Abstract
The front-line management of stage IV indolent non-Hodgkin's lymphoma has ranged from the watch-and-wait approach to intensive experimental regimens such as high-dose chemotherapy and bone marrow transplant. With this broad spectrum of regimens to choose from the decision has become a challenging exercise for both patients and oncologists. With the recent introduction of new agents such as rituximab, fludarabine, and combinations based on these, the management of relapsed cases can be similarly confusing. More aggressive approaches such as high-dose chemotherapy with autologous bone marrow transplant and more recently allogeneic bone marrow transplant have also been used. Recently the technique of “mini-allo transplants” has been introduced. It utilizes a less myelosuppressive conditioning chemotherapy regimen based on fludarabine which is immunosuppressive enough to allow engraftment of the donor marrow. Since it is less myelotoxic it is better tolerated, and this has allowed us to significantly extend the age cut-off for allogeneic transplants. All these advances provide us with a more extensive armamentarium, but at the same time they confront physicians with new challenges in choosing from a large and continuously growing therapeutic menu.
In this review of the alternative therapies a panel of three expert hemato-oncologists each discuss their approach to the management of a 49-year-old patient with a relapsed indolent follicular lymphoma. Dr. Horning discusses the traditional alternatives available for this patient such as standard chemotherapy combinations or the watch-and-wait approach in Section I. In Section II, Dr. Kaminski reviews the different therapeutic monoclonal antibody options such as rituximab, Bexxar (Iodine-labeled anti-CD20) and Ytrium-labeled anti-CD20 antibody. Allogeneic transplants are increasingly more popular for the treatment of indolent lymphomas because they can provide an immune-mediated graft-versus-lymphoma effect. In Section III, Dr. Richard Champlin reviews various transplant options including autologous, allogeneic and mini-allogeneic transplants.
Case Presentation
Fernando Cabanillas, M.D.*
Department of Lymphoma-Myeloma, M.D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston TX 77030-4009
A 49-year-old male patient presents to you in consultation with a history of having been treated for a stage IV-A follicular center cell grade I non-Hodgkin's lymphoma (NHL) (follicular small non-cleaved cell) with bone marrow involvement. His treatment had consisted of six courses of CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) in April 1997. He had achieved a complete remission but relapsed in a neck node in June 2000. A biopsy done in July 2000 revealed follicular center cell grade II NHL (follicular mixed lymphoma). After you interview him, you don't find any abnormalities on examination and he doesn't have any constitutional symptoms. He has two brothers and one sister, all healthy. You order restaging to be performed including chest x-ray, CT scan of abdomen/pelvis, and bilateral bone marrow biopsies. Routine blood work reveals a normal complete blood count, lactic dehydrogenase and β-2 microglobulin. The CT scan reveals enlarged nodes in the retroperitoneum, the largest being 3 cm in size, the chest x-ray is normal, and the bone marrow biopsy is negative bilaterally.
What would be your next step in management?
Watch and wait
FND (fludarabine, mitoxantrone, dexamethasone)
Rituximab
Radiolabeled anti-CD 20 monoclonal antibody
Autologous bone marrow transplant
Allogeneic bone marrow transplant if one of the siblings is HLA identical
“Mini” (non-myeloablative) allogeneic bone marrow transplant if one of the siblings is HLA identical
The indolent B-cell non-Hodgkin's lymphomas for the most part consist of the grade I-II follicle center lymphomas, the small lymphocytic lymphomas and lymphoplasmacytoid lymphomas, which frequently present as Waldenström's macroglobulinemia. These entities were classified in the Working Formulation as “low-grade NHL,” which we now refer to as “indolent NHL” to avoid confusing histological with clinical grading. They are characterized by slow growth and a propensity to present with advanced Ann Arbor stage III-IV disease. More recently, another group of disorders known as the marginal zone lymphomas has been added in the Revised European American Lymphoma (REAL) Classification that can also be considered as indolent NHLs. This group includes the splenic marginal zone, the monocytoid B-cell, and the extranodal MALT lymphomas. In contrast to most other indolent NHLs, the MALT lymphomas usually present with localized disease; the most common of these is the gastric MALT lymphoma. The treatment of patients with indolent NHLs represents one of the more controversial issues in hematology. The controversy stems from their rather prolonged survival, which ranges from 7-9 years, despite the apparent incurability of their disease when they present with stage IV disease. The management of relapsed indolent NHL represents another even more challenging and controversial area because of the many new options currently available. With the introduction of the purine nucleoside analogues and effective new combinations based on these, as well as newer agents such as rituximab, 131I-tositumomab (Bexxar) and IDEC-Y2B8 (Zevalin), the management of relapsed cases can be disconcerting. More aggressive approaches such as high-dose chemotherapy with autologous bone marrow transplant (BMT) and allogeneic BMT have also been used. Recently the technique of “mini-allo transplants” was introduced. It utilizes a non-myeloablative conditioning chemotherapy regimen based on fludarabine, which can be immunosuppressive enough to allow engraftment of the donor marrow. Since it is less myelotoxic than other approaches, it is better tolerated and this has allowed a significant extension of the age cut-off for allogeneic transplants. All these advances provide us with a more extensive armamentarium but at the same time they confront the hematologist-oncologist with new challenges in choosing from a large and continuously growing therapeutic menu.
I. The Case for Conventional Management
Sandra J. Horning, M.D.*
Division of Oncology, Stanford University Medical Center, 1000 Welch Road, Suite 202, Palo Alto CA 94304-5756
Chemotherapy has long been the mainstay of treatment for indolent follicular lymphoma, which characteristically presents in advanced stage. The timing of treatment and the choice of individual drugs or combinations require a thorough understanding of the natural history of the disease and important differences in clinical presentations. The goals of treatment are to extend longevity and promote quality of life through reduction of symptoms or signs of the disease and its treatment. Current disease management is predicated on the following factors: 1) lack of defined curative therapy for indolent lymphoma; 2) relatively long natural history with median survivals of 6-12+ years; 3) side effects associated with therapy that reduce quality of life; and 4) cumulative effects of therapy that may cause late adverse effects.
Natural History and the Timing of Treatment
The case in point, involving a 49-year-old man with recurrent follicular lymphoma, causes one to ask the question, is the natural history different in young patients? We have recently studied a group of 188 patients less than 50 years old with advanced indolent follicular lymphoma.1 High tumor burden, according to the criteria of the Groupe d'Etude des Lymphomes Folliculaires (GELF) and serum lactate dehydrogenase (LDH) were identified as adverse prognostic factors.2,3 Achievement of a complete response or equivocal complete response (CR or CRe), 70% in this series, was associated with significantly longer survival. At 10 years, 63% of patients were alive, but this proportion varied from 82% for CR/CRe patients with < 2 risk factors (median survival 18 years) to just 12% for patients with both risk factors for whom therapy failed to achieve CR/CRe. In this series the age-adjusted International Index was not significant in multivariate analysis. Although many investigators have found the International Index useful to separate indolent lymphoma patients into different prognostic categories, the very small proportion with low- or high-risk disease diminishes its clinical utility.4 In the less well studied setting of recurrent disease, these prognostic factors and others apply, including the depth of initial remission measured in time as well as completeness and the characteristics of the tumor burden and rate of growth. Just as at presentation, there are recurrent disease settings that may be managed expectantly whereas others suggest the need for more aggressive treatment, possibly associated with greater risk.
Although we lack all the presenting features in our young patient, we do know that he 1) achieved CR with initial treatment, 2) enjoyed a remission of more than 3 years, and 3) has small volume disease at relapse. It should be noted that deferred treatment is an option upon relapse as well as at diagnosis. That is, each and every manifestation of disease need not be treated. At diagnosis and upon recurrence, a significant subset of patients will be asymptomatic and will have a low tumor burden. Deferral of treatment in a large number of such patients at Stanford University was associated with no adverse impact on survival.5,6 Subsequently, two randomized clinical trials have confirmed this observation.2,7 The survival curves for selected patients with advanced stage indolent lymphoma randomized to ProMACE-MOPP chemotherapy plus low-dose total lymphoid irradiation versus watchful waiting may be essentially superimposed at 10 years (Longo, personal communication 1999). Similarly, the GELF found no difference in overall survival or the incidence of histologic transformation in low tumor burden patients randomized to no initial therapy, prednimustine or interferon.2 Therefore, in a non-study setting, conservative management of indolent follicular lymphoma should be considered both as an initial strategy and upon relapse(s).
Choice of Chemotherapy Treatment
The alkylating agents chlorambucil and cyclophosphamide have been the backbone of chemotherapy for indolent lymphoma for several decades. Studies performed in the 1970s at Stanford University demonstrated that daily chlorambucil and cyclic CVP (cyclophosphamide, vincristine, prednisone) resulted in similar time to progression and overall survival.8 The time to response was shorter with combination therapy, suggesting a role for this approach in symptomatic patients or those with bulky disease. Certainly the ease of oral therapy and lack of alopecia recommends chlorambucil, which can be easily and effectively given in a cyclic fashion at 16 mg/m2.
In contrast to aggressive lymphoma, mature results of Southwest Oncology Group (SWOG) studies with CHOP chemotherapy do not indicate a therapeutic superiority for doxorubicin in indolent lymphoma.9 Although there is a theoretic advantage in a lower cumulative dose of alkylating agents with CHOP, this is likely offset by the greater incidence of cardiac damage, which can be significant in patients receiving 8 cycles of treatment.10 Through the years, the question of choice of regimen on the basis of histologic subtype has been raised. This query emanated from a small National Cancer Institute study suggesting improved outcome with CMOPP (cyclophosphamide, vincristine, procarbazine, prednisone) chemotherapy in follicular mixed lymphoma.1 However, this observation was not verified in a subsequent Eastern Cooperative Oncology Group (ECOG) study, and the SWOG experience with CHOP did not demonstrate prolonged remission in any histologic subtype.9,12 In fact, multiple studies have failed to show a difference in natural history or response to treatment according to histologic subtype, now designated Grade I and II in the World Health Organization Classification.4 In my opinion, follicular lymphomas represent a clinical and pathologic spectrum. Pathologic features of a greater proportion of large cells and diffuse areas and clinical features of constitutional symptoms, high tumor burden, and elevated LDH suggest a more aggressive disease process and may be appropriately treated with a doxorubicin-containing combination.
The purine analogs represent an effective, non-crossresistant alternative to alkylating agent therapy. Fludarabine has been studied as a single agent and also in combination therapy.13,14,15,16 Two randomized trials comparing fludarabine and alkylating agents have been presented in abstract form.17,18 Although these studies suggested the superiority of fludarabine as a single agent, both the European and the Canadian studies unfortunately included standard arms in which the alkylating agents were used in low, sub-optimal doses. Thus the results were not impressive on either study arm. Of interest, the European study included a no initial treatment option prior to the institution of chemotherapy. Response rates and overall survival were not different among patients treated immediately or expectantly.
McLaughlin and colleagues reported a remarkable CR rate of 47% with the combination of fludarabine, mitoxantrone, and dexamethasone (FND) among patients with relapsed follicular lymphoma. A group of European centers was unable to confirm this result, possibly related to the fact that a larger proportion of patients in this series was resistant to chemotherapy (Rohatiner, personal communication, May 2000). In a study in previously untreated patients, the SWOG found a projected disease-free survival of 63% at two years with fludarabine and mitoxantrone, a result nearly identical to previous outcomes with CHOP chemotherapy.19 These data demonstrate the need for confirmatory studies and randomized trials to establish a therapeutic advance in follicular lymphoma. Combinations of alkylating agents and fludarabine have also been studied in indolent lymphoproliferative disease. Following on a promising phase I/II experience in which 89% of patients achieved a CR, the ECOG launched a phase III trial comparing fludarabine (25 mg/m2 d 1-5) plus cyclophosphamide (1000 mg/m2 d 1) with CVP.15 Unfortunately, due to excess deaths on the fludarabine arm, the study was prematurely closed.
Fludarabine is associated with significant immunosuppression and myelosuppression. The former leads to an increased incidence of opportunistic infection such that prophylaxis against Pneumocystis carinii is indicated. Newer immunotherapy treatments such as vaccines or adoptive immunotherapy may be rendered ineffective after fludarabine. Fludarabine also contributes to infectious risks and may result in difficulty in collecting sufficient numbers of CD34+ cells, if required, to support high dose treatment.
Combination Chemotherapy and Immunotherapy
Numerous phase III trials have evaluated the combination of chemotherapy with interferon. The results are inconclusive, perhaps related to the variables in chemotherapy, interferon, and the patient population.20 More recently, there have been small studies utilizing chemotherapy to achieve remission in advance of idiotype vaccine.21,22 The encouraging results from these experiences recommend further study in phase III trials, now in progress. In a case presentation questionnaire distributed nationally, 19% of respondents selected a combination of CHOP and rituximab for treatment of first relapse in a 68-year-old woman. The surprising popularity of this combination is based on an experience in nine patients with recurrent disease and 31 individuals with untreated disease.23 CHOP plus rituximab was well tolerated with promising results in a small number of patients. In my view, this combination is an appropriate choice in the context of a clinical trial or in situations where a response is needed and prior treatments have proved ineffective.
The Effects of Chemotherapy on Quality of Life
In their 1998 paper, Webster and Cella state, “...since cure remains elusive, and since many patients with low grade lymphoma may have few or even no symptoms (initially), the decision about whether or not to initiate treatment logically must include quality-of-life issues.”24 Quality-of-life issues for patients with indolent follicular lymphoma include disease symptoms, toxicity of treatment, fear of transformation, increased risk of second cancer, treatment decision making, chronic and late effects of treatment, and psychosocial issues. Psychosocial issues include fear of relapse, living with incurable disease and uncertainty, depression and anxiety, disruption in family and social functioning, employment difficulties, and financial burden. Although no quality-of-life data from large randomized trials in low-grade lymphoma are available, a subset analysis of lymphoma patients drawn from a group with mixed cancers showed an adverse impact of chemotherapy on quality of life. Patients, however, are often willing to trade off quality of life, accepting significant risks, for modest increases in disease-free interval or overall survival. For indolent follicular lymphoma, a disease that does not often produce debilitating symptoms, the advantage provided by a longer time to progression may be a combination of psychological factors and freedom from chemotherapy.
Late Sequelae of Chemotherapy
Secondary leukemia has been associated with higher cumulative doses of alkylating agents. Radiation therapy to large volumes may add to this risk. In the series of 188 young follicular lymphoma patients managed at Stanford University, the median number of cycles of alkylating agent–based chemotherapy was nine.1 Two patients developed secondary leukemia and both of these had received extensive radiotherapy as well. Myelodysplastic changes and an increased risk of leukemia have also been associated with fludarabine.25 Cumulative myelosuppression may complicate the delivery of sequential therapies over time and obviate the ability to collect an adequate number of hematopoietic stem cells for autotransplantation. Further, extensive prior treatment resulting in myelosuppression or a significant cumulative dose of doxorubicin may compromise the ability to treat histologic transformation of disease or participate in clinical trials. For young patients, infertility may be a primary concern of treatment. All patients are at risk for possible reduction in performance status or chronic fatigue that may accompany multiple courses of chemotherapy.
Management Philosophy
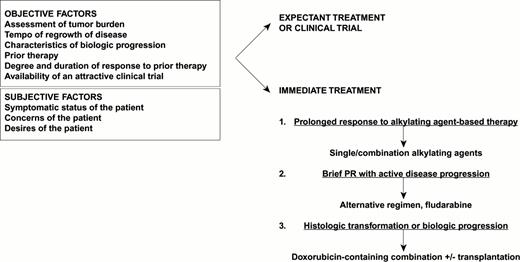
An algorithm for the treatment of recurrent lymphoma is presented in Figure 1. At the time of relapse, it is important to note the following: 1) symptomatic status of the patient, 2) assessment of tumor burden, 3) tempo of regrowth (based on previous assessment of active disease sites), 4) presence or absence of characteristics suggesting transformation or biologic progression (LDH, asynchronous growth in prior disease sites, histologic evidence of progression), 5) prior therapy, 6) degree and duration of response to prior therapy, and 7) availability of an attractive clinical trial.
Dr. Sandra Horning's algorithm for chemotherapy management of recurrent follicular indolent NHL.
Dr. Sandra Horning's algorithm for chemotherapy management of recurrent follicular indolent NHL.
The most important issues to address are whether further treatment should be given based on the considerations above and the desires of the patient. For patients with bulky disease, combination chemotherapy will yield a more rapid response and is more likely to yield a complete or partial response than antibody treatments. Patients who enjoyed a prolonged initial response to alkylating agent–based treatment may be retreated, whereas those with brief partial responses and active, progressive disease should move on to another treatment such as fludarabine. Histologic evidence of transformation or clinical suspicion of more aggressive disease indicate the need for more intensive treatment with doxorubicin-containing chemotherapy, possibly as a prelude to transplantation. Infrequently, persistence or recurrence of disease in an isolated anatomic region argues for the use of radiotherapy.
For our patient, I would recommend observation or participation in a clinical trial if I felt that the latter had a high likelihood for benefit and minimal to modest risk. If the patient was not comfortable with observation and no such trial was available, I would consider rituximab because of its excellent safety profile and a projected response rate of 60% for this patient. There is little question about the positive effect of treatment on the course of follicular lymphoma but the question as to whether any treatment significantly modifies the ultimate duration of disease remains. The art of practicing medicine is a very important aspect of both the timing and selection of treatment for indolent follicular lymphoma.
II. The Case for Rituximab or Radiolabeled Anti-CD20 Antibodies
Mark S. Kaminski, M.D.*
University of Michigan Cancer Center, 1500 E Medical Center Drive, 4-316 CCGC, Ann Arbor MI 48109-0936
Treatment recommendations for any patient in this situation encompass a tremendously wide range of options. Given this range, these recommendations must be tailored to the patient's particular situation and desires. In the case under discussion, a convincing argument can be made to support a watch-and-wait approach (or, as I prefer to call it, a “watch and live” approach) because there do not appear to be any compelling circumstances that would demand immediate treatment (such as symptoms, current or impending involvement of a critical organ, or signs of rapid progression of disease that would suggest histologic transformation). In addition, this patient's case is typical of the expected natural history of the disease, with no signs indicating a worse prognosis than the average.
However, this patient may not be comfortable with this approach for a variety of reasons, including psychological concerns of leaving the disease untreated, despite counseling by the physician about the lack of clearly identifiable curative options and the potential of risks and decreased quality of life associated with treatment. We must remember that this patient is young and that if no innovations emerge that can alter the expected outcome, his median survival is now about 4 years.26
Thus, this patient is likely to ask about newer options other than standard-dose chemotherapy. Even with the introduction of newer drugs like fludarabine in the last several years, it is unlikely that this patient would achieve a remission lasting any longer than his first remission.27 He is also likely to ask about side effects and the impact on quality of life. He may be willing to accept a treatment with severe side effects, such as an auto- or allotransplant, if he could be assured that the treatment would result in cure or at least a major improvement in length of overall survival and that a good quality of life would be expected. Unfortunately, such assurances cannot presently be made with confidence. Moreover, early mortality or complications of treatment (e.g, myelodysplasia or chronic graft-versus-host disease [GVHD]) may preclude a variety of other treatment options in the future. Thus, this patient may opt for a treatment that is effective but not necessarily curative, has a low toxicity profile, assures good quality of life, and has a minimal impact on precluding other promising options in the future.
Recently, the option of using monoclonal antibodies, either in an unmodified form or in a radiolabeled form, has become available for this patient. Among the most successful and thoroughly studied monoclonal antibodies for the treatment of low-grade lymphoma are those with specificity for the CD20 antigen. This antigen is expressed by virtually all B-cell lymphomas. It is also found on the surface of a subset of normal B cells, but not progenitor B cells, mature plasma cells, or any other non-lymphoid cells. By virtue of this specificity, anti-CD20 antibodies are more selective in their therapeutic mode of action than chemotherapeutic agents. This also results in a different side effect profile as well as a different delivery schedule from chemotherapy.
Rituximab (rituximab in the US; MabThera in other countries), a human-mouse chimeric anti-CD20 monoclonal antibody, was approved by the US FDA in 1998 for the treatment of relapsed low-grade or follicular lymphoma. In the pivotal trial that led to approval, 166 patients who had undergone a median of two prior chemotherapies were treated with a schedule of four weekly IV infusions of 375 mg/m2.28 About half of the patients had a response, and 6% had a complete remission. The median duration of response was about one year. When the analysis included only those patients with follicular histologies (as is the case in this patient), the overall response rate was 60%. In a subsequent analysis, patients appeared to have a similar duration of response with rituximab as they did with their last preceding chemotherapy regimen.29
Side effects of treatment were predominantly infusion related (usually Grade 1 or 2 fever and/or chills) and were most commonly seen with the first infusion. Patients with circulating lymphoma cells and/or high tumor burdens were most likely to experience these adverse events. Infusion time was generally about 4 to 6 hours for the first infusion and 3 to 4 hours for subsequent infusions. Myelosuppression was distinctly unusual, as was infection.
With these data in mind, what should our patient expect from rituximab treatment? Because he fits a profile typical of that of patients in the pivotal trial (moderate amount of pretreatment, good performance status, non-bulky disease, a relatively long response to the last prior treatment), he should expect a 60% chance of achieving a remission. The duration of this remission would be predicted to be similar to that achieved with his last chemotherapy, although follow-up data longer than a median of one year with rituximab are not yet available. Moreover, his treatment would be over in one month as opposed to 4 to 6 months of chemotherapy, he would have mild to moderate side effects only with the first infusion, retain his hair (if he had any in the first place), and continue to enjoy his baseline quality of life. If he should not respond, he has lost little in terms of other treatment options. His bone marrow and other organs will have been spared any significant insult that would compromise future chemotherapy or stem cell transplant options. On the other hand, if he should respond, because the chimeric antibody is mostly comprised of human sequences, he would be very unlikely to develop anti-antibodies that would preclude repeating treatment upon relapse. Upon retreatment, it is estimated that he would have a 40% chance of responding again.30
Because the toxicity profiles of rituximab and chemotherapy are generally non-overlapping, and because some data indicate that rituximab may sensitize tumor cells to chemotherapeutic agents, there has been recent interest in combining the two treatments. Clinical studies have indicated that this can be done safely without increasing the toxicity of either of the treatments.23 Although high response rates have been observed, including molecular remissions, these have primarily been in previously untreated patients. Moreover, whether there is truly an advantage to combination treatment remains to be determined from controlled trials comparing chemotherapy alone to the combination.
What about the option of radiolabeled anti-CD20 antibodies? Although not yet FDA-approved, radiolabeled anti-CD20 antibodies have actually been under study for longer than rituximab. Thus, there is longer follow-up with radiolabeled antibodies, which can be useful in assessing durability of responses. This is especially true for Bexxar, an Iodine-131-labeled antibody that has been in clinical trials for over 10 years. More recently, a Yttrium-90-labeled antibody or ibritumomab tiuxetan (Zevalin) has undergone investigation. When both have been evaluated in the setting of relapsed or refractory low-grade lymphoma they have shown superior overall response rates and complete response rates compared to rituximab. Bexxar, for instance, in a review of 124 patients treated with a median of three prior chemotherapy regimens, yielded an overall response rate of 74% and a complete response rate of 30%.31 Preliminary results of a randomized trial comparing Zevalin to rituximab indicate an overall response rate of 80% for Zevalin compared to response rate of 44% for rituximab.32 A similar trial comparing Bexxar to its unlabeled antibody component is showing a similar trend.33 These superior results over rituximab are likely due to the contribution of targeted irradiation of tumor sites and possibly the crossfire effects of the high-energy beta particles emitted by the isotopes killing neighboring cells by irradiation emitted from radiolabeled antibodies that have bound other cells in the vicinity.
Further evidence, although less direct, of the greater anti-tumor effect of radiolabeled anti-CD20 antibodies compared to rituximab comes from a phase III trial of Bexxar in patients with heavily pretreated and chemotherapy-resistant low-grade or transformed low-grade lymphoma (less than 6-month response to last chemotherapy).34 In this study, the response rate and response duration with Bexxar was prospectively compared to that obtained with the last chemotherapy regimen the patient had received. The response rate with Bexxar was 65%, compared to 28% with the last chemotherapy, and response duration was nearly doubled with Bexxar. As noted above, a prolongation of response duration beyond that of prior chemotherapy would not have been expected with rituximab, and resistance to the last chemotherapy predicted for a lower response rate in the pivotal rituximab trial.28
The higher complete response rate achieved with radiolabeled antibodies would be expected to predict for an overall increase in median duration of response. However, the response durations in the two randomized trials comparing radiolabeled anti-CD20 to unlabeled anti-CD20 have not been reported yet. Moreover, entry criteria of previous studies of rituximab and radiolabeled antibodies have differed enough that retrospective comparisons are difficult to interpret. Although the median duration of response in previously treated patients has been reported in the 9- to 12-month range with Bexxar, the entry criteria for the Bexxar studies heavily skewed the population toward patients with no response or short durations of response to preceding chemotherapy. However, the 30% of patients who achieved a complete response had a median remission durations of close to 5 years.31 Furthermore, trends have been noted for the overall response rate and complete response rate to be higher in less heavily pretreated patients—a point quite relevant to the patient under discussion who has had only one prior treatment. For instance, in previously untreated patients the overall response rate has been reported to be 97% and the complete response rate to be 76% using Bexxar.35 In addition, durable molecular remissions have been achieved in these patients, something that would not be expected from standard-dose chemotherapy.
The time period involved in treating a patient with radiolabeled anti-CD20 antibodies is even shorter than with rituximab. With both radiolabeled antibodies, a dosimetric dose is currently given on day 0 and a therapeutic dose is generally given on day 7. Prior to each radiolabeled antibody dose, an unlabeled antibody predose is given to increase the efficiency of tumor uptake of the radiolabeled antibody. In the case of Bexxar, this involves a one-hour infusion of 450 mg of unlabeled antibody followed immediately by a 30-minute infusion of the radiolabeled antibody. On day 0 and on two other occasions over the next week, brief whole-body gamma camera scans are performed to estimate the whole-body clearance rate of I-131-labeled antibody so that an individualized therapeutic dose designed to deliver a specified whole-body radiation dose can be calculated. In the case of Zevalin, rituximab is given as the unlabeled antibody predose and dosimetry is performed using an Indium-111-labeled version of Zevalin. Therapeutic dosing with Zevalin (the yttrium conjugate), however, is not based on dosimetry measurements but on body weight. With either radiolabeled antibody, no further cycles of treatment are administered—again, differing significantly from chemotherapy.
With new Nuclear Regulatory Commission guidelines, Bexxar can be given on an outpatient basis to the vast majority of patients in the U.S. Patients are given a set of simple instructions to follow over the week after therapy to minimize gamma radiation to the community. With Zevalin there are no gamma emissions, so all patients can be treated on an outpatient basis. Both radiolabeled antibodies are in use in major medical centers as well as community hospitals. A program in these settings coordinating the efforts of the medical hematologist/oncologist with Nuclear Medicine or Radiation Therapy is necessary.
The principal toxicity of this treatment is myelosuppression. In our patient, who has had minimal pretreatment and no bone marrow involvement by lymphoma, this toxicity would be expected to be only moderate and unlikely to require any supportive care (transfusions, growth factors, antibiotics). Moreover, since this is a one-cycle treatment, the patient would not exposed to recurring risks with repeated cycles as with chemotherapy. Although some cases of myelodysplasia have been reported in long-term follow-up after Bexxar, a clear association between this occurrence and radiolabeled antibody treatment has not been established.36 Moreover, those patients who developed myelodysplasia had been heavily pretreated with chemotherapy. Thus, the risk to our patient, even if it exists, appears to be low.
In terms of infusional toxicities, these are again of a mild nature, at least for Bexxar. Infusional adverse events have occurred in only a third of patients and the unlabeled antibody is given quickly over an hour, in contrast to 4 to 6 hours with rituximab. Other non-hematologic toxicities are also mild, with no hair loss and generally minimal nausea being.
The unlabeled antibody and 131I-labeled antibody composing Bexxar is purely murine, as are the Indium-111-and Yttrium-90-labeled antibodies of Zevalin. These murine antibodies have the potential of eliciting an anti-murine antibody response. The anti-murine antibodies can form complexes with the murine antibodies resulting in more rapid clearance of the murine antibody that may potentially reduce the efficacy of the treatment. In addition, this may cause allergic symptoms or symptoms similar to serum sickness. Thus, it is generally believed, although not formally tested that patients who develop antimurine antibodies should not be given murine antibodies again. However, in contrast to patients who have been given Bexxar as a first-line treatment (no prior treatment with chemotherapy) where about 62% eventually develop anti murine antibodies,35 patients such as our patient who have received prior chemotherapy develop these antibodies only about 10% of the time.34 Thus, our patient would have a high chance of being able to be retreated upon relapse, although a test for serum anti murine antibodies should probably be performed before embarking on treatment.
What, then, should our patient expect from a treatment with radiolabeled anti-CD20 antibodies? Given the patient's prognostic features, including his minimal pretreatment status, he should expect about an 80% chance of response and at least a 30% chance of a complete response. The overall response and complete response could even be higher, but there are too few patients in the clinical trials of Bexxar that are similar to this patient to predict more accurately. The same problem exists for predicting durability of response, but the data in more refractory patients compared to previously untreated patients would predict a response duration measured in years. If he should not respond, he has still retained other treatment options, including standard- or high-dose chemotherapy. If he should respond and relapse, it is highly likely that he could be retreated with a radiolabeled antibody (given the low rate of development of antibodies against the murine antibody). About 60% of patients would be expected to respond again, and some may have longer responses the second time.36 Alternatively, he could receive rituximab if desired or if he had developed antimurine antibodies.
Another alternative using radiolabeled antibodies should be mentioned—the use of myeloablative doses in conjunction with autologous stem cell transplant. Using this approach with Bexxar, Press et al have shown an overall response rate of 86% and complete response rate of 79% in previously treated patients.37 The progression-free survival rate at 4 years was estimated to be 51% for patients with indolent histologies. To determine whether this approach is superior to the nonmyeloablative strategy would require a controlled comparative trial. It is clear, however, that this approach would be limited in its application to certain centers capable of handling the high doses of radioactivity involved.
On balance, with currently available data, if the patient wishes to be treated, I would recommend treatment with radiolabeled antibodies as part of a clinical trial. His participation in a clinical trial would help define the role of this promising therapy for patients with a more favorable prognosis than those previously studied using this approach.
III. The Case for Hematopoietic Transplantation for Low-Grade Lymphoma
Richard Champlin, M.D.*
M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Box 24, Houston TX 77030-4095
In this patient's case, I would recommend salvage FND chemotherapy, followed by a non-ablative allogeneic transplant within the setting of a clinical trial, if an HLA-identical sibling donor is available. The major concern would be the timing of the transplant. In general, transplant results are better early in the course of a malignancy than after multiple progressions. GVL effects are most effective when the patient has low bulk, chemotherapy-responsive disease. If one delays performing the transplant until later in the patient's course, the patient may be more debilitated and the disease may be less responsive to chemotherapy or have transformed to a more aggressive histology compromising the chance of success.
If an HLA-identical sibling is not available and the patient responds to salvage chemotherapy, an autologous transplant should be considered, particularly if autologous marrow or blood stem cells can be collected that is PCR negative for residual malignant cells. An unrelated donor transplant can also be considered; relatively few have been performed for low-grade lymphomas. The approach has curative potential, but the risks are increased compared to an HLA-identical sibling transplant. A more intensive preparative regimen is required to achieve engraftment with an unrelated donor transplant. Because of these considerations, we would delay consideration of an unrelated transplant until after failure of further salvage therapy.
Autologous Bone Marrow Transplantation
Autologous BMT was originally proposed as a means of restoring hematopoiesis38 to enable the escalation of the doses of myelotoxic chemotherapy and radiation. High-dose therapy, usually cyclophosphamide and total body radiation, with autologous bone marrow or blood stem cell transplantation has been widely employed as cytoreductive treatment for low-grade lymphoma, producing rates of complete remission over 80% in patients with recurrent disease. Use of autologous transplantation is limited by the frequent involvement of the blood and bone marrow as a part of the natural history of these diseases as well as the inability of even high-dose chemoradiotherapy to eradicate systemic disease in most patients. Patients receiving marrow or blood stem cell autografts effectively depleted of malignant cells using anti-B cell monoclonal antibodies may achieve prolonged remissions.39,40 In a recent update of the Dana-Farber results,41 Freedman et al reported 153 patients with recurrent low-grade lymphoma who underwent purged autologous transplantation. Patients were required to have chemotherapy-sensitive, low-bulk disease. At the time of harvest 30% of patients were in complete remission. Disease-free and overall survival was 42% and 66% at 8 years, with significantly better disease-free survival in patients receiving marrow that was PCR negative for bcl-2-rearranged cells. However, whether purging improves clinical outcome is controversial, and its efficacy has never been confirmed in a randomized trial.42 In most studies, there is a continuing risk of relapse following purged autologous transplants with no clear plateau in disease-free survival.43 In addition, there has been a high rate of secondary myelodysplasia in long-term survivors after autotransplants in this disease.44,45,46,47
Allogeneic Myeloablative Bone Marrow Transplantation
High-dose chemoradiotherapy with allogeneic BMT has generally been reserved for far advanced patients with low-grade lymphoma. Van Besien et al reported encouraging results with extended disease-free survival in 12 of 15 heavily pretreated patients.48,49 Other groups have also reported a high percentage of long-term remissions.50,51,52,53 Relapse rates after allogeneic transplants have been substantially lower than with purged autologous transplants.54,55 Verdonck et al published a study of 28 patients with advanced low-grade lymphoma, comparing results of allogeneic transplants to autologous transplants.55 The patients had previously received two to five conventional chemotherapy regimens. All 10 allogeneic BMT patients achieved complete remission, three patients had treatment-related deaths, and seven patients were alive in remission at a median follow-up of 41 months. Of 18 autologous transplant recipients, none died of treatment-related complications, but only three patients are alive and disease-free. The probability of disease progression among allogeneic transplants was 0% versus 83% for autologous recipients (P =.002), and progression-free survival at 2 years was 68% and 22% respectively (P = 0.049). The continuing risk of relapse after purged autologous transplants suggests that preparative regimen does not completely eradicate the malignancy in most patients. The lower relapse rate with allogeneic transplants is likely due to an immune mediated GVL effect.
Van Besien for the International Bone Marrow Transplant Registry recently analyzed results of allogeneic BMT from HLA-identical sibling donors in 113 patients with low-grade lymphoma.56 Median age was 38 (range 15-61) years. The median interval from diagnosis to transplant was 24 months, and the median number of prior chemotherapy regimens was two. At transplant, 14% of the patients were in complete remission and 71% continued to have active disease; 37% were considered chemotherapy resistant. The conditioning regimen contained TBI in 82% of the patients. Three-year probability of disease-free survival was 49% (95% CI: 39-59), and the probability of disease recurrence was 16% (95% CI 9-27). The probability of treatment-related mortality was 28% (95% CI 19-39). In multivariate analysis, a decreased Karnofsky score, presence of chemotherapy-resistant disease, and the use of a non-TBI regimen were adverse independent predictors of survival.
Attal et al recently performed a retrospective case-control analysis of 216 patients reported to the French Bone Marrow Transplant Group Registry from 1986 to 1996.57 Seventy-two allogeneic transplant patients were matched with 144 who had autologous grafts on the basis of age, disease status, and conditioning regimen. Patient characteristics were comparable for sex, age (median 40 years), stage, interval from diagnosis to transplant (33 months), number of prior chemotherapy regimens (2); 53% were refractory to chemotherapy and 75% received a TBI-containing conditioning regimen. A comparable initial complete response rate, 86% and 78%, respectively, occurred after allogeneic or autologous transplantation. Allogeneic transplants had a significantly lower relapse rate of 12% at 60 months with a plateau after 15 months, in contrast to 55% with autologous transplantation without an apparent plateau (p < 0.001). Transplant-related mortality was higher after allogeneic BMT (30% versus 4% with autotransplantation; p < 0.001). The 4-year event-free survival was not significantly different, 53% for allogeneic BMT and 45% for autologous transplantation. The benefit of improved control of the malignancy was largely offset by the higher rate of treatment-related mortality.
These studies indicate that high-dose chemoradiotherapy and allogeneic bone marrow transplantation is potentially curative for patients with advanced low-grade lymphoma. Unlike the case with autologous transplants, there have been very few relapses after 2 years from transplantation, presumably because of the GVL effect.
“Mini” Allogeneic Non-Myeloablative Transplantation
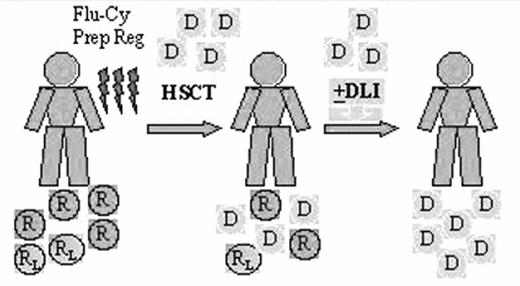
The high-dose chemotherapy and radiation typically used as the preparative regimen for bone marrow transplantation produces considerable morbidity and mortality, and limits the use of this modality to a minority of patients who are young and in good general medical condition. Low-grade lymphoma is typically a disease of older, often debilitated patients, and only a minority have been considered eligible for allogeneic transplantation. Allogeneic transplants are potentially curative, but the benefit is largely offset by a higher rate of treatment-related mortality. An alternative strategy has recently been explored using less toxic nonmyeloablative conditioning with a goal of inducing a GVL effect as the primary treatment. The preparative regimen is designed not to eradicate the malignancy, but to provide sufficient immunosuppression to achieve engraftment of an allogeneic blood stem cell or marrow graft and development of a GVL effect. Patients who achieve engraftment may receive additional donor lymphocytes as necessary to augment the graft-versus-malignancy effects58,59,60,61 (Figure 2).
Nonablative hematopoietic stem cell transplant (HSCT) for low-grade lymphoma.
Abbreviations: D, donor cells; DLI, donor lymphocyte infusions; R, normal host cells; RL, lymphoma cells; Flu-Cy Prep Reg, fludarabine-cyclophosphamide preparative regimen
Nonablative hematopoietic stem cell transplant (HSCT) for low-grade lymphoma.
Abbreviations: D, donor cells; DLI, donor lymphocyte infusions; R, normal host cells; RL, lymphoma cells; Flu-Cy Prep Reg, fludarabine-cyclophosphamide preparative regimen
Fludarabine and related purine analogs are effective agents against indolent lymphomas.62 These drugs also induce lymphocytopenia and are highly immunosuppressive.63 We evaluated the feasibility of this strategy for GVL induction in patients with advanced chronic lymphocytic leukemia or transformed lymphoma who were not eligible for myeloablative transplant regimens because of age, comorbidity, or poor performance status.64 Patients received a fludarabine-containing nonablative preparative regimen followed by allogeneic blood stem cell transplantation to achieve engraftment and mediate GVL effects. Peripheral blood stem cells were used where possible rather than marrow, based upon recent data indicating more rapid hematologic recovery and a low rate of early morbidity after allogeneic blood stem cell grafts.65 Ten patients with low-grade lymphomas received fludarabine and cyclophosphamide with allogeneic hematopoietic transplantation from an HLA-identical sibling.66 Seven were follicular small-cleaved, two follicular mixed, and one small lymphocytic lymphoma. Median age was 50 yrs (range, 36-60). The median number of prior chemotherapy regimens was two (range, 1-6); one patient had hepatitis C and had failed to engraft with an earlier allogeneic blood stem cell transplant from a different donor. Another patient had abnormal creatinine of 1.7 mg%. Eight had chemotherapy-sensitive and two had refractory disease at transplant. The preparative regimen consisted of fludarabine 25 mg/m2/daily × 5 and cyclophosphamide 1 gm/m2/daily × 2. The median time for recovery of absolute neutrophil count (ANC) > 1.0 was 12 days. The median number of days of severe neutropenia (ANC < 0.5) was 6. Five patients never required platelet transfusion. All patients engrafted. More than 80% donor cells were detected by RFLP (restriction fragment length polymorphism) in 50% of patients by day 30 after transplant and in 70% of patients by day 100. No grade 2 or greater toxicity was observed. Acute GVHD grade 2 occurred in only one patient and was limited to the skin. All patients achieved complete remission (CR) and none has relapsed. One patient who had refractory disease pretransplant had partial response by day 30, but achieved CR later with the onset of GVHD. Overall survival and event-free survival were both 100% with a median follow up time of 9.5 (range, 4.5-28) months. These preliminary results indicate that complete remissions can be achieved in low-grade lymphomas with a well-tolerated, nonmyeloablative preparative regimen with allogeneic hematopoietic cell transplantation. Further accrual and longer follow up are needed to critically evaluate this strategy.
Potential for Induction of Graft-versus-Lymphoma as Primary Therapy
These preliminary data demonstrate achievement of engraftment following “standard dose,” nonablative conditioning and marked antitumor responses related to GVL, suggesting the potential efficacy of this approach in older and medically infirm patients with indolent lymphoid malignancies. The major barrier to this approach is the risk of GVHD. A number of approaches have been proposed to separate the GVL from GVHD with donor lymphocyte infusions, including infusing graded doses of T lymphocytes,67 CD8-depleted donor lymphocytes68 or thymidine kinase-transduced donor lymphocytes, which can be ablated by ganciclovir in patients developing GVHD.69 Ideally antigen-specific T cells reactive with the malignancy or lymphoid minor histocompatibility antigens could be utilized, which would eradicate the lymphoma without the potential to produce GVHD. Antiidiotype vaccines are also under study that could be utilized to enhance specific graft-versus-malignancy effects.22,70
This strategy is designed to employ a tolerable, less toxic preparative regimen to reduce the morbidity and mortality associated with allogeneic transplantation and allows treatment of older and medically infirm patients. These preliminary data suggest that nonmyeloablative regimens can reduce treatment-related mortality and still produce a high rate of complete remission. This approach for allogeneic hematopoietic transplantation has promise as a potential curative modality with reduced risk of major morbidity. Further studies with large number of patients and prolonged follow-up are required. Controlled studies are needed to compare this approach with standard chemotherapy and alternative transplant approaches. The use of non-myeloablative preparative regimens may improve the safety of the procedure and will likely serve as a future platform to introduce antigen-specific cellular immunotherapy.
Summary
Fernando Cabanillas, M.D.
When faced with a patient with recurrent indolent NHL, a series of steps should be systematically considered. The first step is to rule out transformation to large-cell lymphoma because the management of that problem would be different. Transformation to a higher grade malignancy is a common event in indolent lymphoma, occurring in at least 30-85% of patients.71,72,73 The frequency in which this is detected directly depends on the frequency of biopsies performed at the time of each relapse. Transformation is frequently underestimated because biopsies are not performed routinely at each relapse. In an autopsy series of patients with the diagnosis of low-grade lymphomas, the vast majority of patients were found to have transformed to a more aggressive histology.71 The introduction of fine needle aspirations has allowed us to easily and frequently sample nodes and masses at the time of relapse.74,75,76,77,78,79,80 Disease transformation should always be suspected at the time of disease progression or recurrence, but more so if the serum LDH is elevated, lymph nodes are rapidly enlarging, constitutional symptoms are present or whenever there is invasion of extranodal sites such lung, pleura, central nervous system or bone.
In this decision-making process it is crucial that the patients, with the help of their physicians, assume an active role. In my own view, two essential points have to be factored into this decision: 1) the patient's age, and 2) the patient's expected survival with conventional management. The choice between a treatment alternative associated with high mortality and morbidity, such as allogeneic BMT, versus more conservative therapy, such as antibody treatment, has to be weighed against the expected benefits for each, such as the potential for cure in allogeneic BMT versus a reasonably long survival with little toxicity as in traditional management, which includes the watch-and-wait approach as well as other standard therapies. In spite of the evidence that suggests a GVL effect from allogeneic non-myeloablative transplantation, the potential for cure with this modality requires a longer follow-up.48,55 On the other hand, high-risk alternatives such as allogeneic BMT should not be reserved as a last resort, as pointed out by Dr. Champlin, because they will inevitably be less effective at that point.
As noted by Dr. Kaminski, the expected survival for this patient is approximately 4 years but survival after relapse depends on various prognostic factors in addition to the number of relapses. In our experience at M.D. Anderson Cancer Center, the median survival of patients with indolent lymphoma after a first or second relapse was 36 months, and after a third relapse it was 14 months.81 Features that were associated with short median survival after relapse were the presence of constitutional (“B”) symptoms, bulky tumor mass, more than two relapses, LDH > 400 U/ml (normal = 225 Uml), and hemoglobin < 10 g/dL.81 Whenever any of these variables was present, the median survival was 28 months, and when two or more were present, it was 8.5 months. When none of these adverse features were present, the median survival was > 6 years. In the case we are discussing today there were no adverse features present at relapse so that his expected survival would be over 6 years provided the biopsy did not show evidence of transformation to an aggressive NHL. In such cases, age plays an important role in making a therapeutic decision.
With allogeneic transplants, patients 55 years old are known to have a mortality rate of approximately 15% (and as high as 30% in some series) and the morbidity is even higher. These results depend in great part on the experience of the transplant center. Thus, the most difficult decision in patients older than 55 years is whether to proceed with an experimental and risky approach such as allogeneic BMT, should an HLA-identical donor be available. If the decision is made to delay the use of such high-risk strategy for the future, other alternatives to be considered include the watch-and-wait approach, autologous transplant, or conventional-dose chemotherapy regimens such as FND as well as monoclonal antibodies. The decision as to when to proceed with an allogeneic BMT may vary from one center to another, and even from one investigator to another within the same center, but it seems reasonable to choose this strategy in young patients who have experienced more than two relapses or who have any of the other unfavorable features mentioned above. Table 1 is offered as a guide and is not to be considered as a fixed rule for therapeutic decision making in patients with relapsed indolent lymphoma.
Preferred initial strategies in patients with relapsed indolent lymphoma.
| No adverse prognostic features* . | > 1 adverse prognostic features* . | ||
|---|---|---|---|
| Any age . | Age < 55 years No serious co-morbidity . | Age 55-65 years No serious co-morbidity . | Age > 65 years or serious co-morbidity . |
| Standard dose chemotherapy | Allogeneic transplant | Autologous transplant | Monoclonal antibodies |
| Monoclonal antibodies | Autologous transplant | Standard dose chemotherapy | Standard dose chemotherapy |
| Watch and wait | Standard dose chemotherapy | Monoclonal antibodies | |
| Monoclonal antibodies | |||
| No adverse prognostic features* . | > 1 adverse prognostic features* . | ||
|---|---|---|---|
| Any age . | Age < 55 years No serious co-morbidity . | Age 55-65 years No serious co-morbidity . | Age > 65 years or serious co-morbidity . |
| Standard dose chemotherapy | Allogeneic transplant | Autologous transplant | Monoclonal antibodies |
| Monoclonal antibodies | Autologous transplant | Standard dose chemotherapy | Standard dose chemotherapy |
| Watch and wait | Standard dose chemotherapy | Monoclonal antibodies | |
| Monoclonal antibodies | |||
Adverse prognostic features include the presence of constitutional (“B”) symptoms, bulky tumor mass, more than two relapses, LDH > 400 mU/ml (normal = 225 mU/ml), and hemoglobin < 10 g/dL
Ultimately this should be a shared decision between the patient, after being provided with all the pros and cons, and the physician. The patient's personality and willingness to undertake the risks involved with each option are essential parts of the equation. The high mortality associated with allogeneic transplantation appears to decrease substantially with the non-myeloablative transplants (“mini-transplants”), but this procedure still has to be considered experimental and many centers are not yet familiar with this technique. It appears reasonable for centers that are investigating this approach to continue to accumulate data on this modality. As more experience is accumulated and longer follow up becomes available with this modality, it might become a standard approach in the future. The management of these patients, especially when high-risk options are contemplated, should be considered in an experimental context and an effort should be made to include these patients in clinical trials.