Abstract
This review discusses the evolution of novel diagnostic and treatment strategies for multiple myeloma based upon increased understanding of basic disease pathogenesis. Although myeloma has remained an incurable illness to date, these new developments will derive treatments to improve outcome and achieve eventual cure.
In Section I, Dr. Kyle reviews the results of current therapy for multiple myeloma, including high dose therapy and stem cell transplantation which have proven to achieve improved response rates, event-free, and overall survival. Supportive therapy, such as erythropoietin to treat disease-related anemia, and methods of prophylaxis against infection, which both lessen toxicities of treatment and improve quality of life for patients, are also addressed.
In Section II, Dr. Dalton with Drs. Landowski, Shain, Jove and Hazlehurst discusses mechanisms of drug resistance in myeloma, with emphasis on novel treatment approaches to prevent development of drug resistance and to overcome drug resistance. Laboratory studies delineating mechanisms whereby myeloma cells resist drug-induced apoptosis provide the framework for related treatment protocols for patients with refractory disease.
In Section III, Dr. Berenson reviews the management of complications in bone, which occur in the majority of patients with myeloma and are the major cause of decreased quality of life. New insights into the mediators of bone resorption and new bone formation in the marrow milieu have already derived effective bisphosphonate therapy. These drugs not only reduce bone complications and related pain, thereby improving quality of life, but also may have intrinsic anti-tumor activity by virtue of inducing tumor cell adherence to marrow, reducing interleukin-6 secretion, inducing tumor cell apoptosis, or inhibiting angiogenesis.
In the last section, Dr. Anderson explores the potential for future therapies which offer great promise to improve patient outcomes. First, drugs which alter the marrow microenvironment include thalidomide and its derivative immunomodulatory drugs, which act directly on tumor cells to induce apoptosis or G1 growth arrest, alter tumor cell adhesion to marrow stroma, inhibit angiogenesis, and trigger a cellular anti-tumor response. The proteasome inhibitors both act directly on tumor cells and also inhibit the transcription factor NFκB-dependent upregulation of IL-6 secretion triggered by tumor cell adhesion. Second, delineation of both growth and apoptotic pathways has derived novel treatment strategies. Third, the preclinical basis and early clinical trial results using vaccination and adoptive immunotherapy to harness autoimmune and alloimmune anti-myeloma responses are presented. This review sets the stage for an evolving new biologically based treatment paradigm in myeloma targeting both the tumor and its microenvironment to improve outcome and achieve eventual cure.
I. Current Therapy of Myeloma
Robert A. Kyle, M.D.*
Mayo Clinic, Room 920 Hilton Bldg., 200 First Street SW, Rochester MN 55905
Although most patients with multiple myeloma (MM) have symptomatic disease at diagnosis and require therapy, some are asymptomatic and should not be treated. If there is doubt about beginning therapy, it is best to reevaluate the patient in two or three months and delay therapy until progressive disease is evident.
If the patient is younger than 70 years, the physician should discuss the possibility of an autologous peripheral blood stem cell transplant with the patient. Hematopoietic stem cells should be collected before the patient is exposed to alkylating agents. Chemotherapy is the preferred initial treatment for symptomatic MM in persons older than 70 years or in younger patients in whom transplantation is not feasible.
Peripheral blood stem cells are preferable to bone marrow transplantation because engraftment is more rapid and there is usually less contamination of the infused cells with tumor cells. The absolute number of CD34+ cells is the most reliable and practical method for determining the efficacy of the stem cell product. Autologous peripheral stem cell transplantation is applicable for more than half of patients with MM. The two major shortcomings are: 1) Eradication of myeloma from the patient rarely occurs even with large doses of chemotherapy and/or radiation and 2) autologous peripheral blood stem cells are contaminated by myeloma cells or their precursors. Fortunately, mortality from autologous transplantation is only 1-2% if patients are appropriately selected.
Most physicians initially treat the patient with vincristine and doxorubicin (Adriamycin) given IV for 96 hours and dexamethasone orally (VAD) for 3-4 months to reduce the number of tumor cells. Dexamethasone with or without thalidomide is being evaluated for initial therapy. Peripheral blood stem cells are collected following granulocyte colony-stimulating factor (G-CSF) with or without high-dose cyclophosphamide. One can then proceed with the transplant following high-dose chemotherapy and/or total body irradiation (TBI) followed by infusion of the peripheral blood stem cells. The other choice is to treat the patient with alkylating agents after stem cell collection until a plateau is reached and then give the patient alpha-2-interferon (α2IFN) or no therapy until early relapse. At that time, the patient is given highdose melphalan with or without total body radiation and the previously collected peripheral blood stem cells are infused. Early or late transplantation are reasonable options. In one study, 185 patients were treated with three or four courses of VAD and then randomized to highdose chemotherapy and autologous stem cell transplantation or to conventional chemotherapy. There was no difference in median survival of the two groups (65 vs. 64 months). The main advantage of early transplantation was a shorter period of chemotherapy.1
A randomized trial by the French Myeloma Group compared high-dose chemotherapy followed by autologous bone marrow transplantation with conventional chemotherapy in 200 previously untreated myeloma patients under the age of 65 years.2 Data was analyzed on an intention-to-treat basis. Twenty-five percent of the patients randomized to transplantation did not receive a transplant. Response rate (81 % vs 57%) and complete response rate (22% vs. 5%) were superior in the transplant group. The 5-year event-free survival (28% vs 10%) and overall survival (52% vs 12%) were superior in the transplant group. It must be kept in mind that patient selection plays an important role in response and survival. In one report, 77 patients with MM who fulfilled the criteria for transplant (age < 66 years, Stage II or III, good performance status and disease responsive to initial chemotherapy) but who were treated with conventional chemotherapy had a survival of 5 years, which is similar to that seen in autologous stem cell transplantation.3
In a series of 177 patients < 75 years of age with IgG myeloma, C-VAMP followed by high-dose chemotherapy with or without stem cell rescue and maintenance interferon were reported. The median survival was 4.9 years. Those with β2-microglobulin <2.7 mg/L and those < 52 years of age had a more favorable response.4
The role of TBI in the preparative regimen is controversial. In a comparison of melphalan, 140 mg/m2 plus TBI vs melphalan 200 mg/m2, there was no difference in response rate, event-free survival and overall survival. However, the toxicity of melphalan 200 mg/m2 was significantly lower than melphalan plus TBI.5 Consequently, many have discontinued TBI and give only melphalan, 200 mg/m2 for the preparative regimen.
The role of double or tandem autologous stem cell transplants is controversial. In an uncontrolled series of 231 newly diagnosed MM patients who received a second transplant, 51 % achieved complete response and 95% had a complete or partial response. The authors felt that the double transplant extended both event-free and overall survival even in patients with unfavorable cytogenetics and β2-microglobulin values.6 In a randomized trial of 400 patients from France, there was no difference in event-free or overall survival between single and double autologous stem cell transplants when evaluated at two years. The two groups were similar from the standpoint of age, gender, stage, Ig isotype, β2-microglobulin value, C-reactive protein level and bone marrow plasmacytosis. The complete response rate was 32% with a single transplant and 33% with a double transplant. At 2 years, the event-free survival was 54% vs. 57% while the overall survival was 71% vs 67%.7 In a subsequent evaluation, patients with a low β2-microglobulin value at diagnosis appeared to have better results with the double transplant.
Almost all patients will relapse following an autologous stem cell transplant. A preliminary analysis of a randomized trial of 85 patients with MM who were treated with high-dose melphalan and autologous bone marrow transplantation followed by α2-IFN maintenance therapy suggested both a relapse-free survival and overall survival benefit. However, the final analysis of this trial demonstrated no significant difference in relapse-free or overall survival among patients randomized to maintenance therapy with α2-IFN.8 Idiotype-treated autologous dendritic cells are being used to prolong response duration.9
In an effort to prolong survival, highly purified CD34+ cells did not influence the achievement of clinical or molecular complete remission or remission duration or overall survival.10 Thus, tumor cell purging does not appear to be beneficial.
The use of α2-IFN for more than six months following autologous stem cell transplantation resulted in delay of platelet recovery.11 Patients refractory to VAD had the same overall survival as those who responded to VAD.12 In a retrospective study, high-dose therapy for newly diagnosed myeloma resulted in prolongation of survival for patients < 60 years old when compared to historic controls treated with chemotherapy (61 vs 46 months).13
Allogeneic Bone Marrow Transplantation
The major advantage of allogeneic transplantation is that the graft contains no tumor cells that can lead to a relapse. Unfortunately, over 90% of patients with multiple myeloma are ineligible because of their age, lack of an HLA-matched sibling donor or inadequate renal, pulmonary or cardiac function. Furthermore, there is a mortality of at least 25% at present.
In a report of 266 patients from the European Blood and Bone Marrow Transplantation registry, 51% obtained a complete response. The overall treatment mortality rate was approximately 40%. The actuarial survival was 30% at 4 years and 20% at 10 years.14
It is obvious that the mortality rate for allogeneic transplantation must be reduced before it can assume a major role in the treatment of multiple myeloma. The use of a “mini-allo” transplant15 or depletion of T cells in an effort to reduce transplant mortality are promising approaches. Graft-versus-myeloma effect has been noted after donor peripheral blood mononuclear cells were given for relapse following allogeneic transplantation. Eight of 13 patients with relapsed myeloma following an allogeneic bone marrow transplantation responded to donor lymphocyte infusions.16
Chemotherapy
Various combinations of therapeutic agents have been used because of the shortcomings of melphalan and prednisone. Melphalan and prednisone produces an objective response in 50-60% of patients. In an overview of individual data from 4,930 persons from 20 randomized trials comparing melphalan and prednisone with a variety of combinations of chemotherapeutic agents, the response rates were higher with combination chemotherapy (60%) than melphalan and prednisone (53%) (p < 0.00001). However, there was no significant difference in overall survival and there was no evidence that any group of patients benefited from receiving combination chemotherapy.17
Chemotherapy should be continued until the patient is in a plateau state or for at least one year. Continued chemotherapy may lead to the development of a myelodysplastic syndrome or acute leukemia. The possible benefit of maintenance therapy with α2-IFN following conventional chemotherapy is controversial due to conflicting results and frequency of undesirable side effects. In a large meta-analysis Wheatley reported a survival benefit in both induction (p = 0.05) and maintenance (p = 0.03) with an increase in median response duration of six months in both settings.18 Patients should be monitored closely during the plateau phase and the same chemotherapy regimen should be reinstituted if relapse occurs after six months. The use of prednisone, 50 mg orally every 48 hours, appears to prolong the plateau state as well as the overall survival.19
Treatment of Refractory Multiple Myeloma
Patients who are initially refractory or who become refractory to alkylating agent therapy have a modest response rate to subsequent chemotherapy and a limited survival. The highest response rates in patients with MM resistant to alkylating agents have been with vincristine, doxorubicin and dexamethasone (VAD). Vincristine and doxorubicin are given by continuous infusion for four days and dexamethasone (40 mg daily) is given on days 1-4, 9-12, and 17-20 each month. Dexamethasone is often given only on days 1-4 in even-numbered cycles because of toxicity. Dexamethasone can be given as the only therapeutic agent since it accounts for approximately 80% of the effect of VAD. Vincristine (0.4 mg) and doxorubicin (9 mg/m2) as a rapid intravenous infusion daily for 4 days plus dexamethasone (40 mg) produced an objective response in 67% of 134 previously untreated myeloma patients. This suggests that 4-day infusion of vincristine and doxorubicin is unnecessary.20 Intravenous pulse methylprednisolone (2 g three times weekly) is helpful for patients with pancytopenia and refractory disease. We find fewer side effects from this approach than with dexamethasone.21 Vincristine (2 mg), carmustine (BCNU; 30-40 mg) and doxorubicin (30-40 mg) intravenously on day 1 and prednisone daily for 5 days every 3 to 4 weeks produces benefit in about one-third of patients. Thalidomide produced responses in 32% of 84 patients with previously treated, progressive MM. After 12 months of follow-up, 22% of patients remained event free and 58% were alive.22 Thalidomide was given in an initial dosage of 200 mg daily and gradually increased to 800 mg daily. Constipation, weakness or fatigue, sleepiness, skin rash and peripheral neuropathy were undesirable side effects. In the majority of patients, response occurred within six weeks and with only 400 mg of thalidomide daily. The use of thalidomide in conjunction with dexamethasone is being explored.
Supportive Care
Renal failure
Approximately 20% of patients with MM have a creatinine level ≥ 2 mg/dL at diagnosis. The two major causes of renal insufficiency are “myeloma kidney” and hypercalcemia. Dehydration, infection, nonsteroidal anti-inflammatory agents and roentgenographic contrast media may contribute to acute renal failure. Amyloid deposition occurs in 10% of patients who have MM and often causes nephrotic syndrome, renal insufficiency or congestive heart failure.
Maintenance of a high urine output (3 L/day) is important for preventing renal failure. Prompt treatment of hypercalcemia as well as correction of dehydration and electrolyte imbalance is crucial. Alkalinization of the urine may be useful.
Acute renal failure should be treated with appropriate fluid and electrolyte replacement and VAD in an effort to reduce the tumor mass as quickly as possible. A trial of plasmapheresis in younger patients with acute renal failure is a reasonable approach.23 Hemodialysis or peritoneal dialysis is necessary in the event of symptomatic azotemia.
Anemia
Infection
Patients should receive pneumococcal and influenza vaccinations despite their suboptimal antibody response. Prompt and appropriate therapy of bacterial infections is essential. Prophylactic daily oral penicillin often benefits patients with recurrent pneumococcal infections. Since many infections occur in the first two months after instituting chemotherapy, the use of daily oral trimethoprim/sulfamethoxazole is helpful.27 Intravenously administered gamma globulin may be beneficial for patients with recurrent infections, but it is inconvenient and very expensive.
Spinal cord compression
This complication should be suspected in patients with severe back pain who develop weakness or paresthesias of the lower extremities or bladder or bowel dysfunction. Magnetic resonance imaging or computed tomography must be done immediately. An MRI is particularly useful in demonstrating extramedullary plasmacytoma. Radiation therapy and dexamethasone are usually effective, and surgical decompression is rarely necessary.
Hyperviscosity
This is characterized by oral or nasal bleeding, blurred vision, paresthesias, headache or congestive heart failure. It may occur from high concentrations of IgA or, rarely, IgG. The serum viscosity levels do not correlate well with the symptoms or clinical findings. Consequently, a decision to perform plasmapheresis depends on the symptoms and changes in the ocular fundus. Plasmapheresis promptly relieves the symptoms and should be done regardless of the viscosity level if the patient has signs or symptoms of hyperviscosity.28
Emotional support
All patients with MM need substantial and continuing emotional support. The physician's approach must be positive in emphasizing the potential benefits of therapy. It is reassuring for patients to know that some survive for 10 years or more. It is vital that the physician caring for patients with MM has the interest and capacity to deal with an incurable disease over the period of years with assurance, sympathy and resourcefulness.
II. Determinants of Drug Response and Drug Resistance in Multiple Myeloma
William S. Dalton, M.D., Ph.D.,* Terry Landowski, Kenneth Shain, Richard Jove and Lori Hazlehurst
Clinical Investigations, H. Lee Moffitt Cancer Center, 12902 Magnolia Drive, MRC 3043, Tampa FL 33612-9497
Effective therapy for multiple myeloma began in the early 1960s with the development of alkylating agents, in particular, melphalan and cyclophosphamide.1 Even today, the combination of oral melphalan and prednisone is considered to be the mainstay of treatment for myeloma producing responses in approximately 50-60% of patients. During the past four decades, various drugs have been found to be effective for the treatment of myeloma; most of these drugs belong to the following pharmacologic classifications: alkylating agents (melphalan, cyclophosphamide, BCNU), topoisomerase II inhibitors (doxorubicin and etoposide), glucocorticoids (prednisone and dexamethasone), and the anti-tubulin agent, vincristine. With the exception of the glucocorticoids, most of these agents are relatively ineffective as single agents and are best administered as a combination of agents. Since the introduction of melphalan and prednisone, several drug combinations have been investigated. A popular combination of drugs proven to be effective is the M2 protocol consisting of vincristine, carmustine, melphalan, cyclophosphamide and prednisone (VBMCP).2 While this combination produces higher response rates than melphalan and prednisone, there is only a marginal improvement in overall survival that appears to occur chiefly for patients with a poor prognosis.3 Similar observations have been made for other drug combinations including vincristine, melphalan, cyclophosphamide, prednisone (VMCP) alternating with vincristine, carmustine (BCNU), doxorubicin and prednisone.4 The combination of VAD (vincristine, doxorubicin, and dexamethasone) produces responses in 60-70% of patients who become resistant to alkylating agents, but ultimately patients will develop resistance to all known chemotherapy regimens.5 The problem of drug resistance is a major obstacle to curing myeloma and understanding factors that determine drug response and the development of drug resistance should prove useful in developing means of preventing or overcoming this problem.6
Substantial progress has been made in the last decade in the identification of cellular mechanisms that confer clinical drug resistance in myeloma. Research to date has chiefly focused on intrinsic cellular mechanisms of drug resistance; in other words, mechanisms that reside within the myeloma cell itself. Examples of intrinsic cellular drug resistance include the following: (a) reduction of intracellular drug concentration due to the overexpression of membrane pump proteins such as P-glycoprotein, (b) altered drug metabolism or enhanced drug detoxification, (c) alterations in the drug target that reduce drug efficacy, and (d) enhanced cellular repair of drug-induced damage.6 Any one of these mechanisms could occur within a myeloma cell, and in fact, it is likely that a combination of these mechanisms occurs simultaneously within the same myeloma cell. Studies both in the laboratory and the clinic have demonstrated that eliminating or preventing a single mechanism of resistance, such as reduced intracellular drug concentration due to P-glycoprotein, selects for alternative mechanisms of drug resistance. Based on this evidence, attempting to overcome a single mechanism of intrinsic drug resistance is not likely to produce long-standing clinical remissions. In order to make substantial progress in the treatment of myeloma, it is important to develop a better understanding of the biological principles that govern myeloma cell survival and growth. Understanding these principles will allow investigators to develop therapeutic approaches that capitalize on the uniqueness of myeloma cells, thereby targeting the malignant cell and sparing normal cells.
Targeting Intrinsic Molecules and Pathways in the Myeloma Cell
One approach that may prove to be successful in improving myeloma therapy is the development of small molecules that inhibit or interrupt cellular targets or pathways that regulate myeloma cell growth and survival. These pathways may be intrinsic to the myeloma cell itself, such as altered signal transduction pathways due to Ras mutations. Mutations in the Ras family of genes are relatively common in myeloma patients. Neri and colleagues first reported in 1989 that 32% of patients with myeloma had Ras mutations.7 The most frequent mutation occurred in the N-Ras gene, particularly at codon 61. The second most frequent mutation was found at codon 12 for K-Ras. The frequency of Ras mutations increased from 27% at diagnosis to 46% with disease progression. Interestingly, these investigators found that patients with mutated Ras were less likely to respond to chemotherapy compared to patients with wild-type Ras. In a more recent study of 346 newly diagnosed, untreated patients, Liu et al reported a total incidence of 39% Ras mutations.8 Patients with Ras mutations had a median survival of 2.1 years compared to 4.0 years for patients with wild-type Ras. These studies demonstrate that Ras mutations adversely affect survival and may reduce response to chemotherapy; therefore, targeting Ras signaling may be a novel therapeutic approach to the treatment of myeloma.
One approach to the interruption of Ras signaling in the myeloma cell is to prevent Ras “processing,” a requirement for the activation of Ras.9 The enzyme farnesyltransferase is responsible for transferring the farnesyl group from farnesyl diphosphate, a cellular chemical used in the synthesis of cholesterol, to certain proteins such as Ras. This process is important for Ras activation because it allows the protein to attach to the inner plasma cell membrane. Preventing Ras from going to the plasma membrane by blocking its farnesylation short-circuits oncogenic growth signals to the nucleus. Inhibition of the farnesylation process is a prime target for drugs aimed at preventing Ras activity.10 Recently, several drugs have been synthesized to inhibit protein farnesylation. These drugs are called farneslytransferase inhibitors (FTIs) and preclinical studies have shown them to be effective in tumors with Ras mutations. A phase II clinical trial using the Janssen FTI (R11 5777) has been approved by CTEP for the treatment of myeloma and should begin soon. A second approach to inhibiting Ras processing may be the use of amino-bisphosphonates. Shipman et al found that the bisphosphonate incadronate caused apoptosis in human myeloma cells by inhibiting the mevalonate pathway.11 This pathway is essential for the biosynthesis of sterols and long-chain isoprenoid lipids including farnesyl PPi and geranylgeranyl PPi. These latter two compounds serve as substrates for farnesyltransferase and geranylgeranyl transferase, respectively, and are transferred to small GTP-binding proteins such as Ras. Inhibiting isoprenylation of these critical proteins may induce apoptosis in myeloma cells.
Targeting Extrinsic Molecules or Pathways Involved in Myeloma Cell Survival and Growth
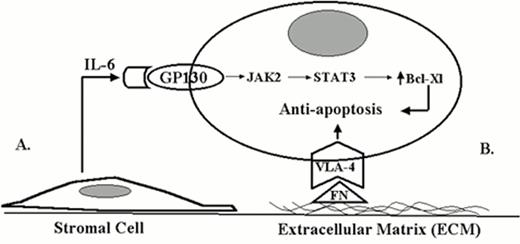
In addition to the intrinsic molecules or pathways discussed above, there are signal transduction pathways that rely on external communication between the myeloma cell and its environment. Similar to the intrinsic pathways, these extrinsic factors also regulate myeloma cell survival and growth and are potential targets for novel therapy of myeloma. For example, the primary source of interleukin 6 (IL-6) is from the bone marrow stroma and not the myeloma cell itself.12 Inhibiting IL-6 production, binding to its receptor, or downstream signaling may block myeloma cell proliferation and result in apoptosis. Myeloma cells express cell adhesion molecules that allow attachment and communication between the myeloma cell and the bone marrow microenvironment.13 This communication influences myeloma cell survival and growth; interrupting cellular adhesion may induce programmed cell death and enhance the efficacy of standard treatment of myeloma. In addition, it has recently been reported that myeloma cell growth and dissemination may depend upon angiogenesis.14 Interactions between the microenvironment and the myeloma cells may regulate angiogenesis and stimulate myeloma growth and progression. Blocking intercellular communications by blocking cell adhesion may represent a new approach for the treatment of myeloma.
Figure 1 demonstrates two possible forms of interaction between myeloma cells and the bone marrow microenvironment. A soluble form of interaction is represented by the interaction between IL-6, a cytokine produced by the bone marrow stromal cells, and the myeloma cell. Interaction between the myeloma cell and the bone marrow stroma mediated by cell adhesion molecules represents a direct form of interaction. For example, the interaction between cell surface integrins and extracellular matrix components, such as fibronectin, may be involved in prolonging cell survival and dissemination of disease. Both types of interactions (soluble and direct) may be potential targets for novel therapeutic approaches for myeloma.15
Two different forms of tumor-microenvironment interactions influence drug response in cancer.
A. Soluble form of tumor-microvenvironment interaction (IL-6).
B. Direct contact form of tumor-microvenvironment interaction (ECM).
Two different forms of tumor-microenvironment interactions influence drug response in cancer.
A. Soluble form of tumor-microvenvironment interaction (IL-6).
B. Direct contact form of tumor-microvenvironment interaction (ECM).
Inhibiting IL-6 Signaling
IL-6 is a critical growth factor for B-cell growth and development.16 Although IL-6 is involved in normal B-cell development, overproduction of this cytokine is considered to be an important component of the pathogenesis and progression of myeloma.17 The most common source of IL-6 in myeloma is believed to be the bone marrow stromal cells, suggesting that IL-6 is a paracrine rather than an autocrine growth factor in myeloma.17 In light of these findings, it is generally believed that inhibiting IL-6 signaling may be a novel approach to the treatment of myeloma. Theoretically, this could be accomplished by inhibiting the production of IL-6, blocking the binding of IL-6 to its cell surface receptor, or by blocking down-stream signaling events within the myeloma cell itself.12 One approach to blocking IL-6 receptor activation is to generate IL-6 variants that block the receptor in an inactive form. One IL-6 variant in particular, called Sant7, is a super-antagonist found to be effective at blocking IL-6.18 Preclinical testing of this super-antagonist is ongoing in hopes that Sant7, or similar agents, may soon be tested in the clinic (G. Ciliberto, personal communication).
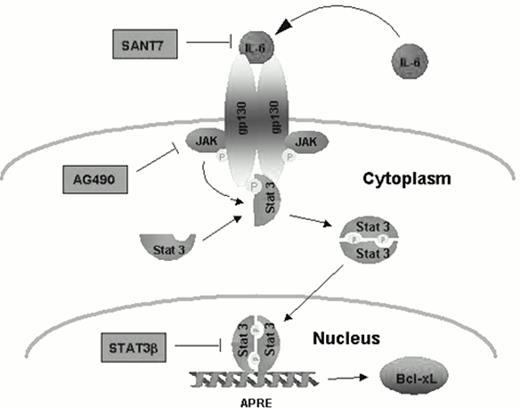
Another approach to inhibiting IL-6 is to interrupt the intracellular signaling associated with cytokine receptor activation. IL-6 induces intracellular signaling through multiple pathways, but one pathway utilized by IL-6 to influence cell survival and resistance to apoptosis is the JAK/STAT pathway. Engagement of cell surface cytokine receptors activates the Janus kinase (JAK) family of tyrosine kinases, which in turn phosphorylate and activate the cytoplasmic STAT (signal transducers and activators of transcription) proteins.19 Activated STATs dimerize upon activation by JAKs and translocate to the nucleus where they bind specific DNA response elements and regulate the expression of certain genes. Seven mammalian STAT family members have been cloned and share common structural characteristics. One particular STAT member, Stat3, has been associated with oncogenesis when it is constitutively activated.20 A recent report by Jove and colleagues demonstrated that Stat3 is constitutively activated in bone marrow mononuclear cells in patients with myeloma.21 In addition, Jove and co-workers demonstrated high levels of activated Stat3 in the myeloma cell line U266 known to produce and utilize IL-6 for survival. Moreover, this cell line is resistant to Fas-mediated apoptosis in spite of high levels of Fas receptor being expressed in U266 cells. Using specific inhibitors of the IL-6 receptor (Sant7), a Jak2 inhibitor (AG490), and a dominant negative of the Stat3 gene (Stat3b), these investigators were able to delineate an IL-6-signaling pathway from Jak2 to Stat3 to the bcl-xl gene promoter in myeloma cells (Figure 2). Utilizing these inhibitors to block activation of Stat3 in U266 cells inhibited Bcl-xL expression, induced apoptosis, and overcame resistance to Fas-mediated apoptosis. These findings provide evidence that Stat3 activation contributes to the pathogenesis of myeloma by preventing apoptosis and conferring a survival advantage. Interrupting Stat3 signaling may be a potential target for therapeutic intervention in myeloma.
Constitutive activation of STAT3 prevents apoptosis in human myeloma cells. From Dalton and Jove. Immunity, 1999.21
Constitutive activation of STAT3 prevents apoptosis in human myeloma cells. From Dalton and Jove. Immunity, 1999.21
Myeloma and Cell Adhesion-Mediated Drug Resistance
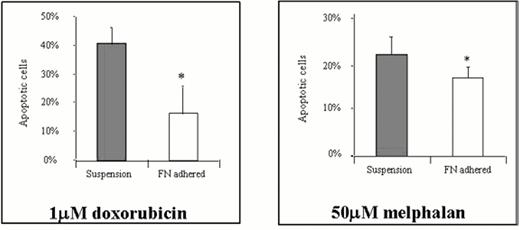
Intercellular interactions have long been known to contribute to tumor cell survival and progression.22 Recently, it has been recognized that cell-cell and/or cell-ECM (extracellular matrix) adhesion may regulate apoptosis and cell survival in a wide variety of tumor types.23 Damiano and colleagues recently presented evidence that the integrin receptors on myeloma cells may be responsible for the cell adhesion-mediated drug resistance (CAM-DR) observed when cells are adhered to fibronectin (FN).24 These investigators showed that the drug-sensitive myeloma cell line RPMI 8226 known to express both VLA-4 (α4β1) and VLA-5 (α5β1) integrin FN receptors, was relatively resistant to the apoptotic effects of doxorubicin and melphalan when cells were pre-adhered to FN compared to cells exposed to drug while in suspension media (Figure 3). Recent data demonstrates that adhesion to FN protects cells from DNA damage induced by DNA intercalating agents (e.g. doxorubicin), alkylating agents (e.g. melphalan), and radiation treatments. More recently, Hazlehurst and colleagues demonstrated that the cyclin dependent kinase (cdk) inhibitor, p27kip1 is overexpressed when cells are adherent to FN and this cdk inhibitor may play an important role in effecting the CAM-DR phenotype.25 These findings provide evidence that antagonists of cellular adhesion, or signaling events related to adhesion, may serve as a means of inducing myeloma cell apoptosis or improving the efficacy of anti-cancer therapy for myeloma.
Fibronectin-induced drug resistance.
* p < 0.05 by Student's T-test; effect reversed by addition of α4 and 15-blocking Ab. Damiano et al.24
Fibronectin-induced drug resistance.
* p < 0.05 by Student's T-test; effect reversed by addition of α4 and 15-blocking Ab. Damiano et al.24
Conclusions
There are at least three different approaches that may be used to improve therapeutic outcome for patients with myeloma: (1) enhancing the efficacy of currently available chemotherapeutic drugs by identifying and overcoming drug resistance mechanisms, (2) identifying new targets that regulate cell survival and growth of myeloma cells, and (3) developing means to enhance host immune response against myeloma cells. However, no matter what new approach clinicians may choose to investigate, a thorough understanding of the biology of myeloma will be necessary for developing effective therapeutic approaches. Understanding the biological principles that regulate myeloma cell survival and growth will likely provide new targets for therapy and perhaps improve the efficacy of already available myeloma treatments.
III. Advances in the Biology and Treatment of Myeloma Bone Disease
James R. Berenson, M.D.*
Department of Medicine, Cedars-Sinai Medical Center, 8700 Beverly Blvd., Bev. Mod.-1, Room 120, Los Angeles CA 90048
Biology
The major clinical manifestations of multiple myeloma are related to loss of bone. This bone loss often leads to pathologic fractures, spinal cord compression, hypercalcemia and bone pain. As a result, these patients often require analgesics, radiation therapy and surgery to bone. This enhanced bone loss occurs because of the stimulation of the cells responsible for bone resorption, the osteoclasts, and results from the interplay between the tumor cells, other nonmalignant cells in the bone marrow microenvironment and the bone-resorbing osteoclasts. Specific soluble factors have been identified in the bone marrow from myeloma patients that stimulate osteoclasts. Recent studies have elucidated the specific factors involved in osteoclast stimulation and the ways that the different cell populations interact in the bone marrow to produce enhanced bone resorption in these patients.
Bone Resorbing Factors
Initially, Mundy and colleagues identified a number of proteins known as OAFs, osteoclastic activating factors, which were thought to be the proteins responsible for enhanced bone loss in myeloma patients. These factors including lymphotoxin (tumor necrosis factor) (TNF) β) and interleukin 1 β (IL-1) β were identified in the supernatants from cultures from myeloma cell lines and fresh myeloma bone marrow in these early studies. However, more recent studies have suggested that other factors are important in leading to the loss of bone among these patients.
The role of lymphotoxin in myeloma bone disease has been downplayed by more recent studies failing to find significant differences in the amount of this cytokine in supernatants derived from bone marrow cultures or fresh bone marrow plasma derived from myeloma patients compared to controls. In addition, antibodies to lymphotoxin do not reduce the bone resorbing activity of fresh bone marrow plasma from myeloma patients. Another tumor necrosis factor, TNFα, is found at higher levels in supernatants from these patients' bone marrow cultures, and is capable of stimulating osteoclast formation. Its effects are mediated by stimulation of the proteolytic breakdown of IκB that leads to the release of NFκB. This enhancer translocates into the nucleus where it induces transcription of specific genes, some of which are involved in enhancing bone resorption. The importance of NFκB in bone resorption is supported by recent studies showing that NFκB knockout mice show osteopetrotic bones.
A recently identified receptor for activation of NFκB, a member of the TNF receptor family, and its ligand, RANKL, has been shown to be key players in the development of osteoclasts. Unlike other soluble bone resorbing factors, the activity of these molecules requires direct cell-to-cell contact. It has been known for some time that osteoclastogenesis requires the direct interaction of osteoblasts or stromal cells with osteoclasts. The identification of RANK expressed on the surface of osteoclasts and RANKL on osteoblasts and stromal cells explains how this interaction leads to osteoclast development. TNF itself is capable of stimulating osteoblasts to increase expression of RANKL. Malignant plasma cells from myeloma patients have been recently shown to express RANKL so that it is possible that the tumor cells themselves may directly stimulate osteoclast development in the myeloma bone marrow environment. Importantly, a soluble decoy receptor called osteoprotegerin (OPG) exists that binds RANKL and prevents the binding of the ligand to RANK. In fact, animals lacking OPG show profound osteoporosis. It is the delicate balance between soluble OPG and RANKL that determines the amount of bone loss. Because of its profound inhibitory effect on bone loss, OPG is now being evaluated in early clinical trials.
Despite the early findings of Mundy identifying IL-1β as one of the OAFs in multiple myeloma patients, its role in myeloma bone disease has become less clear. Supernatants from myeloma patients' bone marrow cultures show increased levels of this cytokine.3 The role of IL-1β in resorbing bone has been shown in bone organ cultures. Although inhibitors of this protein and its receptors are capable of inhibiting bone resorption generated by supernatants derived from myeloma bone marrow, other groups have been unable to show that this cytokine plays a role in myeloma bone disease.2 Fresh bone marrow plasma from myeloma patients does not show higher levels of IL-1β, and antibodies to this protein do not change the ability of myeloma bone marrow plasma to induce osteoclast formation.
IL-6, which is mainly produced by nonmalignant cells in the bone marrow of myeloma patients, is a cytokine capable of stimulating growth and preventing apoptosis of the malignant cells in myeloma patients. However, it also has been shown to stimulate bone loss by inhibiting bone formation and stimulating osteoclast formation especially in the presence of IL-1 or the soluble IL-6 receptor (sIL-6R). Stromal cells, osteoclasts and osteoblasts are all major sources of IL-6 in the bone marrow microenvironment. Recent studies show that malignant cells from myeloma patients increase IL-6 production by osteoblasts both by direct cell-to-cell contact and release of soluble factors. Since some studies report that malignant cells from myeloma patients produce IL-1 and TNF and both of these cytokines stimulate IL-6 production by osteoblasts,9,10 either or both may be the soluble factor(s) involved in myeloma cell-induced release of IL-6 by osteoblasts. Thus, these studies suggest the role of bone cells not only in bone-related changes in these patients but also in the promotion of growth and prevention of apoptosis of the tumor cells themselves as mediated by IL-6.
IL-11 stimulates osteoclastogenesis and inhibits bone formation.11 It has been shown to be produced by osteoblasts, and present in culture supernatants of bone marrow cells from myeloma patients. This cytokine stimulates RANKL expression by osteoblasts. In addition, recent studies have shown that hepatocyte growth factor (HGF) which is known to be produced by malignant plasma cells,12 may also induce IL-11 secretion by osteoblasts.13 Other cytokines such as IL-1 are capable of potentiating the effect of HGF on IL-11 secretion. High serum levels of HGF are associated with a poor prognosis in myeloma patients.
It is clear that macrophage colony-stimulating factor (M-CSF) is involved in early events in the development of osteoclasts. Although this factor along with RANKL are all that is required for osteoclastogenesis to occur, its role in myeloma bone disease remains unclear. Some studies show higher amounts of this factor in the serum of myeloma patients and its correlation with tumor load.
Recently, macrophage inhibitory protein-1α (MIP-1α), has been identified as an important factor involved in myeloma bone disease.2 Levels of this cytokine are elevated in the bone marrow of these patients. This chemokine is capable of inducing osteoclast formation in vitro, and antibodies to this protein block the induction of osteoclast formation by fresh bone marrow plasma from myeloma patients. In addition, this chemokine attracts and activates monocytes, and is a potent inhibitor of early hematopoiesis.
There is evidence for an increasing role of angiogenesis in the pathogenesis of multiple myeloma. It is clear that vascular endothelial growth factor (VEGF) is produced by malignant plasma cells, and the receptors that bind this factor are expressed on bone marrow stromal cells.14 In fact, recent results show that VEGF increases IL-6 production by bone marrow stromal cells from myeloma patients. This may indirectly lead to enhanced bone loss in these patients. Until recently, it was not clear that VEGF had any direct role in bone resorption. However, it has now been shown that VEGF can replace M-CSF in leading to early osteoclast development.15
Adhesion Molecules
The critical role of the integrin αvβ3 in bone resorption has been demonstrated in several recent studies.16,17 Mice lacking the β3 molecule show reduced bone resorption, and antibodies and blocking ligands to αvβ3 reduce osteoclastic activity.16,17 The αvβ3 molecule binds to a tripeptide, RGD, within the extracellular matrix. When this integrin is bound to specific extracellular matrix proteins that contain this peptide, a chain of events occurs within the osteoclast that results in a cell that is actively resorbing bone. Both the protein tyrosine kinase Src and another specific kinase, Pyk-2, are activated upon αvβ3 binding to the matrix proteins. The pivotal role of Src in bone resorption is supported by the development of osteopetrosis in mice without Src.18 These animals contain abnormal osteoclasts and lack bone-resorbing activity. In addition to osteoclasts, αvβ3 has also been shown to be present on tumor cells and endothelial cells.19,20 This molecule is expressed by neovascularized blood vessels and its expression by tumor cells correlates with invasiveness. Thus, this integrin is important in bone resorption, angiogenesis, and tumor invasion.
Bone Resorption Markers
A variety of markers of bone resorption and formation have been used to assess bone disease in myeloma patients.21,22,23,24 Patients with multiple myeloma show the expected increases in bone resorption markers such as C-terminal telopeptide of type I collagen, pyridinoline and deoxypyridinoline and decreases in bone formation markers such as osteocalcin. In addition, a decrease in osteocalcin level or higher ICTP concentrations predicts a shortened survival in myeloma. In a recent placebocontrolled randomized Finnish clinical trial involving oral clodronate, higher baseline levels of the amino-terminal propeptide of type I procollagen (PINP), a product of growing osteoblasts, ICTP and alkaline phosphatase (AP) were associated with a worse survival. PINP and ICTP levels decreased dramatically during clodronate treatment. Similarly, treatment with oral risedronate reduced urinary pyridinoline/creatinine and deoxypyridinoline/creatinine ratios as well as the bone formation markers AP and osteocalcin plasma levels. Monthly administration of intravenous pamidronate is also associated with a decrease in both bone resorption and bone formation markers. In the Finnish clodronate trial, a decrease in these markers during clodronate therapy was associated with a better survival. In current clinical trials evaluating newer bisphosphonates, it is being determined whether baseline values or changes in these markers predict for either the development of new skeletal complications or whether these agents will be clinically effective in individual cases. There is some evidence to support this from two recent studies. In breast cancer patients with lytic bone metastases receiving the aminobisphosphonate pamidronate who normalized urinary N-telopeptide, a newer bone resorption marker, levels, there was a reduction in the development of new fractures as well as progression of bone metastases.25 In a recent study evaluating another nitrogen-containing bisphosphonate, ibandronate, in myeloma patients, increased suppression of urinary bone resorption markers was associated with reduction in the development of bony complications.26
Treatment of Myeloma Bone Disease
Although analgesics, surgery and radiotherapy may effectively palliate patients with complications from myeloma bone disease, these treatments do little to slow the progressive bone loss that occurs in these patients. Chemotherapy may reduce tumor burden but has little impact on the underlying bone disease. The demonstration that bisphosphonates could reduce the skeletal complications and effectively palliate the symptoms related to bone disease in myeloma patients has resulted in a dramatic change for the better in the lives of these patients. Importantly, because these agents lack significant bone marrow suppressive effects, they can be administered as an adjunct to other cytotoxic therapy. Recent laboratory studies suggest that these drugs have potential anti-myeloma effects, and this is supported by clinical studies showing an improvement in the survival of some patients receiving these drugs. Newer more potent aminobisphosphonates are in active clinical trials and offer the potential to further reduce bone-related problems while improving the overall outcome of myeloma patients. In addition to the bisphosphonates, a number of other types of anti-bone resorptive agents are entering clinical trials.
Bisphosphonates
Pharmacology
Bisphosphonates are analogues of endogenous pyrophosphate in which a carbon atom replaces the central atom of oxygen.27 This carbon substitution makes these compounds resistant to hydrolysis, and allows two additional chains of variable structure. One of these side chains usually contains a hydroxyl moiety that allows high affinity for calcium crystals and bone mineral. The differences at the other side chain produce marked differences in the anti-resorptive potency of different bisphosphonates. In fact, the newer bisphosphonates, such as ibandronate and zoledronic acid, show 10,000-100,000-fold more potency than do the older agents such as etidronate. These drugs have limited bioavailability (usually < 1%) and are also poorly tolerated orally, with significant gastrointestinal toxicity, particularly esophagitis and esophageal ulcers. The bisphosphonates are almost exclusively eliminated through renal excretion. Although significant nephrotoxicity can occur, this is clearly related to the drug dose and rate of intravenous infusion. Importantly, this renal dysfunction results from the bisphosphonate backbone so that the newer, more potent bisphosphonates can be administered at therapeutic doses more rapidly without significant risk of nephrotoxicity.
Bisphosphonates preferentially bind to bones that have high rates of bone turnover (i.e. undergoing increased bone resorption or formation). Thus, these agents are concentrated at the exposed bone surface that undergoes active remodeling. Once these drugs are integrated into a region of bone that is not undergoing remodeling, the bisphosphonates lose their ability to inhibit bone resorption. As a result, continued administration of these agents is required to achieve an enduring reduction in bone resorption in a patient with lytic bone disease.
Mechanisms of action
The inhibition of bone resorption occurs as a result of the effect of these drugs on osteoclasts both directly and indirectly. Bisphosphonates were first shown to reduce osteoclast development from their precursors as well as inhibit movement of osteoclasts to the bone surface where they would normally resorb bone. These drugs are also capable of inducing apoptosis of osteoclasts as well as tumor cells from myeloma patients.28,29 Interestingly, this has been shown to occur as a result of inhibition of the mevalonic acid pathway particularly in nitrogen-containing bisphosphonates.29 Interestingly, the statin drugs that lower cholesterol also block enzymes in this same pathway. Both types of drugs prevent prenylation of a number of proteins including guanidine triphosphatases such as ras and rho. Specifically, the addition of geranyl-geranylated derivatives rather than farnesylated compounds is able to overcome the apoptosis-inducing effects of aminobisphosphonates and statin derivatives.29 In addition, a reduction in the production of the cytokine IL-6 from myeloma bone marrow stromal cells exposed to bisphosphonates has also been found.30,31 This cytokine is not only capable of stimulating bone resorption but also is an important growth factor and anti-apoptotic factor in myeloma.7 Thus, reducing the availability of this cytokine in the bone micro-environment by exposure to bisphosphonates may not only reduce bone loss but may also have an antimyeloma effect. Recent animal studies have shown that the aminobisphosphonates also have potent anti-angiogenic activity. Anti-angigoenesis agents such as thalidomide have recently been shown to be effective antitumor agents in myeloma.32 Thus, the anti-angiogenic effect of the aminobisphosphonates may contribute to these drugs' antibone resorptive effect (see above) as well as provide additional mechanisms by which these drugs may have antimyeloma effects as well. A new potential anti-tumor mechanism for these compounds was recently reported for aminobisphosphonates.33 These drugs were shown to induce expansion of γδT cells in peripheral blood mononuclear cell cultures, and enhance cytotoxicity of malignant plasma cells in bone marrow cultures by these T lymphocytes. Thus, there is increasing evidence that bisphosphonates, especially the nitrogen-containing compounds, can lead to direct and indirect effects that result not only in less bone loss but less tumor burden as well. In support of this, Epstein and colleagues34 have shown a reduction in both lytic bone metastases and improvement in survival in severe combined immunodeficiency mice implanted with fresh human myeloma bone marrow and fetal bone after treatment with pamidronate. However, treatment with ibandronate in a murine model of myeloma35 showed only a reduction in lytic bone disease, without an impact on tumor burden.
Clinical studies in myeloma patients
Small, open-label trials involving bisphosphonates suggested their potential role in these patients. The first two randomized double blind placebo-controlled trials involved use of daily oral etidronate (5 mg/kg/d)36 or clodronate (2,400 mg/d)37 in newly diagnosed patients who also received oral melphalan and prednisone. Neither of these trials showed any significant clinical benefit with these less potent oral agents, although there were fewer patients developing new lytic lesions in the clodronate trial who received the bisphosphonate. Oral clodronate has also been the subject of another randomized double blind trial from the Medical Research Council. 38 In addition to their chemotherapy, 536 newly diagnosed multiple myeloma patients received either 1,600 mg of clodronate or placebo daily. Although fewer patients treated with clodronate experienced severe hypercalcemia and vertebral as well as nonvertebral fractures, there were no differences in the time to first skeletal event or requirement for radiotherapy. The drug had no significant effect on pain except back pain at a single time point (24 months), and performance status was also unaffected except at this same time point. Patients receiving clodronate showed no difference in survival compared to the placebo group. Oral pamidronate (300 mg/d) was compared to placebo in 300 newly diagnosed myeloma patients also receiving intermittent oral melphalan and prednisone, and had no effect on skeletal-related morbidity or survival.39 Several small open-label studies suggested the possible benefit of infusional pamidronate in myeloma patients. As a result, 392 myeloma patients with Durie-Salmon Stage III multiple myeloma and at least one lytic lesion were randomized to receive monthly 4-hour infusions of either placebo or pamidronate (90 mg) for 21 cycles.40,41
Patients were stratified prior to randomization according to the amount of prior antimyeloma therapy at study entry: stratum 1, first-line chemotherapy; stratum 2, second-line or greater chemotherapy. Initial results after nine cycles showed a marked reduction in the proportion of patients having any skeletal event with pamidronate treatment,40 and this difference was also observed for patients in both stratum 1 and stratum 2. Patients receiving pamidronate also showed decreases in bone pain and no increase in analgesic usage or deterioration in performance status or quality of life, in contrast to placebo patients. The results after an additional 12 cycles of randomized treatment continued to show that the proportion of patients developing any skeletal event remains smaller in the pamidronate group.41 Among all 392 patients, there was no difference in overall survival between the pamidronate and placebo groups. However, patients receiving pamidronate in stratum 2 showed a median survival of 21 months compared with 14 months for placebo patients. Thus, it is clear from this large randomized trial that intravenous pamidronate (90 mg), when administered monthly as an adjunct to chemotherapy, results in a significant reduction in the skeletal complications as well as palliation of symptoms among myeloma patients with lytic bone disease. It is unclear whether this drug or other bisphosphonates will be similarly effective for patients without lytic bone disease or prevent patients with solitary plasmacytoma from developing multiple myeloma. Third-generation bisphosphonates (e.g., zoledronic acid and ibandronate), that appear to be more than 100 times more potent than second-generation aminobisphosphonates, have recently entered clinical trials. Very small doses of these agents effectively restore normocalcemia in patients with tumor-induced hypercalcemia.42,43 Recent results show the superiority of zoledronic acid (4 or 8 mg) compared to pamidronate (90 mg) in reversing hypercalcemia of malignancy.44 Clinical evaluation of zoledronic acid and ibandronate in bone metastases is in progress. Zoledronic acid can be given safely over several minutes and produces similar anti-resorptive effects, as assessed by bone resorption marker, as 90 mg of pamidronate.45 Preliminary reports from a randomized phase II study comparing monthly infusions of zoledronic acid (0.4, 2 or 4 mg as a 5-minute infusion) compared to pamidronate (90 mg as a 2-hour infusion) in 280 patients with lytic bone metastases (109 myeloma) have been reported.46 The proportion of patients with any SRE was lower (30-35%) in the 2-mg and 4-mg zoledronic acid and the pamidronate groups compared to the 0.4 mg zoledronic acid group (46%). This phase II trial was not “powered” to show superiority of zoledronic acid compared to pamidronate. An ongoing, larger phase III randomized trial compares higher doses of zoledronic acid to 90-mg pamidronate in multiple myeloma or breast cancer with lytic disease. Ibandronate is another newer potent bisphosphonate. A phase III placebo-controlled trial of 214 myeloma patients with Durie Salmon Stage II or III was recently completed.26 Patients either received monthly bolus injections of 2 mg of ibandronate or placebo injections in addition to their antineoplastic therapy. Ninety-nine patients in each group were evaluable for efficacy. The mean number of events per patient year on treatment was similar in both groups (ibandronate 2.13 vs placebo 2.05). However, in the subgroup of ibandronate-treated patients showing a sustained reduction in bone resorption markers, less SREs/year occurred. There was no difference in overall survival. Thus, this dose of ibandronate is inadequate to show significant effects on preventing skeletal complications in myeloma.
New Agents
A number of new agents are entering clinical trials for the treatment of metastatic bone disease. An oral αvβ3 antagonist, the RGD peptidomimetic SD-7784, is entering a phase I trial in myeloma patients. αvβ3 antagonists have previously been shown to have potent anti-tumor and anti-osteoclastic effects in animal models.16,17,20 Thus, these drugs have the potential to both reduce bone loss as well as reduce tumor burden in myeloma patients. An OPG analog, an inhibitor of RANK-RANKL interaction, is now being evaluated in a clinical trial in patients with bone metastases. In addition, inhibitors of src activity show marked anti-resorptive capability in animal models47 and may enter clinical trials soon. The cholesterol-lowering statin drugs have recently shown to increase bone formation in vitro and in vivo in rodent models,48 possibly by their induction of bone morphogenetic protein (BMP)-2 as well as their ability to induce apoptosis of osteoclasts. Some studies have suggested that patients receiving statins to lower serum cholesterol also show a reduced risk of developing fractures. The apoptotic-inducing effect of statins has also been shown to occur in myeloma cells in vitro through their blockage of the mevalonic acid pathway.29 Whether either the bone-enhancing or anti-tumor effects of statins is clinically observed in myeloma patients is being assessed in clinical trials. New attempts are underway to encourage new bone formation using BMPs, gene therapy and mesenchymal stem cells.49 Thus, the potential exists to not only halt the relentless bone loss in myeloma patients but to repair their already damaged bones.
IV. Novel Biologically Based Therapies for Myeloma
Kenneth C. Anderson, M.D.*
Dana-Farber Cancer Institute, 44 Binney Street, Boston MA 02115
Novel Therapeutic Targets
Multiple myeloma is incurable with current therapies, but several recent biological advances have provided the framework for novel therapeutic strategies. First, multiple lines of evidence suggest that the precursor cell in multiple myeloma is a cytoplasmic μ-positive B cell that has undergone antigen selection and somatic hypermutation in the lymph node, but which has not yet undergone isotype class switching. Chromosomal translocations involving the immunoglobulin (Ig) switch region are common, and multiple partner chromosomes have been described. Given that abnormalities in Ig gene rearrangement, IgH class switching, and DNA damage repair are hallmarks of myeloma, we have undertaken studies of Ku expression and function in human myeloma cells.1 Ku is a heterodimer composed of Ku70 and Ku86 subunits that binds with high affinity to altered DNA and is essential for double stranded DNA break (DSB) repair and normal Ig V(D)J recombination. Our studies to date have identified a 69 kD variant of Ku86 (Ku86v) in some myeloma cells, which neither binds DNA-dependent protein kinase (DNA-PKcs) nor activates kinase activity and therefore may account for decreased DNA repair and increased sensitivity to radiation and chemotherapy; conversely, Ku86 in myeloma cells confers resistance to therapy and may represent a therapeutic target.
Myeloma cells home to the bone marrow (BM) microenvironment, where excess plasma cells characteristic of this disease accumulate. We have demonstrated mechanisms whereby tumor cells specifically adhere both to extracellular matrix (ECM) proteins and bone marrow stromal cells (BMSCs), as well as changes in cell adhesion molecule profile correlating with egress of tumor cells into the peripheral blood (PB) in the context of progressive disease and plasma cell leukemia (PCL).2 Adhesion molecules not only localize tumor cells within the BM microenvironment but also have multiple functional sequelae. Adherence to BMSCs confers resistance to apoptosis,3 and agents that block adhesion, i.e. bisphosphonates, can confer sensitivity to treatment. Adherence of tumor cells to BMSCs upregulates IL-6 transcription dependent in part on the transcription factor NFkB, as well as IL-6 secretion within BMSCs,4 and also allows for tumor cell secretion of cytokines, i.e., transforming growth factor β, which further enhances IL-6 transcription and secretion in BMSCs.5 This is of central importance since our studies have shown that IL-6 is both a growth and survival factor for myeloma cells.6 Proteasome inhibitors are novel drugs that inhibit activation of NFκB7 ; they induce apoptosis of myeloma cells which are resistant to conventional therapy and importantly, inhibit the NFκB-dependent upregulation of IL-6 in BMSCs and related paracrine growth of adherent tumor cells.8
We have shown that proliferation of myeloma cells triggered by IL-6 is mediated via the mitogen-activated protein kinase (MAPK) cascade,9 suggesting therapeutic strategies based upon blocking this pathway in tumor cells. Apoptosis triggered by gamma irradiation (IR), Fas, and Dexamethasone (Dex) is mediated via distinct signaling cascades. For example, Dex (but not IR or Fas)-induced apoptosis is mediated via activation of related adhesion focal tyrosine kinase (RAFTK).10 IL-6 is also a survival factor for human myeloma cells, specifically activating protein tyrosine phosphatase (SHP2) and thereby blocking the activation of RAFTK and related apoptosis in response to Dex.11 Blocking SHP2 activation with small molecule SHP2 inhibitors may therefore relieve this protective effect. Further delineation of these pathways will derive strategies for triggering apoptosis, overcoming Dex resistance, and inhibiting survival signals, which will provide the framework for related novel treatment approaches.12
Our recent studies also suggest that adhesion of myeloma cells to BMSCs also upregulates vascular endothelial growth factor (VEGF) secretion by BMSCs and myeloma cells. Therefore, in addition to examining the effect of VEGF on BM angiogenesis, we are evaluating whether VEGF is a growth and/or survival factor for myeloma cells. Preliminary studies suggest that VEGF induces MAPK activation and proliferation of some myeloma cells, and that VEGF receptor inhibitors block proliferation of tumor cells and may therefore be useful clinically. This increase in VEGF may in part account for increased angiogenesis in human myeloma BM. Based upon its anti-angiogenic activity, thalidomide (Thal) was recently used very successfully to treat patients with myeloma, even those refractory to conventional therapy.13 Although Thal may be acting in myeloma as an anti-angiogenic agent, there are multiple other potential mechanisms of action of Thal and/or its in vivo metabolites.14 First, Thal may have a direct effect on the myeloma cell and/or BMSC cell to inhibit growth and survival. For example, free radical-mediated oxidative DNA damage may play a role in the teratogenicity of Thal and may also have anti-tumor effects. Second, adhesion of myeloma cells to BMSCs both triggers secretion of cytokines that augment myeloma cell growth and survival and confers drug resistance; thalidomide modulates adhesive interactions and thereby may alter tumor cell growth, survival, and drug resistance. Third, cytokines secreted into the BM microenvironment by myeloma and/or BMSCs, such as IL-6, IL-1β, IL-10 and TNFα, may augment myeloma cell growth and survival, and Thal may alter their secretion and bioactivity. Fourth, VEGF and basic fibroblast growth factor (bFGF)-2 are secreted by myeloma and/or BMSCs and may play a role both in tumor cell growth and survival, as well as BM angiogenesis. Given its known anti-angiogenic activity, Thal may inhibit activity of VEGF, bFGF-2, and/or angiogenesis in myeloma. Finally, Thal may be acting against myeloma via its immunomodulatory effects, such as induction of a Th1 T cell response with secretion of interferon gamma (IFN-γ) and IL-2. Understanding which of these mechanisms mediate anti-myeloma activity will be critical both to optimally define its clinical utility and to derive analogues with enhanced potency and fewer side effects. Already two classes of Thal analogues have been reported, including phosphodiesterase 4 inhibitors that inhibit TNFα but have little effect on T cell activation, and others that are not phosphodiesterase inhibitors but do markedly stimulate T cell proliferation as well as IFN-γ and IL-2 secretion.15 In recent studies, we have delineated mechanisms of anti-tumor activity of Thal and its potent analogoues (immunomodulatory drugs, IMiDs).16 Importantly, these agents act directly, via inducing apoptosis or G1 growth arrest, in myeloma cell lines and patient myeloma cells that are resistant to melphalan, doxorubicin, and Dex. Moreover, Thal and the IMiDs enhance the anti-myeloma activity of Dex and, similar to Dex, apoptotic signaling triggered by Thal and the IMiDs is associated with activation of RAFTK. Most recent studies suggest that treatment with these drugs augments their adherence to BMSCs and fibronectin, but abrogates the upregulation of IL-6 and VEGF induced by tumor cell binding. Finally, these drugs appear to upregulate natural killer cell-mediated killing of myeloma cells. These studies establish the framework for the development and testing of Thal and the IMiDs in a new treatment paradigm to target both the tumor cell and the microenvironment, overcome classical drug resistance, and achieve improved outcome in this presently incurable disease.
Immune-Based Strategies
Although high response rates can be achieved using high-dose therapy followed by stem cell grafting, the majority of patients are destined to relapse and few, if any, are cured. Major obstacles to cure are the excessive toxicity noted after allografting in myeloma, contaminating tumor cells in autografts, and most importantly, the persistence of minimal residual disease (MRD) after high-dose therapy followed by either allogeneic or autologous stem cell transplantation. In this context, we are developing improved strategies to treat MRD after high-dose therapy followed by allogeneic or autologous stem cell grafting. Most importantly, we are developing multiple approaches for the generation and enhancement of allogeneic and autologous anti-myeloma immunity in vitro and in animal models. Based upon these studies, we are designing clinical trials that couple our treatments to achieve MRD with these novel immune-based therapies post transplant in an attempt to achieve long-term disease-free survival and potential cure of multiple myeloma.
Allografting
We have carried out high dose therapy followed by T (CD6) cell-depleted allografting using histocompatible sibling donors in 61 patients with myeloma whose disease remained sensitive to conventional chemotherapy.17 This included 39 men and 22 women with median age of 44 (32-55) years. Most patients presented with advanced stage myeloma. The majority of patients achieved either complete (28%) or partial (57%) response; importantly, only 17% patients developed ≥ grade 2 graft-versus-host disease (GVHD), and the transplant-related mortality was only 5%. Therefore we have shown that allografting can be done safely in myeloma. Indeed in our Center the over-all and progression-free survival of allograft and autograft recipients is equivalent, with approximately 40% patients surviving at 3 years. However, only 20% patients are disease free at ≥ 4 years posttransplant. Excitingly, data from our centers and others unequivocally demonstrate that donor lymphocyte infusions (DLI) mediate a graft-versus-myeloma effect (GVM) which can effectively treat relapsed myeloma post allografting.18,19 Unfortunately GVHD is a frequent cause of morbidity and mortality after DLI. However, at our Myeloma Center five of seven patients who had relapsed post-CD6-depleted allografting responded (including three complete responses) to CD4+ T cell enriched DLI, in some cases in the absence of GVHD. This raised the possibility that distinct T cell clones may be mediating GVM versus GVHD. Given the high response rates but inevitable relapses observed in the setting of allografting for myeloma, we are now testing in a clinical protocol whether CD4+ DLI at 6 months post-CD6-depleted allografting may mediate GVM, which will effectively treat MRD and thereby improve outcome. To date 21 patients have undergone CD6-depleted allografting, 18 of whom developed only grades 0-1 GVHD. Eleven of these 18 patients are > 6 months posttransplant and have received CD4+ DLI. Eight of the 11 patients who received DLI demonstrated further response (including four complete responses), suggesting the potential of DLI to treat MRD. Therefore our studies already suggest that GVM can be adoptively transferred in this fashion. We are also examining T cell repertoire, based upon Vβ T cell receptor gene rearrangement, to identify those clonal T cells associated with GVM and their target antigens on tumor cells.20,21 Already we have shown that T cells mediating GVM can target idiotypic antigens and are presently identifying other target antigens. The goal of these studies is to characterize, isolate, and expand GVMT cell clones for antigen-specific adoptive immunotherapy.
Autografting
Although randomized studies convincingly demonstrate a survival advantage for myeloma patients treated with high-dose therapy and autografting compared to those receiving conventional chemotherapy, this treatment is not curative. Two sites of MRD contribute to the failure of autografting: MRD in the autograft and MRD in the patient post myeloablative therapy. At our Center we have to date carried out high dose therapy and stem cell autografting in 105 patients who presented with advanced stage myeloma but whose disease remained sensitive to chemotherapy. As in our allografting experience, the majority of patients responded, including 30% complete and 62% partial responses. However, none of these patients are cured. We have produced monoclonal antibodies in the laboratory that have been used to deplete tumor cells from myeloma autografts.22 We have also evaluated CD34 selection techniques to select normal hematopoietic progenitor cells within autografts.23 However, these methods deplete only 2-3 logs of tumor cells, and > 50% autografts still contain MRD. Based upon our laboratory data that myeloma cells express Muc-1 and adenoviral receptors, we have specifically transduced tumor cells within myeloma autografts with the thymidine kinase (tK) gene using an adenoviral vector with a tumor selective (Muc-1) promotor, followed by purging tumor cells exvivo by treatment with ganciclovir.24 Pilot studies suggest that > 6-7 logs of tumor cells can be purged under conditions that do not adversely affect normal hematopoietic progenitor cells, setting the stage for a clinical trial of adenoviral purging prior to autotransplantation.
We are also attempting to generate and expand anti-myeloma specific autologous T cells ex vivo for adoptive immunotherapy of MRD in the patient post autotransplant. It is now possible to clone the gene for the patient's specific idiotypic protein, use computer programs to identify gene sequences encoding for peptides predicted to be presented within the groove of Class I HLA of a given patient's HLA type, and expand peptide specific T cells ex vivo.25 A similar strategy can be used to expand T cells against peptides within shared antigens that are overexpressed on myeloma cells, such as telomerase catalytic subunit (hTERT),26 Muc-1,27 or CYP1B1.28 Strategies are also being tested to enhance the immunogenicity of the whole tumor cell. Our laboratory studies have shown that autologous T cells do not proliferate to the patients' own tumor cells as targets in an autologous MLR. However, CD40 activation of myeloma cells upregulates Class I and II HLA, costimulatory, GRP94, and other molecules; and CD40 activated myeloma cells trigger a brisk autologous T cell response.29 T cells can therefore be harvested from myeloma patients before autografting, expanded ex vivo using CD40-activated autologous myeloma cells as stimuli, and given as adoptive immunotherapy to treat MRD post transplant. Finally, we are developing and examining the clinical utility of a variety of myeloma vaccines. First, based upon our observation that CD40-activated myeloma cells trigger a brisk autologous T cell response, we will examine the utility of vaccinations of patients with autologous CD40 activated tumor cells. Second, based upon our demonstration of the expression of Muc-1 core protein on freshly isolated myeloma cells,27 we will construct and evaluate two vaccines: recombinant vaccinia virus containing the Muc-1 gene and autologous dendritic cells (DCs) transduced using adenoviral vectors with Muc-1. Excitingly, we have recently shown that myeloma cells can be fused to DCs and that the use of the myeloma cell-DC fusion as an antigen presenting cell presents the entire myeloma cell as foreign. In a syngeneic murine myeloma model, vaccinations with myeloma cell-DC fusions, but not with either myeloma cells or DCs alone, demonstrated both protective and therapeutic efficacy. Most importantly, we have shown that patient myeloma cells can be fused to autologous DCs, which are readily isolated from either patient bone marrow and peripheral blood,30 and that autologous myeloma cell-DC fusions can trigger specific cytolytic autologous T cell responses in vitro.31 We will therefore translate these findings to the bedside in clinical trials of myeloma-DC fusion vaccines to assess in vivo myeloma-specific T and B cell responses as well as clinical efficacy. Ultimately, vaccinations will be coupled with adoptive immunotherapy in an attempt to treat MRD post autografting and thereby improve outcome.