results have been obtained in animals,16 and there is, as yet, no consensus about the clinical utility of this approach.17,18,19,20,21
An alternative hypothesis has been offered for the mechanism of protecting spermatogenesis by GnRH agonists. Usually, a residue of spermatogonia survives even after high doses of cytotoxins, but they remain dormant and fail to produce progeny for sperm maturation. It has been suggested that the microenvironment may be inappropriate and that high levels of testosterone found in the seminiferous tubules are inhibitory.22 We must bear in mind, however, that if this strategy does assist the recovery of spermatogenesis there is a potential risk of generating more sperm with damaged DNA and mutations. A similar concern arises from attempts to prevent germ cell apoptosis.
In the mouse ovary, the bax gene is crucial for executing the cell death program in follicular oocytes. When bax-/- mice were created by targeted mutagenesis, follicular reserves were found to be larger in old mice than in controls and, moreover, the animals were more resistant to doxorubicin.23,24 In theory, Bax protein inhibitors could prevent early ovarian failure in young patients undergoing sterilizing chemotherapy, but the quality of their oocytes may be compromised.
Radiotherapy to the subdiaphragmatic areas in Hodgkin's disease is usually in the form of an `inverted Y' field that includes the ovaries. In an attempt to protect the ovaries from radiation, the ovary can be transposed to an extra-pelvic site to avoid the radiation field (lateral ovarian transposition). Traditionally, this is done by laparotomy, either as a part of surgical staging when radiotherapy is anticipated or as a separate procedure.25,26,27,28,29 Lateral ovarian transposition by laparotomy is associated with preservation of ovarian function in 83% of patients after pelvic radiation26 and is more effective than transposing the ovaries behind the uterus and protecting them with a lead block.27 In addition, the lead block also may shield affected nodes. Another method is exteriorization of the ovaries under the skin through an opening in the flank. This approach is not widely used and has been associated with ovarian cyst formation.25 A more complicated technique is heterotopic ovarian transplantation.28 In this procedure, vascular anastomosis was performed and the ovary was implanted on the inner face of the arm. Ovarian transposition by laparotomy is associated with a larger abdominal incision, a long hospital stay and an increased risk of adhesion formation and intestinal obstruction.
In 1998, we described laparoscopic ovarian transposition in a 34-year-old woman with rectal carcinoma.29 It is a simple procedure without dissection of the cecum. In this case, we transected the ovarian ligament and transposed the ovaries without cutting the fallopian tubes. The ovaries were positioned laterally and anteriorly to the level of the anterior-superior iliac spines, and we also cryopreserved a portion of the left ovary. Despite a large dose of pelvic irradiation in combination with intrathecal brachytherapy, the patient's menstrual cycles were never interrupted and she continued to menstruate regularly every 28 days. She conceived spontaneously 2 years after surgery and, at the time of writing, is in her third trimester of pregnancy. A similar technique in a 17-year-old female with non-Hodgkin's lymphoma was reported, and 3 months after irradiation she was having regular menses with normal day 3 serum FSH.30 There is, of course, a small risk that the transposed ovary may harbor metastases,31 but this is a very unusual site of spread in Hodgkin's disease.
Low Temperature Banking of Gametes and Germ Cells
Current practices
Sperm cryopreservation is standard for young men prior to sterilizing chemotherapy and irradiation. Given that it is routine, inexpensive and technically undemanding, it is tempting to regard it as an infallible procedure whereas in practice it has shortcomings. For a start, it cannot be used for pre-adolescents. Fertile men sometimes have difficulty producing a specimen under the stress of recent cancer diagnosis and may produce inferior specimens because of pyrexia or other disease-associated effects Moreover, the supply of frozen straws is limited and artificial insemination has a relatively low success rate per cycle even under optimal conditions using donor sperm.32 Donor sperm for conception is generally regarded as a last resort, which is why development of methods for retrieving mature or immature spermatozoa from the male genital tract has been widely welcomed. A testicular biopsy often produces enough sperm for intracytoplasmic sperm injection (ICSI) of their partner's oocytes collected after controlled ovarian stimulation.33
The female equivalent of sperm banking is oocyte cryopreservation, but this is not (yet) a routine procedure. Worldwide, more than 40 babies have been conceived after freeze-thawing oocytes, although, to our knowledge, none of them were born to former cancer patients. Following a few case reports in the 1980s, there were warnings of metaphase spindle defects and hardening of the zona pellucida after cooling human oocytes or exposing them to cryoprotective agents.34 Recently, there has been a flurry of isolated reports of success with oocyte cryopreservation, attributed to ICSI and different cryoprotective agents to overcome hardening and other problems. We shall probably see further improvements in success rates using either or both equilibrium cooling and ultra-rapid freezing (vitrification) for oocytes, but these will not guarantee fertility conservation until in vitro fertilization (IVF) technology makes more efficient use of precious oocytes. Nor, indeed, does embryo cryopreservation provide a full safeguard, even though it is much more established and routine in clinically assisted reproduction.36
Frozen embryo banking was introduced for storing surplus embryos after in vitro fertilization to avoid the need to collect more fresh oocytes by repeating a full treatment cycle.36 A typical cycle requires 4 or more weeks to down-regulate the pituitary gonadotrophs with GnRH agonists followed by controlled stimulation with FSH until the most advanced follicle has a diameter of 18+mm and the oocytes (∼10) can be aspirated for IVF.37,38 There have been a few case reports of pregnancy after embryo banking for cancer patients,39 but several limitations are acknowledged. First, creation of embryos requires a male partner or donor sperm and, second, oocyte collection from prepubertal girls is inappropriate. Third, there are concerns that embryos could become orphaned and, fourth, there may not be enough time to carry out the procedure before chemotherapy begins. And, lastly, the average chances of a live birth pregnancy from a single IVF treatment cycle are not high, judging from UK national statistics for healthy women with fresh embryos (∼11.0%).40 For these reasons, attention is turning to the opportunities of banking immature germ cells from either ovaries or testes.
New opportunities
Frozen banking of spermatogonia and immature ovarian follicles are still at an experimental stage; clinical trials have hardly begun although the potential of these methods for helping patients is clear. While appearing to be new concepts, it was over forty years ago in London that Ruth Deansley, Alan Parkes and Audrey Smith demonstrated that spermatogenesis and follicular maturation resume in rat tissues after freeze-thawing and grafting fragments to an abdominal or subcutaneous site.41,42 With automated freezers and applying modern understanding of cryoinjury, better results can be now be obtained, with the possibility of retrieving sperm or oocytes for assisted fertilization (ICSI) or even restoring natural fertility.
A testicular biopsy can be prepared for banking either as intact seminiferous tubules or after isolation of spermatogonial stem cells. The second strategy requires enzymatic disaggregation of seminiferous tubules to produce a semi-purified suspension of germ cells, including the stem cells. When isologous germ cells were reintroduced by microinjection into the seminiferous tubules or via the rete testis of sterilized laboratory rodents, sperm production resumed a few weeks later,43 and efforts are underway to apply these methods to larger species, including humans.44 What is more, the stem cells restore sperm production after freeze-thawing and transfer.45
Cryopreservation of germ cells is slightly more advanced in females than in males, though it should still be regarded as experimental until the results of prospective trials are announced. Being densely fibrous, the human ovary does not appear ideal for cryopreservation, but this pessimism now seems misplaced, and there is a case report of a woman who ovulated after her cryopreserved ovarian autograft was hormonally stimulated.45 This result follows a substantial number of studies in laboratory and farm animals showing that grafts can function for extended periods and produce normal offspring. Autografting frozen-thawed tissue to the orthotopic site restored ovulatory cycles and potential fertility for at least 22 months in sheep,47 and oocytes have been retrieved for fertilization from heterotopic grafts.48 Cryopreserved grafts were capable of providing long-term fertility, at least in mice, and the offspring were normal.49
Such encouraging results have led to the establishment of ovarian tissue banking in many centers around the world, but we shall have to wait a few more years before the value of this procedure can be gauged objectively.50 Tissue storage does have some limitations. The store of primordial follicles declines with age and the chances of long-term graft function is likely to decline pari passu. It is not possible to define an upper age limit, short of the menopause, though it is likely that banking will turn out to be most successful with children's ovaries. At any age, there will be some patients who are unsuitable for autografting because their cancer has disseminated to the ovaries, and there is a theoretical risk that their original disease will relapse from a transplant.51 Growing oocytes in immunodeficient mice, provided the disease does not kill the host,12,52 might avert the danger. Methods for growing immature follicles in vitro for harvesting oocytes for IVF are still in their infancy.53 The attraction of this approach is that it will be possible to exclude malignant cells during embryo transfer to the uterus and perhaps utilize scarce oocytes more economically. On the other hand, the patient would not be able to enjoy any of the physiological benefits that a hormonal graft might provide, and she would have to continue receiving hormone replacement therapy.
Lastly, there is a small cohort of immature oocytes represented by antral follicles at every stage of the cycle, even at prepubertal ages, which can be retrieved without prior hormonal stimulation. If cryotechnology of germinal vesicle oocytes became sufficiently reliable, in vitro oocyte maturation would provide one more option for fertility. Our group has recently shown that high pregnancy rates can be obtained in women with polycystic ovaries who undergo in vitro maturation and fertilization of oocytes followed by embryo transfer.54,55 It is partly because the number of options emerging for the conservation of fertility (Figure 1) gives us confidence that cancer patients in future need not surrender all hope of genetic parenthood.
Practical and theoretical options for conserving fertility by cryopreservation.
Practical and theoretical options for conserving fertility by cryopreservation.
III. Secondary Neoplasms after Hodgkin's Disease
Steven L. Hancock, M.D.*
Stanford University Medical Center, Department of Radiation Oncology, A089, Stanford CA 94305
Support: This work was supported in part by National Institutes of Health grants no. CA-56060, and CA-63001.
The incidence of secondary neoplasms among survivors of Hodgkin's disease has exceeded the expected incidence of primary tumors in the general population. Death from secondary neoplasms has contributed more than other causes except Hodgkin's disease to the excess mortality rates observed in large populations of treated patients. Experience reported from single institutions and larger population data compiled from multiple centers and cancer registries have confirmed an excess incidence of leukemia, non-Hodgkin's lymphoma, lung cancer, breast cancer, gastric cancer, melanoma, thyroid cancer, and sarcomas of bone and soft tissue in survivors of Hodgkin's disease.1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24 Tucker et al summarized the experience with secondary cancers in the Hodgkin's disease population at Stanford in 1988.16 This study was based upon 83 cases among 1,507 patients treated between 1968 and 1985, representing 9,239 person-years of observation and a mean follow-up of 6.2 years. These data have been updated to include 197 secondary cancers observed in a population of 2162 patients.25,26 The follow-up interval at the time of analysis averaged 9.3 years, representing 20,100 person-years of observation. Treatments varied: 1,141 patients initially received radiation therapy alone, 312 of whom required subsequent chemotherapy for recurrent disease; 848 patients received both chemotherapy and irradiation as initial therapy; 71 received radioactive colloidal 198Au to increase the radiation dose to the liver in advanced stage III or IV disease, with half of these patients also receiving chemotherapy; 102 patients received chemotherapy alone as initial treatment, 27 of whom required subsequent irradiation. The follow-up interval averaged 10.9 years for those exposed only to irradiation, 8.8 years for those who received chemotherapy for recurrence, 8.2 years after initial combined modality, and 5.5 years after chemotherapy alone, due to more recent accrual and higher early mortality related to advanced presentations of Hodgkin's disease. The mean age at treatment for all patients was 29 years and was similar for all treatment groups.
Since the earlier study by Tucker et al, the risk for all cancers relative to general population risk for an initial primary malignancy has risen from 5.2 to 6.4 (Table 7).16 The relative risk for leukemia remained quite high at 37.7. With more prolonged follow-up the relative risk for non-Hodgkin's lymphoma has increased from 18 to 35.3. The relative risk for secondary solid tumors increased from 3.2 to 4.3. Specific solid tumor risks are summarized in Table 8. Solid tumors in which the relative risks have increased included lung cancer (from 7.7 to 10.3), melanoma (from 8.9 to 11.6), and soft tissue sarcoma (from 15 to 24.3). The relative risk decreased, somewhat, for gastric cancer (from 10 to 4.9) and osteogenic sarcoma (from 31 to 12.3), but remained significantly elevated. Several tumors that had not been observed in excess in the earlier analysis developed significant risk elevation with more prolonged follow-up. These include the breast (from 1.8 to 4.1), thyroid (10.6), pancreas (3.9), and salivary glands (37.9). The relative risk for all urogenital cancers combined became significantly elevated at 2.2. However, no specific urogenital site was associated with a significantly increased risk.
Risks for secondary cancers after Hodgkin's disease.
| Tumor Site . | Observed/Expected . | Relative Risk . | 95% Confidence Intervals . | Absolute Risk* . |
|---|---|---|---|---|
| All cancers-Incidence¶ | 197 / 30.8 | 6.4 | 5.5 - 7.3 | 84.4 |
| Leukemia¶ | 38 / 1.0 | 37.7 | 25.7 - 49.7 | 18.4 |
| Acute myelogenous leukemia | 35 / 0.24 | 144.1 | 96.4 - 191.9 | 17.3 |
| Non-Hodgkin's lymphoma | 33 / 0.9 | 35.3 | 23.2 - 47.3 | 16.0 |
| All solid tumors | 126 / 29.4 | 4.3 | 3.5 - 5.0 | 48.8 |
| All cancers-Mortality | 104 / 16.6 | 6.3 | 5.1 - 7.5 | 43.5 |
| Tumor Site . | Observed/Expected . | Relative Risk . | 95% Confidence Intervals . | Absolute Risk* . |
|---|---|---|---|---|
| All cancers-Incidence¶ | 197 / 30.8 | 6.4 | 5.5 - 7.3 | 84.4 |
| Leukemia¶ | 38 / 1.0 | 37.7 | 25.7 - 49.7 | 18.4 |
| Acute myelogenous leukemia | 35 / 0.24 | 144.1 | 96.4 - 191.9 | 17.3 |
| Non-Hodgkin's lymphoma | 33 / 0.9 | 35.3 | 23.2 - 47.3 | 16.0 |
| All solid tumors | 126 / 29.4 | 4.3 | 3.5 - 5.0 | 48.8 |
| All cancers-Mortality | 104 / 16.6 | 6.3 | 5.1 - 7.5 | 43.5 |
Absolute risk is the excess number of observed cancers (or deaths) per 10,000 patients per year according to the formula: Absolute risk = [(observed - expected) / person-years] × 10,000.
Risks for all cancers and for leukemia or myelogenous leukemia exclude 3 cases of myelodysplastic syndrome because this disease is excluded from the general population rates published by S.E.E.R., which was used for these relative risk calculations.
Risks for secondary solid tumors after Hodgkin's disease treatment according to primary site.
| Tumor Site . | Observed/Expected . | Relative Risk . | 95% Confidence Intervals . | Absolute Risk* . |
|---|---|---|---|---|
| Lung/pleura | 36 / 3.5 | 10.3 | 7.0 - 13.7 | 16.2 |
| Breast | 25 / 6.1 | 4.1 | 2.4 - 5.7 | 21.5 |
| Melanoma | 9 / 0.8 | 11.6 | 5.7 - 21.3 | 4.1 |
| Soft tissue (sarcoma) | 8 / 0.3 | 24.3 | 11.3 - 48.3 | 3.8 |
| Bone (osteosarcoma) | 2 / 0.2 | 12.3 | 2.1 - 41.6 | 0.9 |
| Stomach | 8 / 1.6 | 4.9 | 2.3 - 9.4 | 3.2 |
| Salivary gland | 5 / 0.1 | 37.9 | 13.8 - 84.2 | 2.4 |
| Thyroid | 5 / 0.5 | 10.6 | 3.9 - 23.6 | 2.3 |
| Urogenital sites§ | 15 / 6.8 | 2.2 | 1.3 - 2.9 | 4.1 |
| Pancreas | 3 / 0.8 | 3.9 | 1.0 - 10.5 | — |
| Colon or rectum | 6 / 4.7 | 1.3 | 0.5 - 2.7 | — |
| Unknown primary | 3 / 0.9 | 3.4 | 0.8 - 9.2 | — |
| Oral cavity | 1 / 1.5 | 0.7 | 0.0 - 3.3 | — |
| Tumor Site . | Observed/Expected . | Relative Risk . | 95% Confidence Intervals . | Absolute Risk* . |
|---|---|---|---|---|
| Lung/pleura | 36 / 3.5 | 10.3 | 7.0 - 13.7 | 16.2 |
| Breast | 25 / 6.1 | 4.1 | 2.4 - 5.7 | 21.5 |
| Melanoma | 9 / 0.8 | 11.6 | 5.7 - 21.3 | 4.1 |
| Soft tissue (sarcoma) | 8 / 0.3 | 24.3 | 11.3 - 48.3 | 3.8 |
| Bone (osteosarcoma) | 2 / 0.2 | 12.3 | 2.1 - 41.6 | 0.9 |
| Stomach | 8 / 1.6 | 4.9 | 2.3 - 9.4 | 3.2 |
| Salivary gland | 5 / 0.1 | 37.9 | 13.8 - 84.2 | 2.4 |
| Thyroid | 5 / 0.5 | 10.6 | 3.9 - 23.6 | 2.3 |
| Urogenital sites§ | 15 / 6.8 | 2.2 | 1.3 - 2.9 | 4.1 |
| Pancreas | 3 / 0.8 | 3.9 | 1.0 - 10.5 | — |
| Colon or rectum | 6 / 4.7 | 1.3 | 0.5 - 2.7 | — |
| Unknown primary | 3 / 0.9 | 3.4 | 0.8 - 9.2 | — |
| Oral cavity | 1 / 1.5 | 0.7 | 0.0 - 3.3 | — |
Absolute risk is the excess number of observed cancers per 10,000 patients per year according to the formula:
Absolute risk = [(observed - expected) / person-years] × 10,000.
Absolute risks are not specified when 95% confidence intervals for relative risk include 1.0.
Urogenital tumors included cancers of the endometrium (3), cervix (2), ovary (2), vulva (1), prostate (3), testis (1), kidney (2) and bladder (1). Relative risks were not significantly elevated for any specific urogenital tumor site.
The relative risk of developing a secondary cancer at any site was somewhat higher among patients treated with initial combined modality therapy (7.9) than among patients treated with either initial chemotherapy alone or initial radiation alone with or without subsequent treatments for relapse (5.5) (Table 9). As noted in the earlier analysis, the relative risk was highest after treatment with irradiation and colloidal 198Au hepatic infusion (9.9), with or without chemotherapy.16
Secondary cancer risk according to initial treatment for Hodgkin's disease.
| Cancer type / Therapy . | Observed Cases . | Relative Risk . | 95% Confidence Intervals . |
|---|---|---|---|
| All Cancers | |||
| Radiation alone | 81 | 5.4 | 4.2 - 6.6 |
| Radiation + Salvage Chemotherapy | 24 | 6.1 | 3.7 - 8.5 |
| Radiation ± Salvage Chemotherapy | 105 | 5.5 | 4.5 - 6.6 |
| Radiation + Adjuvant Chemotherapy | 67 | 7.9 | 6.0 - 9.8 |
| Radiation + 198Au ± Chemotherapy¶ | 15 | 9.9 | 5.7 - 13.2 |
| Chemotherapy alone† | 11 | 5.5 | 2.8 - 9.8 |
| Leukemia | |||
| Radiation alone | 3 | 6.3 | 1.6 - 17 |
| Radiation + Salvage Chemotherapy | 5 | 39.1 | 14.3 - 87 |
| Radiation + Adjuvant Chemotherapy | 23 | 76.6 | 45.3 - 108 |
| Radiation + 198Au ± Chemotherapy¶ | 3 | 62.8 | 15.7 - 171 |
| Chemotherapy alone† | 4 | 72.1 | 22.7 - 174 |
| Non-Hodgkin's lymphoma | |||
| Radiation alone | 14 | 31.5 | 17.8 - 45 |
| Radiation + Salvage Chemotherapy | 7 | 56.8 | 24.8 - 112 |
| Radiation + Adjuvant Chemotherapy | 11 | 43.8 | 23.7 - 73 |
| Chemotherapy alone† | 1 | 21.1 | 0.0 - 104 |
| Solid Tumors | |||
| Radiation alone | 64 | 4.4 | 3.3 - 5.5 |
| Radiation + Salvage chemotherapy | 12 | 3.2 | 1.7 - 5.4 |
| Radiation + Adjuvant chemotherapy | 33 | 4.1 | 2.7 - 5.5 |
| Radiation + 198Au ± Chemotherapy¶ | 12 | 8.4 | 4.5 - 14 |
| Chemotherapy alone† | 5 | 1.7 | 1.0 - 6.4 |
| Cancer type / Therapy . | Observed Cases . | Relative Risk . | 95% Confidence Intervals . |
|---|---|---|---|
| All Cancers | |||
| Radiation alone | 81 | 5.4 | 4.2 - 6.6 |
| Radiation + Salvage Chemotherapy | 24 | 6.1 | 3.7 - 8.5 |
| Radiation ± Salvage Chemotherapy | 105 | 5.5 | 4.5 - 6.6 |
| Radiation + Adjuvant Chemotherapy | 67 | 7.9 | 6.0 - 9.8 |
| Radiation + 198Au ± Chemotherapy¶ | 15 | 9.9 | 5.7 - 13.2 |
| Chemotherapy alone† | 11 | 5.5 | 2.8 - 9.8 |
| Leukemia | |||
| Radiation alone | 3 | 6.3 | 1.6 - 17 |
| Radiation + Salvage Chemotherapy | 5 | 39.1 | 14.3 - 87 |
| Radiation + Adjuvant Chemotherapy | 23 | 76.6 | 45.3 - 108 |
| Radiation + 198Au ± Chemotherapy¶ | 3 | 62.8 | 15.7 - 171 |
| Chemotherapy alone† | 4 | 72.1 | 22.7 - 174 |
| Non-Hodgkin's lymphoma | |||
| Radiation alone | 14 | 31.5 | 17.8 - 45 |
| Radiation + Salvage Chemotherapy | 7 | 56.8 | 24.8 - 112 |
| Radiation + Adjuvant Chemotherapy | 11 | 43.8 | 23.7 - 73 |
| Chemotherapy alone† | 1 | 21.1 | 0.0 - 104 |
| Solid Tumors | |||
| Radiation alone | 64 | 4.4 | 3.3 - 5.5 |
| Radiation + Salvage chemotherapy | 12 | 3.2 | 1.7 - 5.4 |
| Radiation + Adjuvant chemotherapy | 33 | 4.1 | 2.7 - 5.5 |
| Radiation + 198Au ± Chemotherapy¶ | 12 | 8.4 | 4.5 - 14 |
| Chemotherapy alone† | 5 | 1.7 | 1.0 - 6.4 |
37 of 71 patients treated with radiation and 198Au received chemotherapy; 10 of 15 cancers arose in patients with both chemotherapy and radiation exposure.
26 of 102 patients initially managed with chemotherapy alone had subsequent irradiation; 3 of 9 second cancers arose in patients with both chemotherapy and radiation exposure.
Secondary Leukemia
In the more recent data from Stanford, 35 of the 38 cases of leukemia were of the acute myelogenous or myelomonocytic type (AML). Since earlier analyses of leukemia risk,27 the relative risk for AML has become significantly increased after radiation alone with two reported cases (17.5; 95% C.I.: 2.9-58). Chemotherapy exposure conferred higher leukemia risks. The relative risk was 126 (95% C.I.: 39.9-305) for patients who received chemotherapy for recurrent Hodgkin's disease after initial radiation, 290 (95% C.I.: 169-412) for those managed with combined modality therapy, 281 (95% C.I.: 70-766) after radiation and colloidal 198Au, and 360 (95% C.I.: 113-868) after chemotherapy alone. Twenty-nine cases of AML arose among 922 patients who received MOPP. Four cases developed among 286 patients who received procarbazine, melphalan, and vimblastine (PAVe) with irradiation. Myelodysplastic syndrome developed in two patients after initial combined modality therapy and in one patient treated with irradiation alone.
Many studies have implicated chemotherapy regimens containing alkylating agents or epipodophyllotoxins as the primary factor determining the risk of secondary leukemia after Hodgkin's disease therapy.3,5,7,10,12 ,14,15,16 ,21 Among the patients treated at Stanford with irradiation alone, 82.3% received total lymphoid irradiation or subtotal lymphoid irradiation, in which the external iliac, inguinal and femoral lymph node groups were omitted from treatment. Despite exposure of a substantial proportion of the marrow cavities in the axial skeleton to relatively high radiation doses, radiation has been a comparatively weak leukemogen. In a study from the BNLI Swerdlow et al reported a relative risk for acute leukemia of 2.5 (95% C.I.: 0.1-14.1) after treatment with radiation alone, 27.9 (95% C.I.: 12.7-52.9) after chemotherapy alone and 21.5 (95% C.I.: 7.9-46.8) after combined chemotherapy and irradiation.21 In an analysis from the International Database on Hodgkin's Disease Henry-Amar reported no significant increase in the risk of acute leukemia after localized, regional or extended field irradiation of Hodgkin's disease (RR 1.0, 1.2 and 1.3, respectively).19 The relative risk of leukemia was increased with chemotherapy alone (3.5 for MOPP-like regimens and 4.3 for other regimens) and was higher with combined radiation and MOPP-like chemotherapy regimens (5.3). Among 1,939 patients treated for Hodgkin's disease in the Netherlands, Van Leeuwen et al found no significant increase in the relative risk of leukemia after treatment with radiation alone (3.1; 95% C.I.: 0.1-17.3).12 The risk was 93.4 with initial chemotherapy only (95% C.I.: 25.4-239), 50.6 with initial irradiation and chemotherapy (95% C.I.: 18.6-110) and 51.7 for initial irradiation and chemotherapy at relapse (95% C.I.: 19.0-113). In a subsequent case-control analysis from this population, Van Leeuwen et al found no evidence that radiation use in combined modality treatment regimens increased the risk of acute leukemia above that associated with chemotherapy alone.3 The extent of the irradiation fields added to chemotherapy made little difference. The relative risk was 1.1 (95% C.I.: 0.15-7.8) with treatment of involved fields only, 0.67 (95% C.I.: 0.08-6.0) with sub-total lymphoid irradiation, and 2.5 (95% C.I.: 0.32-18.8) after total nodal irradiation.12 Similar conclusions regarding the lack of apparent interaction between irradiation and subsequent leukemia risk were reached by Kaldor et al in an earlier analysis derived from analysis of 163 cases of leukemia among 29,552 patients with Hodgkin's disease.14 A contrary finding was reported by Andrieu et al, who found a low 2.2% actuarial risk of leukemia with MOPP chemotherapy alone, or combined with mantle, supra-diaphragmatic irradiation and a 9.0% risk for those who received inverted-Y, sub-diaphragmatic irradiation, sub-total lymphoid, or total lymphoid irradiation.28 Splenectomy was associated with a higher risk of leukemia in some analyses3,8,11,12,14 but not in others.22 This potential risk factor could not be assessed in the Stanford data because staging laparotomy was routinely employed during the period in which chemotherapy regimens based upon alkylating agents were commonly used. As has been reported in most studies, the peak incidence of secondary leukemia occurs in the first decade after therapy with risk declining after 10 years.3,14,16,19,21 In the Stanford series, all cases of leukemia developed from 1 to 12.5 years after therapy.
Non-Hodgkin's Lymphoma
In contrast to acute leukemia, the risk of developing a non-Hodgkin's lymphoma persists or increases during the second decade after treatment for Hodgkin's disease and appears to be relatively independent of the type of initial therapy or total treatment exposure.12,16,17,19,22,29,30 Among patients treated at Stanford, the relative risk of a secondary non-Hodgkin's lymphoma was 31.5 after treatment with irradiation alone, 56.8 after radiation and chemotherapy at relapse, and 43.8 after initial chemotherapy and irradiation, but was not significantly elevated in the small cohort managed with chemotherapy alone (21.1). In the BNLI data, the risk for non-Hodgkin's lymphoma after Hodgkin's disease was the same for cohorts of relatively comparable size whether treated with chemotherapy alone, radiation and chemotherapy, or radiation alone.22,29 The risk for secondary non-Hodgkin's lymphoma is thought to relate to compromised immune function associated with Hodgkin's disease and its therapy, because non-Hodgkin's lymphomas are also seen with increased frequency in other illnesses associated with significant immune compromise.17
Secondary Solid Tumors
The increased risk of developing a secondary solid tumor has generally been attributed to the direct effects of irradiation. Some of the tumors have arisen within or at the margin of irradiated tissue. The latency period between treatment and the development of a solid tumor has tended to be prolonged, which is consistent with theories of radiation carcinogenesis.17 Several studies have identified extended field irradiation and young age at treatment as major factors contributing to solid tumor risk after treatment.1,17,19 In the population of patients treated at Stanford, the relative risk of solid tumors tended to be highest for patients treated with radiation therapy and colloidal 198Au hepatic infusions with or without chemotherapy.16,25 The relative risk of a secondary solid tumor was 8.4 in this group (95% C.I.: 4.5-14). Six of the 12 solid tumors that have developed in this small population were lung cancers. The high risk for lung cancer observed in this group has been attributed to uptake of the colloidal 198Au by the reticuloendothelial cells in the lung.16 The relative risk for a secondary solid tumor was nearly comparable for treatment with irradiation alone (4.4; 95% C.I.: 3.3-5.5), initial irradiation with chemotherapy at relapse (3.2; 95% C.I.: 1.7-5.4) or initial chemotherapy and irradiation (4.1; 95% C.I.: 2.7-5.5). The risk of a secondary solid tumor was not significantly elevated in the small cohort of patients treated with chemotherapy alone (1.7; 95% C.I.: 1.0-6.4). Swerdlow et al have reported similar relative risks for lung cancers and other solid tumors among cohorts of patients treated with chemotherapy alone, extensive radiotherapy, or combined modality therapy in the BNLI experience.22 In a case-control study derived from population-based cancer registries and cancer centers, Kaldor et al found that patients treated with chemotherapy alone for Hodgkin's disease had twice the risk of secondary lung cancer of those treated with radiotherapy alone.31 Boivin et al studied secondary neoplasms identified among Hodgkin's disease patients from 14 cancer centers in the United States and Canada.8 They found that chemotherapy exposure was associated with a significantly increased risk of respiratory or intra-thoracic tumors in the first 5 years after therapy (2.2; 95% C.I.: 1.1-4.3). Radiation exposure was associated with an increased relative risk of lung cancer only in the cohort at risk more than 10 years beyond therapy (2.7; 95% C.I.: 1.1-6.8). In an analysis of Hodgkin's disease patients in the Surveillance, Epidemiology, and End Results (SEER) Program Travis et al identified an increased relative risk for lung cancer of 2.68 for patients treated initially with chemotherapy alone, 3.09 after treatment with radiation alone, and 3.03 after initial irradiation and chemotherapy.32 However, the comparability of risk remains in question in this study because information on subsequent therapies was not available to clearly evaluate risk in those patients who never received irradiation.
Lung cancers or pleural malignancies have been the most common secondary solid tumor observed among patients treated for Hodgkin's disease at Stanford.25 The relative risk was 10.3 (95% C.I.: 7.0-13.7). Cigarette smoking was implicated as a probable cofactor in secondary lung cancers in the earlier analysis of the Stanford experience and in cases studied by List et al.16,33 In a case-control study from the Netherlands, Van Leeuwen et al reported a relative risk of lung cancer of 4.1 (95% C.I.: 0.48-36) after irradiation when compared to patients treated with chemotherapy alone.34 Lung cancer risk increased with radiation dose to the lung and with exposure to cigarette smoke. Among the smoking variables evaluated, the number of pack-years smoked after diagnosis of Hodgkin's disease was most closely correlated to increased lung cancer risk. Among patients who received a radiation dose to the lung of 5 Gy or more, those with less than 1 pack-year of smoking had a relative risk for lung cancer of 2.5 (95% C.I.: 0.21-29.4). Those reporting greater than one pack-year of smoking after diagnosis had a relative risk for lung cancer of 9.1 (95% C.I.: 0.78-106). Chemotherapy exposure was not clearly associated with increased lung cancer risk in this study.
Secondary breast cancer has emerged as a significant concern for women after treatment of Hodgkin's disease and appears most closely associated with exposure of breast tissue to irradiation at young age.5,8,9,12 ,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 In the Stanford experience, women who were treated for Hodgkin's disease before 20 years of age had a relative risk of developing breast cancer that exceeded 19 and risk declined with increasing age.35 No excess risk was identified among women treated after 30 years of age. The risk of developing breast cancer within 15 years of treatment was significantly increased for those treated with MOPP chemotherapy with radiation (relative risk 6.3; 95% C.I.: 3.1-11.6). Early risk was not significantly increased among those treated with radiation alone (relative risk 0.8; 95% C.I.: 0.1-2.5). However, risks appeared comparable beyond 15 years for those treated with irradiation alone or with chemotherapy and irradiation. In a recent update, 50 cases of breast cancer were identified among 1,064 women who had been treated for Hodgkin's disease at Stanford.37 The relative risk for secondary breast cancer was 4.7 (95% C.I.: 3.4-6.0). The relative risk became significantly increased between 5 and 9 years after therapy (2.9; 95% C.I.: 1.1-5.9) and rose dramatically between 15 and 19 years after treatment (11.1; 95% C.I.: 6.4-15.8). Within 15 years of treatment the relative risk was not increased for women after irradiation alone (1.8; 95% C.I.: 0.6-3.9), but was significantly increased for women who received irradiation with MOPP or PAVe (3.7; 95% C.I.: 1.8-6.8). This trend to increased risk with chemotherapy exposure persisted for women who were more than 15 years beyond therapy with a relative risk of 12.1 (95% C.I.: 6.6-17.3) after irradiation and MOPP or PAVe chemotherapy, compared to 8.8 (95% C.I.: 5.1-10.9) after irradiation alone. The importance of young age at exposure in determining risk of secondary breast cancer in women was also observed in studies by Mauch et al from Harvard, who found an excess relative risk of breast cancer confined to women treated before 25 years of age, and by Bhatia et al in analysis of secondary cancers among children treated for Hodgkin's disease in the Late Effects Study Group cohort.40,41 The prolonged latency of breast cancer risk was also reported by Van Leeuwen et al, who observed significantly increased risk confined to women who were followed more than 15 years after irradiation.12 Although many of the breast cancers observed after treatment in the Stanford study developed at prolonged latent intervals, 80% of the women affected were under 50 years of age at the diagnosis of a secondary breast cancer.38 The use of mammography has been controversial for screening individuals in the general population before 50 years of age. However, 12 of the secondary cancers observed in women at Stanford were identified solely by mammography performed before 50 years of age. Dershaw et al have also reported a favorable rate of identification of breast cancer using mammography in young women who had been treated for Hodgkin's disease.50
The risk for malignant melanoma was elevated in the recent and previous Stanford experience as well as in reports of several other large populations of patients treated for Hodgkin's disease.1,5,9,16,17 Tucker et al analyzed eight melanomas arising in six patients who had been treated for Hodgkin's disease.51 All appeared to be invasive tumors, and most arose within 5 years of treatment. Melanomas appeared to develop equally in irradiated or in unirradiated sites. They showed little histologic evidence of a host immune response, similar to the melanomas observed in renal transplant recipients.52 Although these findings suggested that compromised host immunity may contribute to the increased risk of melanoma, five of the patients displayed manifestations of dysplastic nevus syndrome, which predisposes to malignant melanoma.
Late thyroid cancers have been infrequently observed and seem to feature a prolonged latent interval after Hodgkin's disease therapy.53 Radiation exposure at young age has been clearly implicated in the risk of this neoplasm.54 A relative risk of 16 was observed in the predominantly young adult population treated for Hodgkin's disease at Stanford. In a recent update of secondary cancers in 697 patients treated before 21 years of age at Stanford, Wolden et al reported a relative risk of thyroid cancer of 9.7 (95% C.I.: 2.4-26.4) based upon three cases that arose between 8.2 and 10.7 years after therapy.37 A somewhat higher relative risk of 68 was observed among children treated for Hodgkin's disease in the Late Effects Study Group cohort.54
The further increase in the relative risks of second cancers in the more recent Stanford data derives from additional person-years of observation at prolonged intervals from Hodgkin's disease treatment. Treatments have evolved substantially over the past 40 years. In the early 1960s most patients received aggressive primary radiation treatments alone, which were typically administered at a rate of 2.2 to 2.75 Gy per fraction to one of two opposed fields each day to a total dose of 44 Gy or more. Daily radiation doses for Hodgkin's disease have decreased to 1.5 to 1.8 Gy with normal tissue doses equalized and minimized by treating both opposed fields each day. Most patients now receive limited field irradiation to lower total radiation doses—particularly in younger patients who have had the highest risks for radiation-induced tumors. Chemotherapy has also evolved from alkylating agent-intensive regimens to combinations that appear to be less damaging to bone marrow and fertility. It is hoped that such regimens may cause fewer adverse effects in other normal tissues.55,56,57,58,59,60 However, the risk of developing a secondary neoplasm after treatment of Hodgkin's disease has changed modestly. Among 1,458 patients treated for Hodgkin's disease at Stanford between 1960 and 1980, the actuarial freedom from developing a secondary cancer within 15 years of treatment was 85.9% ± 1.1% compared with 87.1% ± 1.7% for 1,216 patients treated since 1980.
The relative stability of the secondary neoplasm risk after therapy for Hodgkin's disease has led some to search for germ-line abnormalities that may pre-dispose to malignancy. Ben-Yehuda et al reported increased microsatellite instability and a relatively high (38%) rate of p53 gene mutations in a small cohort of individuals with therapy-related secondary leukemia.61 This finding suggested a possible “mutator” phenotype that would predispose to an increased risk of malignancies. Felix et al observed the contrary in a cohort of children with therapy-related secondary leukemia.62 One of 19 children showed evidence of a germ-line p53 mutation that might influence the risk for neoplasia. Otherwise, p53 mutations appeared to be infrequent. Among 11 patients who developed secondary lung cancer, De Benedetti et al identified four mis-sense and two silent p53 point mutations in five patients.63 The authors reported that the pattern of mutational change observed seemed more typical of responses to oxidative injury or radiation than to smoking. The increased frequency of secondary neoplasms has suggested the possibility of a more radiation-sensitive phenotype to some investigators. Nichols et al searched for evidence of heterozygous germ-line mutations in the ataxia-telangiectasia gene but found no cases in 52 patients who had developed one or more neoplasms after treatment of Hodgkin's disease.64 Thus far, no intrinsic genetic factor has been identified to explain the increased risk of secondary neoplasms after Hodgkin's disease.
The prevalence of secondary tumors underscores the importance of continued careful follow-up for all individuals after treatment of Hodgkin's disease. Follow-up care should include aggressive programs to minimize smoking and careful screening programs, such as breast self-examination and mammography in women irradiated at young age, thyroid examination, skin examinations and periodic chest imaging to identify secondary tumors when they have the greatest potential for cure.