Abstract
The myelodysplastic syndromes (MDS) constitute a challenge for the biologist as well as for the treating physician. In Section I, Dr. Willman reviews the current classifications and disease mechanisms involved in this heterogeneous clonal hematopoietic stem cell disorder. A stepwise genetic progression model is proposed in which inherited or acquired genetic lesions promote the acquisition of “secondary” genetic events mainly characterized by gains and losses of specific chromosome regions. The genetic risk to develop MDS is likely multifactorial and dependent on various constellations of risk-producing and -protecting alleles. In Section II Dr. Barrett with Dr. Saunthararajah addresses the immunologic factors that may act as important secondary events in the development of severe pancytopenia. T cells from patients with MDS may suppress autologous erythroid and granulocytic growth in vitro, and T cell suppression by antithymocyte globulin or cyclosporine may significantly improve cytopenia, especially in refractory anemia. Recent studies have also demonstrated an increased vessel density in MDS bone marrow, and a phase II trial of thalidomide showed responses in a subgroup of MDS patients especially in those with low blast counts. In Section III Dr. Hellström-Lindberg presents results of allogeneic and autologous stem cell transplantation (SCT), intensive and low-dose chemotherapy. The results of allogeneic SCT in MDS are slowly improving but are still poor for patients with unfavorable cytogenetics and/or a high score according to the International Prognostic Scoring System. A recently published study of patients between 55-65 years old showed a disease-free survival (DFS) at 3 years of 39%. Consolidation treatment with autologous SCT after intensive chemotherapy may result in long-term DFS in a proportion of patients with high-risk MDS. Low-dose treatment with 5-azacytidine has been shown to significantly prolong the time to leukemic transformation or death in patients with high-risk MSA. Erythropoietin and granulocyte colony-stimulating factor may synergistically improve hemoglobin levels, particularly in sideroblastic anemia. Recent therapeutic advances have made it clear that new biological information may lead to new treatment modalities and, in combination with statistically developed predictive models, help select patients for different therapeutic options.
I. Biologic and Genetic Features of the Myelodysplastic Syndromes
Cheryl L. Willman, M.D.*
University of New Mexico Cancer Center, 2325 Camino de Salud NE, Room 101, Albuquerque NM 87131
Recent scientific advances have provided new insights into the etiology and pathogenesis of the myelodysplastic syndromes (MDS). Despite heterogeneous morphologic, genetic, biologic, and clinical features, all forms of MDS are clonal hematopoietic stem cell disorders characterized by ineffective hematopoiesis and peripheral cytopenias. Although a substantial proportion of MDS cases evolve to acute myeloid leukemia (AML), the natural history of these syndromes ranges from more indolent forms of disease spanning years to those with a rapid evolution to AML. Thus, MDS is best considered a preleukemic disorder in which the neoplastic clone that has been established may or may not fully progress to acute leukemia. Although the relationship between MDS and de novo AML has been controversial and current disease classification systems (Table 1) are considered unsatisfactory, most hematologists now consider MDS and AML as part of the same continuous disease spectrum rather than as distinct disorders. This review will briefly high-light current controversies in the classification of MDS and AML, the cytogenetic and molecular genetic features of MDS, the biologic features that characterize MDS including abnormal apoptosis and an altered marrow microenvironment, and new and highly interesting insights into the complex genetic predisposition to MDS. Excellent, well-referenced reviews are also available.1,2
MDS Classification Systems.1
| FAB Classification System2 . | WHO Classification System3 . | IPSS Risk-Based Classification System4 . | |
|---|---|---|---|
| Refractory Anemia (RA): Cytopenia of one PB lineage; normo- or hypercellular marrow with dysplasias; < 1% PB Blasts and < 5% BM Blasts. | Myelodysplastic Syndromes | Overall IPSS Risk Score Based On: | |
| Refractory Anemia (RA) | Marrow Blast Percentage | ||
| With ringed sideroblasts (RARS) | Blast % | IPSS Score | |
| Refractory Anemia with Ringed Sideroblasts (RARS): Cytopenia, dysplasia and the same % blast involvement in BM and PB as RA. Ringed sideroblasts account for >15% of nucleated cells in marrow. | Without ringed sideroblasts | < 5 | 0 |
| 5-10 | 0.5 | ||
| Refractory Cytopenia (MDS) with Multilineage Dysplasia (RCMD) | 11-20 | 1.5 | |
| 21-30 | 2.0 | ||
| Refractory Anemia with Excess Blasts (RAEB): Cytopenia of two or more PB lineages; dysplasia involving all 3 lineages; < 5% PB blasts and 5-20% BM Blasts. | Refractory Anemia with Excess Blasts (RAEB) | Cytogenetic Features5 | |
| Karyotype | IPSS Score | ||
| 5q-Syndrome | Good prognosis (-Y, 5q-,20q-) | 0 | |
| Intermediate prognosis | 0.5 | ||
| Myelodysplastic syndrome, unclassifiable | Poor prognosis (abn. 7; Complex) | 1.0 | |
| Refractory Anemia with Excess Blasts in Transformation (RAEB-T): Hematologic features identical to RAEB. > 5% Blasts in PB or 21-30% Blasts in BM or the presence of Auer rods in the blasts. | Myelodysplastic/Myeloproliferative Diseases | Cytopenias6 | |
| Cytopenia | IPSS Score | ||
| None or 1 Type | 0 | ||
| Chronic Myelomonocytic | 2 or 3 Types | 0.5 | |
| Chronic Myelomonocytic Leukemia (CMML): Monocytosis in PB (>1 × 109 per liter); <5% blasts in PB and up to 20% BM blasts | Leukemia (CMML) | ||
| Atypical Chronic Myelogenous | Overall IPSS Score and Survival | ||
| Leukemia (aCML) | Overall Score | Median Survival | |
| Low (0) | 5.7 Yrs. | ||
| Juvenile Myelomonocytic | Intermediate | ||
| Leukemia (JMML) | 1 (0.5 or 1.0) | 3.5 Yrs | |
| 2 (1.5 or 2.0) | 1.2 Yrs. | ||
| High (≥ 2.5) | 0.4 Yrs. | ||
| FAB Classification System2 . | WHO Classification System3 . | IPSS Risk-Based Classification System4 . | |
|---|---|---|---|
| Refractory Anemia (RA): Cytopenia of one PB lineage; normo- or hypercellular marrow with dysplasias; < 1% PB Blasts and < 5% BM Blasts. | Myelodysplastic Syndromes | Overall IPSS Risk Score Based On: | |
| Refractory Anemia (RA) | Marrow Blast Percentage | ||
| With ringed sideroblasts (RARS) | Blast % | IPSS Score | |
| Refractory Anemia with Ringed Sideroblasts (RARS): Cytopenia, dysplasia and the same % blast involvement in BM and PB as RA. Ringed sideroblasts account for >15% of nucleated cells in marrow. | Without ringed sideroblasts | < 5 | 0 |
| 5-10 | 0.5 | ||
| Refractory Cytopenia (MDS) with Multilineage Dysplasia (RCMD) | 11-20 | 1.5 | |
| 21-30 | 2.0 | ||
| Refractory Anemia with Excess Blasts (RAEB): Cytopenia of two or more PB lineages; dysplasia involving all 3 lineages; < 5% PB blasts and 5-20% BM Blasts. | Refractory Anemia with Excess Blasts (RAEB) | Cytogenetic Features5 | |
| Karyotype | IPSS Score | ||
| 5q-Syndrome | Good prognosis (-Y, 5q-,20q-) | 0 | |
| Intermediate prognosis | 0.5 | ||
| Myelodysplastic syndrome, unclassifiable | Poor prognosis (abn. 7; Complex) | 1.0 | |
| Refractory Anemia with Excess Blasts in Transformation (RAEB-T): Hematologic features identical to RAEB. > 5% Blasts in PB or 21-30% Blasts in BM or the presence of Auer rods in the blasts. | Myelodysplastic/Myeloproliferative Diseases | Cytopenias6 | |
| Cytopenia | IPSS Score | ||
| None or 1 Type | 0 | ||
| Chronic Myelomonocytic | 2 or 3 Types | 0.5 | |
| Chronic Myelomonocytic Leukemia (CMML): Monocytosis in PB (>1 × 109 per liter); <5% blasts in PB and up to 20% BM blasts | Leukemia (CMML) | ||
| Atypical Chronic Myelogenous | Overall IPSS Score and Survival | ||
| Leukemia (aCML) | Overall Score | Median Survival | |
| Low (0) | 5.7 Yrs. | ||
| Juvenile Myelomonocytic | Intermediate | ||
| Leukemia (JMML) | 1 (0.5 or 1.0) | 3.5 Yrs | |
| 2 (1.5 or 2.0) | 1.2 Yrs. | ||
| High (≥ 2.5) | 0.4 Yrs. | ||
Abbreviations: PB, peripheral blood; BM, bone marrow; abn, abnormality
Reference 21.
Reference 30.
IPSS Cytogenetic Classification28 : Good prognosis: -Y only, normal, del(5q) only, del(20q) only; Intermediate prognosis: +8, Single miscellaneous abnormality, double abnormalities; Poor prognosis: Complex (i.e. ≥ 3 abnormalities), any chromosome 7 abnormality.
IPSS Types of Cytopenia28 : Hemoglobin <10g per deciliter; Absolute neutrophil count <1500 per cubic millimeter; Platelet count < 100,000 per cubic millimeter.
MDS and AML Disease Classification Systems: Unresolved Controversies
The French-American-British (FAB) Classification, proposed in 1977, provided hematologists with the first consistent framework for morphologic classification of MDS (Table 1), the myeloproliferative disorders, and the acute leukemias.3,4 However, the separation of MDS as a distinct disorder from AML in the FAB classification scheme has been perceived by many to have scientifically impeded our understanding of the full spectrum of leukemic progression.1 Indeed, the initial failure to recognize and classify MDS as a “neoplastic” pre-leukemic disorder and part of the same disease spectrum as AML resulted in the exclusion of MDS cases from virtually all US cancer registries and the NCI-sponsored Surveillance, Epidemiology, and End Results (SEER) program (www.seer.cancer.gov). This has greatly impeded studies of the true incidence, natural history, and epidemiology of MDS in the US. Importantly, however, European epidemiologic studies suggest that the incidence of MDS is at least as high as that of AML, particularly AML cases that arise in older individuals.5 In the US, the age-specific incidence rate for AML in males aged 50 years at diagnosis is 3.5 per 100,000, increasing dramatically to 15 at age 70 and 35 at age 90.6 (See also www.seer.cancer.gov.) With the exponential increase in the incidence of AML with age and the aging of our populations, the median age at diagnosis of AML in the US is currently 63 years. Thus, the majority of AML cases, like MDS, occur in older individuals. Further linking MDS and AML, several studies have noted that the biologic, morphologic, and genetic features of AML arising in older individuals are similar to 1) primary MDS; 2) AML arising secondary to antecedent MDS; 3) AML arising secondary to prior therapy, particularly alkylating agent exposure; and 4) AML cases that arise from documented environmental or occupational exposures to agents such as benzene, petroleum, organic solvents, and arsenical pesticides.7,8,9,10,11,12,13
In the FAB Classification (Table 1), the two primary distinguishing features between the various MDS subtypes, chronic myelomonocytic leukemia (CMML) and AML, are blast cell percentage and the presence of dysplastic features. CMML is now considered a myeloproliferative/leukemic-like disorder and frequently associated with t(5;12)(q33;p13),1,14 and AML is defined as ≥ 30% marrow blasts with the various MDS subtypes ranging from < 5% to < 30% blasts. However, the previous distinction between MDS and AML has become blurred with the recognition of several common features of the two diseases: 1) MDS is now recognized to be a clonal pre-leukemic hematopoietic stem cell disorder frequently associated with specific recurrent cytogenetic abnormalities15,16,17,18 ; 2) multi-lineage dysplasia is now recognized to occur in the majority of AML cases presenting clinically as “de novo” disease in older individuals7 ,19,20,21 ; 3) AML arising in older individuals and primary, secondary, or therapy-induced MDS are now known to share strikingly similar biologic and genetic features7,8,9,10,11,12,13 ; 4) de novo AML cases such as those with t(8;21) and inv(16) may present clinically with less than 30% marrow blasts and may have dysplastic features21 ; and 5) transgenic and “knock-in” murine models of leukemia made with fusion genes from translocations associated primarily with de novo AML [t(15;17), t(8;21), inv(16)] are often characterized by hematopoietic dysplasia or an MDS-like disease antecedent to the development of AML.22,23,24,25 These more recent clinical and biologic studies indicate that disorders previously classified as MDS are part of the same disease continuum as AML and that MDS is best considered a pre-leukemic disorder with variable frequencies and rates of progression to AML.
As we now recognize that MDS and AML are part of the same continuous biologic and genetic spectrum of disease, the use of arbitrary “thresholds” for the distinction of AML from MDS for the purposes of disease classification and therapeutic decision making has become particularly problematic. At what blast cell percentage should a clinician institute AML-based therapies in an MDS patient progressing to RAEB-T and from RAEB-T to AML? Should AML-based therapies be instituted in a patient whose marrow has dysplastic morphologic features, a blast cell percentage < 20%, and a t(8;21)-containing clonal population of cells? While the treshold of 30% blasts used by the FAB Classification to distinguish AML from MDS (Table 1) is clearly arbitrary, a reduction in this threshold to 20% blasts and the resultant elimination of RAEB-T as a distinct clinical stage in the evolution of MDS to AML as proposed in the new WHO Classification21 (Table 1) has been perceived by many hematologists to be even more problematic.26,27 While RAEB-T and AML arising clinically as “de novo” disease in older patients share highly similar cytogenetic features,13,26 they have differing clinical and biologic features and therapeutic responsiveness.26,27,28 Although not directly tested in a randomized fashion, in several, if not all, studies RAEB-T patients appear to have a worse response to intensive chemotherapy when compared historically to AML cases with similar biologic and cytogenetic features.28,29 Thus, it will be particularly important to retain the distinct RAEB-T MDS subtype in order to compare future therapeutic advances in AML/MDS to historical controls. Additional concerns that have arisen with the proposed WHO Classification (Table 1) include29 : 1) the proposal that a diagnosis of RA and RARS be restricted to patients who have abnormalities solely involving the erythroid lineage, even though a diagnosis of MDS requires dysplasia in at least two hematopoietic lineages; 2) the creation of vague new MDS diagnostic categories (“refractory cytopenias with multilineage dysplasia (RCMD)” and “MDS, unclassifiable”) which have no biologic, clinical, or genetic basis; and 3) the general lack of clinical and prognostic relevance in the proposed WHO classification scheme. Unfortunately for clinicians and diagnosticians alike, these controversies will likely continue until our knowledge has increased to the degree that disease classification systems can be developed on clinical features, genetics/genomics, and functional biology. And as our knowledge continues to evolve, classification systems will necessarily require constant revision.
Taking an alternative approach, others have worked to develop risk-based classification systems for MDS in order to facilitate clinical decision-making.30 The International Scoring System for Evaluating Prognosis (IPSS) in MDS assigns IPSS scores to varying degrees of those clinical and biologic features that today provide the most prognostic significance in MDS: marrow blast cell percentage, karyotype, and degree of cytopenia (Table 1).29 An overall IPSS score developed using these variables has a strong correlation with predicted median survival.29 The IPSS system has proven to be a highly useful method for evaluating prognosis in MDS patients, has achieved international acceptance, and is being used to design clinical trials.
Genetic Features of MDS: Models for Genetic Progression and Clues to Etiology
MDS is a clonal hematopoietic stem cell disorder characterized by step-wise genetic progression
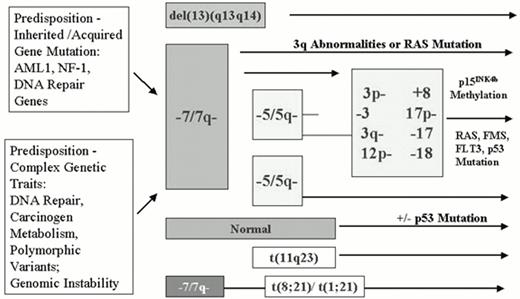
Initially demonstrated by studies of expression of the various isoforms of the X-linked gene G6PD and more recently by molecular methods that detect non-random patterns of X-inactivation, evidence for clonality has been found in all forms of MDS, even in their very earliest stages.17,18 Interestingly, cytogenetic and molecular data provide evidence, in some MDS patients, for the existence of a clonal phase of disease prior to the acquisition of the characteristic cytogenetic abnormalities associated with MDS.18 Similarly, MDS patients who evolve to acute leukemia may after therapy revert to a cytogenetically normal but persistently clonal remission. Such findings have led to the hypothesis that the recurrent cytogenetic abnormalities associated with MDS, previously considered the “primary” cause of disease, are actually “secondary” cytogenetic abnormalities that arise due to cytogenetically undetectable initiating lesions in a clonal hematopoietic stem cell population.30 Such initiating events are likely to be heterogeneous and could either be inherited or result from acquired somatic DNA damage, genomic instability, defective DNA repair, or perturbations in cell signal transduction pathways that give rise to stem cell clones that have a growth or survival advantage. In contrast to the de novo acute leukemias that occur primarily in younger patients (particularly those associated with balanced translocations and inversions such as t(8;21), t(15;17), inv(16), t(9;11), etc. lacking dysplastic features), MDS and AML arising in older individuals appear to have a different model of genetic progression (Figure 1).31,32 In this proposed model, initiating genetic lesions (which may be inherited or acquired) promote the acquisition of “secondary” genetic events that are primarily characterized by stepwise gains and losses of specific chromosomal regions (particularly chromosome 3p-, 3q-, 5q-, 7q-, 12p-, -17, -18, 20q11-12, +8). Such gross chromosomal changes are ultimately accompanied by sub-microscopic DNA mutations of genes such as p53, FLT3, or RAS, methylation of specific gene promoters, and in some cases by the reciprocal translocations and inversions more frequently associated with AML. This model of step-wise genetic progression for MDS and related AML is strikingly reminiscent of those proposed for human solid tumors, such as colon cancer. Three lines of evidence support this model and the existence of a genetic predisposition to MDS: 1) the occurrence of AML and MDS in families with inherited defects in DNA repair or neurofibromatosis-type I33,34,35,36,37 ; 2) genetic mapping studies in the rare families with “familial” MDS and AML38,39,40,41 ; and 3) studies of the association of various genetic polymorphisms with AML and MDS.42,43,44,45,46,47,48
An inherited genetic predisposition to MDS
Support for an inherited predisposition to MDS and related AML has long been evident from studies of inherited constitutional genetic defects (such as Schwachman-Diamond syndrome, the defective DNA repair of Fanconi anemia, or deregulation of the RAS signal transduction pathway in neurofibromatosis-type 1) that are present in a large proportion of children who develop MDS and AML.33,34,35,36,37 Indeed, recent studies indicate that up to 30% of children affected by MDS and related myeloproliferative disorders have an inherited constitutional genetic disorder.34 The original studies by Shannon and colleagues35,36 of the genetic basis of familial MDS with chromosome 7q abnormalities (the “monosomy 7 syndrome”) are important in that they first revealed that abnormalities of chromosome 7q were not the “primary” cause of the syndrome; indeed, these investigators concluded that the predisposition locus mapped to some other as yet unidentified chromosomal location. Thus, the foundation was laid for the putative existence of genetic loci that could “predispose” to chromosomal instability, secondary loss of specific chromosomal regions (such as 5q, 7q, and 20q), and the ultimate development of MDS and AML in adults and children. This original hypothesis was recently supported the findings of Gilliland and colleagues who determined the genetic basis of familial platelet disorder with leukemia (FPD/AML), an autosomal-dominant congenital thrombocytopenia characterized by platelet aggregation abnormalities.38,39 Affected individuals in the seven pedigrees studied to date all have a striking propensity to develop MDS, AML, and more rarely, chronic myelogenous leukemia (CML). Interestingly, the MDS and AML cases that develop in these pedigrees have the cytogenetic abnormalities classically associated with MDS, particularly abnormalities of chromosomes 5q and 7q and complex abnormalities. After mapping the FDP/AML predisposition locus to chromosome 21q22 in 1998, Gilliland and colleagues went on to determine that the causative gene for this disorder was CBFA2 (AML1), the gene whose function is most frequently disrupted in the acute leukemias by various reciprocal translocations and inversions, including the t(8;21), inv(16), t(3;21), and t(12;21).49 Heterogeneous point mutations and small deletions of a single AML1 allele were found in these different pedigrees.39 Despite this molecular heterogeneity, each mutation characteristic of each pedigree affected the DNA-binding domain of one AML1 allele, particularly targeting the two arginine residues at positions 166 and 201 that bind to DNA. The change of arginine to glutamine resulted in a loss of DNA-binding activity.38 These data thus support the hypothesis that AML1 may surprisingly function as a tumor suppressor gene and that loss of one AML1 allele (hemizygous loss) is sufficient to initiate tumorigenesis and establish a neoplastic clone in affected individuals. This loss of function of a single AML1 allele appears to also confer a susceptibility to the acquisition of secondary mutations and the gain and/ or loss of the chromosomal regions frequently associated with AML and MDS. This discovery has led to a particularly attractive model for MDS/AML whereby AML1 mutations predispose to chromosome instability leading to the eventual loss of chromosomes 5q, 7q; such models are currently being developed in mice in which the mutated AML1 allele has been introduced (Downing and Gilliland, personal communication). These pivotal studies also further cement a genetic and etiologic link between MDS and AML (and even CML). Not unexpectedly, a low percentage of sporadic AML, ALL, and CML cases (5-8%) have recently been reported to have similar AML1 mutations50 ; whether such AML1 mutations can be observed in primary “sporadic” MDS cases is currently under investigation. Whether AML and MDS cases with AML1 mutations are indeed “sporadic” or represent AML and MDS cases that have arisen in individuals with inherited AML1 mutations is as yet unknown. Given the functional role and association of CBFβ (mapping to chromosome 16q22) with AML1 in normal and neoplastic hematopoiesis,51 it is tempting to further speculate that CBFβ might be the causative gene for the second predisposition locus in AML and MDS in those familial cases where the predisposition has been mapped to chromosome 16q21-23.2.40
Models for the development of sporadic MDS: Cumulative environmental exposures in genetically predisposed individuals
While genetic and familial mapping studies have clearly demonstrated that mutations in a specific gene, such as AML1, NF1, or genes mediating DNA repair, can predispose to the acquisition of secondary cytogenetic abnormalities and MDS, it is likely that such inherited genetic mutations will account for only a minority of MDS cases. How the majority of “sporadic” MDS cases arise is as yet undetermined. However, epidemiologic case-control studies of MDS (and related AML) have demonstrated associations between MDS and smoking, exposure to chemical compounds (particularly petroleum products and diesel derivatives, exhausts, organic solvents, fertilizers, and nitro-organic explosives), semi-metals (arsenic and thallium), stone dusts (such as silica), and cereal dusts.52,53 In light of these epidemiologic studies, it is interesting that evidence is increasing for a complex genetic predisposition to MDS involving naturally occurring DNA polymorphisms in genes that mediate DNA repair and metabolize environmental carcinogens.42,43,44,45,46,47,48 These studies are leading to a model, also diagrammed in Figure 1, in which MDS arises as a result of cumulative environmental exposures in genetically predisposed individuals. In this case, the genetic predisposition is a more complex genetic trait: a constellation of genetic variants in critical polymorphic genes. The initial attempts to dissect this complex genetic predisposition have focused on the association of MDS with naturally occurring polymorphisms in genes that mediate carcinogen metabolism.42,43,44,45,46,47,48 Following initial reports of the association of a non-functioning 609C→T polymorphic allele of the NAD(P)H:Quinone Oxidoreductase (NQO1) gene that plays a critical role in detoxifying benzene metabolites with an increased incidence of hematologic malignancies in Chinese workers exposed to benzene,43 several groups have attempted to determine the incidence of this polymorphism in primary and secondary leukemia cases.44,45 Larson and colleagues first reported an increased frequency of the NQ01 609C→T polymorphism in patients with therapy-related AML, particularly in AML patients with abnormalities involving chromosomes 5 and 7, 88% of whom were homozygous for the non-functional allele.44 Interestingly, studies of de novo AML in children45 and adults (C. L. Willman et al, manuscript in preparation and M.A. Smith et al, personal communication) have failed to demonstrate an association of this NQO1 polymorphism with abnormalities of chromosome 5 and 7, but have instead demonstrated strong associations with balanced translocations and inversions, particularly involving MLL and chromosome 11q23. While it is tempting to speculate that the NQO1 609C→T polymorphism could predispose to the development of MDS, no such studies focusing on MDS cases have been reported; our own limited studies of 120 primary MDS cases have failed to reveal such an association. Similar complexities have arisen in studies of the association of MDS and polymorphisms in the glutathione S-transferases (GST) that mediate exposure to cytotoxic and genotoxic agents, specifically the “null” variant allele GST theta 1 (GSTT1).46,47,48 Chen and colleagues initially reported that the frequency of the GSTT1 null genotype was higher among MDS cases than controls.46 However, Fenaux and colleagues47 and other groups48 did not confirm these initial observations and actually reported that the incidence of the GSTT1 null genotype tended to be higher in unexposed MDS patients and controls. Thus, while the hypothesis and model that MDS arises due to cumulative environmental exposures in genetically predisposed individuals is indeed attractive, these studies of natural human genetic variation and disease association are only in their infancy. Moreover, it is likely that true genetic risk will not be simply determined through studies of one gene, but through the constellation of risk-producing and risk-protecting alleles present in each individual. Thus, it will ultimately be necessary to study polymorphic variants in many human genes in a large number of affected individuals and controls and carefully monitor environmental exposures in order to dissect what is likely to be a very complex genetic predisposition.
Cloning and identification of genes disrupted by the recurrent cytogenetic abnormalities associated with MDS
The identification of potential “initiating” genetic lesions in MDS and related AML patients has lead to the hypothesis that the cytogenetic abnormalities traditionally associated with MDS (involving chromosomes 7q, 5q, 20q11-12, trisomy 8, 12p, and 3q) are “secondary” genetic events. However, these secondary cytogenetic abnormalities are likely no less critical for disease progression, and identification of the gene(s) involved in these regions remains very important. Unfortunately, despite years of mapping and definition of minimally deleted chromosomal regions on chromosomes 5, 7, and 20, no investigator has yet succeeded in identifying the “single” tumor suppressor gene that is responsible for MDS and AML on any of these chromosomes. Recent detailed cytogenetic and molecular mapping studies reveal that rearrangements and deletions involving these chromosomes are very complex and that multiple distinct regions may contribute to the disease phenotype or progression: at least two different regions are implicated on chromosome 7q (7q22 and 7q32-34) and more than four different regions may be involved on chromosome 5q (5q11, 5q13-q21, 5q31, and distal 5q33-35).54,55,56,57,58,59,60,61 One issue with many mapping studies is that in most instances little attention was paid to “phenotype” rather than “genotype.” In other words, patient samples were selected for molecular studies based on the presence of a specific cytogenetic abnormality (such as a 5q- or a 7q- with or without additional cytogenetic abnormalities) without regard to the specific form of disease or mode of disease presentation (primary MDS, secondary AML, de novo AML, or tAML/MDS). Both cytogenetic and molecular genetic studies are not only revealing the tremendous heterogeneity in different breakpoints but also the need to focus on a pure genotype and phenotype for mapping studies. Very detailed cytogenetic studies by Pederson in 1996 revealed that while chromosome region 5q31 was deleted in all patients with MDS, other chromosome 5 regions were deleted more often than 5q31 in AML patients; the chromosome 5q13-q21 region was particularly involved in the genetic progression of RA to RAEB and 5q22-5q33 for further progression to AML.61 Recent studies by Westbrook and colleagues have focused on a single AML patient with a very small deletion in the 5q31 region. In this patient, the D5S500-D5S594 region was identified to be the minimal deletion interval, and this interval was shown to contain nine transcriptional units with five unknown expressed sequence tags (ESTs) and the genes CDC25, HSPA9, EGR1, and CTNNA1.58 While all of these sequences are interesting candidates and are expressed in hematopoietic cells, none has yet been identified as “critical” for disease. It also remains possible that loss of more than one gene in this region, as well as the other distinct regions on chromosome 5, could actually be responsible for the disease phenotype. Boultwood and colleagues56,57 have focused on the identification of the gene(s) deleted in MDS patients who have the isolated “5q- syndrome,” a clinically distinct form of RA associated with more indolent disease and a lower rate of progression to AML, chronic macrocytic anemia, thrombocytosis, and dysplastic megakaryocytes. Interestingly, the region on chromosome 5 specifically associated with this disease presentation appears to be more distal to 5q31; novel transcriptional units have also been recently identified in this distinct region.57
In light of the complexity of this cytogenetic and molecular data, it is attractive to hypothesize that in MDS, an initiating abnormality gives rise to genome instability leading to the deletion and rearrangement of particularly susceptible chromosomal regions, such as those on chromosome 5q and 7q. Cytogenetic studies have revealed the continued instability of these regions during disease progression in individual patients.62 While it may be that loss of function of a single gene in each of these relatively large regions is responsible for disease progression, more recent studies have given more credence to the possibility that hemizygous loss of the function of several genes in each of these regions could contribute to the disease phenotype.
Molecular mutations and genome methylation in MDS
In addition to the complex cytogenetic abnormalities seen in MDS, several molecular defects have also been reported. Studies of MDS in children, with or without Neurofibromatosis-type 1, have provided evidence that abnormalities in the RAS signal transduction pathway cooperate with a loss of genes on chromosome 7 to promote myeloid leukemogenesis.63 In adults with MDS, disease progression has been associated with the mutations in genes such as RAS, p53, and FLT364,65,66,67 and with progressive methylation and transcriptional inactivation of critical cell cycle regulatory genes such as p15INK4b that normally function to inhibit cyclin-dependent kinase activity at the G1 phase of the cell cycle.68,69 MDS patients have also been shown to have defective activation of signal transduction pathways, particularly involving STAT5, in response to erythropoietin,70 which may account in part for the defective erythropoiesis and persistent anemia. These molecular abnormalities associated with MDS all further contribute to an escape from normal cell cycle regulation, a disruption of the faithful maintenance of DNA integrity and repair leading to continued genetic instability, and the altered activation of various signal transduction pathways.
Biologic Features of MDS
An abnormal marrow microenvironment: Cytokines, adhesion, apoptosis
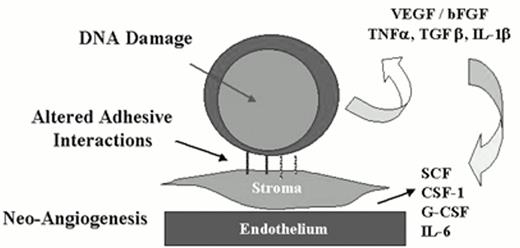
While there is strong evidence supporting the view that MDS arises from an intrinsic or acquired genetic defect in hematopoietic stem cells leading to clonal expansion of a stem cell population, it is also clear that other epigenetic abnormalities such as aberrant cytokine production, altered stem cell adhesion, or an abnormal marrow microenvironment contribute to the biology of the disease and may provide important therapeutic targets (Figure 2). While studies reporting aberrant cytokine expression profiles in MDS patients have been criticized for their lack of controls, lack of a suitable ex vivo system to study the true clonogenic hematopoietic stem cell and marrow stromal cell interactions, and the failure to precisely identify the cellular origin of particular cytokines, abnormalities and elevations in tumor necrosis factor-α (TNFα), transforming growth factor-β (TGFβ), and interleukin-1β (IL-1β) have all been reported.2 In particular, an overabundance of IL-1β and a relative lack of its antagonist IL-1β(ra) have been hypothesized to support the clonal expansion of aberrant hematopoietic stem cells.2
MDS—An altered marrow microenvironment.
Cytokines, adhesion defects, angiogenesis, accelerated apoptosis, adhesion dependence of proliferation and survival.
MDS—An altered marrow microenvironment.
Cytokines, adhesion defects, angiogenesis, accelerated apoptosis, adhesion dependence of proliferation and survival.
Several recent studies have revealed that in its early stages, MDS is characterized by accelerated apoptosis of hematopoietic progenitor cells.71,72 While some controversy remains, most studies of MDS samples using various techniques have demonstrated a lowered apoptotic threshold of marrow CD34+ cells to TNF-α, interferon-γ (IFN-γ), and anti-FAS antibodies.70,71 Excessive apoptosis is an attractive explanation for how a clonal expansion of marrow progenitor cells could result in effective hematopoiesis and marrow failure. However, how such accelerated apoptosis is initiated or acquired is not yet understood. Apoptosis may be triggered in cells by intrinsic DNA damage or in cells that have an abnormal growth factor milieu; both mechanisms could contribute to the accelerated apoptosis observed in MDS. Another biologic mechanism that can induce apoptosis is a lack of appropriate adhesive interactions between cells and their stromal support (Figure 2). In the case of MDS, perturbed adhesive interactions between clonogenic hematopoietic stem cells and the underlying marrow stroma or endothelium could clearly trigger excessive apoptosis. The migration, retention, and survival of hematopoietic stem cells in the marrow microenvironment is controlled by critical adhesion receptors including CD34, CD44, selectins, and integrins; β1 integrins play a particularly critical role in this process and virtually all hematopoietic stem cells express high levels of α4, α5, α6, and β1 integrins.73,74 Unfortunately, this is a relatively unexplored area of MDS and is worthy of continued investigation. Interestingly, while MDS is characterized by excessive apoptosis, further genetic progression in this disease and the transition to AML appears to be associated with a loss of functional apoptosis, though additional studies documenting this progressive change in individual patients with disease progression are clearly necessary.
Summary and Future Scientific Questions
Exciting scientific studies have provided phenomenal insights into the striking biologic and genetic heterogeneity of MDS and related AML. As these scientific studies lead to the further refinement of genetic models for the development of MDS, we may gain new insights that will ultimately lead to the design of more effective prevention strategies and therapeutic regimens. Our ongoing attempts to improve our knowledge, classification, and therapy of MDS and AML would be best served by appreciating that these disorders are linked clonal diseases and represent stages in the progressive transformation of clonal neoplastic hematopoietic stem cells. Particularly fruitful areas for future investigation include the application of genomic and proteomic technologies to understand alterations in the global patterns of gene expression and protein function in MDS and the alterations in these patterns during the variable progression of MDS to AML, studies of global genome methylation and aberrant methylation in the development of MDS, continued studies of the complex genetic predisposition to MDS and how such genetic predispositions and environmental exposures contribute to disease etiology and pathogenesis, studies of the adhesive interactions of hematopoietic stem cells and their underlying stroma and how these interactions are perturbed in MDS, and the role of neoangiogenesis in the development of MDS.
II. Immune Mechanisms and Modulation in MDS
A. John Barrett, M.D., FACP,*and Yogen Saunthararajah*
Hematology Branch, National Heart, Lung and Blood Institute, National Institutes of Health, 9000 Rockville Pike, Bldg. 10 Room 7C103, Bethesda MD 20892-0003
Despite improvements in the supportive care of patients with MDS with transfusions and antibiotics, nearly 50% of patients die before transformation to acute leukemia from complications of thrombocytopenic bleeding or infection. Treatment of the marrow failure alone should therefore bring survival advantages to a significant proportion of patients with MDS. To improve the treatment of the cytopenia associated with MDS requires an understanding of the pathophysiology of the disease. It is well accepted that MDS is a clonal stem cell disorder, characterized by cytopenia, progressive de-differentiation of myeloid lineages and ultimately unregulated blast proliferation. Recent research reveals a further dimension to this pathophysiology, with extrinsic immunological and microenvironmental factors compounding the intrinsic stem cell defect and contributing to the pancytopenia and possibly to leukemic progression. In this section, pathophysiological findings in MDS that underpin experimental trials of immune suppression, cytokine regulation and inhibition of angiogenesis are reviewed.
Dysplasia, Apoptosis and Cytokines
Dysplasia of erythroid, granulocytic and megakaryocytic lineages are the diagnostic hallmarks of MDS. Despite increased proliferation of the marrow, there is an increased rate of programmed cell death. Indeed some of the dysplastic appearance may be explained by apoptotic changes.1,2 Kinetically the apoptosis prevails over the increased proliferation, causing the peripheral cytopenia.3,4 Cytokines derived from unselected marrow mononuclear cells are believed to be extrinsic factors predisposing to apoptosis in MDS.5,6,7,8,9,10 In MDS many studies link overexpression of TNF-α to cell death.7,11,12 TNF-α produced by MDS mononuclear cells is inhibitory to both normal and MDS colony growth indicating that residual normal hematopoiesis can also be blocked in MDS.7,8 IFN-γ, IL-1, and TGF-β4,13 as well as undefined factors produced by stromal cells have also been implicated in causing apoptosis14 but their role in causing marrow failure is not well established. The identification of TNF-α as a key cytokine in cell death regulation and the increased susceptibility of MDS cells to TNF-α is the basis for several clinical trials of TNF-α inhibitors.4
Immune Dysfunction in MDS
Hamblin and others have pointed out an association of MDS with an autoimmune process, noting the occurrence of autoantibody production, and monoclonal gammopathy in some patients with MDS.15 The occasional finding of T cell clonality in MDS has been interpreted as evidence of T cell involvement in the stem cell disorder16 ; however, more recent evidence suggests that clonal T cell expansion is a feature of autoimmunity: we have observed a high frequency of T cell receptor (TCR) V-β repertoire skewing in MDS patients indicating the presence of a persisting clonally-expanded T cell population, characteristic of an autoimmune process. Interestingly, it is not uncommon to find MDS in association with T-cell large granular lymphocyte (T-LGL) leukemia, a condition characterized by oligoclonal or clonal T cell expansions arising in a background of autoimmune disease.17 MDS also shares some of the features of acquired aplastic anemia (AA), a disease with an established autoimmune pedigree.18 Both in AA and MDS, plasma TNF-α and IFN-γ levels are high and T cell-mediated myelosuppression occurs.19 Three groups studying MDS identified T cells inhibitory to autologous granulocyte20,21 or erythroid22 colony growth. These observations strongly suggest that, as in AA, an autoimmune T cell-mediated myelosuppression contributes to the cytopenia of MDS. The use of immunosuppressive treatment to restore marrow function in patients with AA has been the stimulus to consider similar immunosuppressive treatment in MDS.23,24,25 ,32 Evidence for immune-based myelosuppression in MDS is summarized in Table 2.
Evidence for an immune-mediated suppression of the marrow in myelodysplastic syndrome (MDS).
|
| Abbreviations: CFU-E, colony-forming units-erythroid; CFU-GM, colony-forming units-granulocyte/macrophage |
|
| Abbreviations: CFU-E, colony-forming units-erythroid; CFU-GM, colony-forming units-granulocyte/macrophage |
Marrow Microenvironment and Angiogenesis
In MDS the marrow shows a non-homogeneous distribution of cell types, seen as islands of pure erythroid cells, granulocytes, megakaryocytes26 or blast cells termed “abnormally localized immature precursors” (ALIPS).27 This appearance may reflect an underlying abnormality in the marrow stroma. In MDS, vascular endothelial growth factor (VEGF) is strongly expressed especially in megakaryocytes, and investigators have found an increased density of blood vessels and an association of increasing marrow vascularity with leukemic transformation.28,29 This is the basis for using angiogenesis inhibitors to correct abnormalities in MDS and possibly to retard disease progression.
A Model of Pathophysiological Changes of MDS
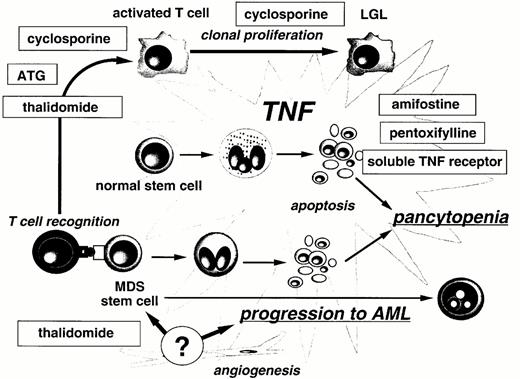
A hypothetical model of MDS pathophysiology and the role of experimental treatments is presented in Figure 3.1 Transformation of normal stem cells induces danger signals or antigenic change and hence an autoimmune T cell response directed against the marrow. Both MDS and normal marrow cells at various stages of differentiation are directly inhibited by CD8+ CTL causing varying degrees of stem cell failure. The MDS cells may be relatively resistant to this immune attack than normal stem cells, and consequently the abnormal immune environment may provide selective pressure in favor of the abnormal cells.2 T cell expansions are clonal and can become autonomous in some individuals as illustrated by the coincidence of MDS with T cell LGL.3 The persisting autoimmune attack results in chronic overproduction of pro-apoptotic cytokines, especially TNF-α. This affects cells differentiated at or beyond the CD34+ stage of differentiation and may contribute to a dysplastic morphology and increased apoptosis in the marrow. Despite the increased cell proliferation in MDS, the marrow fails to export sufficient cells into the blood because intramedullary apoptosis mechanism prevails over proliferation. Increased TNF-α levels lead to changes in the MDS cells: increased FAS, downregulation of Fap-1, and an increase in caspases causing apoptosis. Non-hematopoietic cells in the marrow microenvironment contribute to the process in two ways: (1) stromal cells induce apoptosis by a non TNF-dependent mechanism; (2) VEGF production by the MDS cells may stimulate angiogenesis that could favor disease progression by altering the microenvironment to favor unregulated cell growth.
Mechanism of action of immune modulators in MDS.
Abbreviations: TNF, tumor necrosis factor; LGL, large granular lymphocytes; ATG, antithymocyte globulin
Mechanism of action of immune modulators in MDS.
Abbreviations: TNF, tumor necrosis factor; LGL, large granular lymphocytes; ATG, antithymocyte globulin
Experimental Treatments for MDS
Previous research in MDS has focused on increasing marrow cell proliferation and differentiation by growth factors and differentiating agents. Perhaps because of the importance of inhibitory factors extrinsic to the MDS stem cell itself, these treatment approaches have produced only partial beneficial effects.
The results of experimental treatments that affect extrinsic mechanisms of cytopenia and disease progression in MDS are outlined below.
It is important to recognize that MDS represents a diversity of subtypes with different degrees of severity of cytopenia and different rates of progression to leukemia. Determining if a novel treatment has altered the natural disease progression requires a knowledge of the rate of progression and survival probability of MDS subtypes with comparable prognostic features. Currently the IPSS provides the most widely accepted yardstick to measure disease progression and survival.30 Numerical changes in cell counts are frequently used to describe responses to cytopenia. However, even the finding of a 50% increase in neutrophils or platelets is only of clinical relevance if treatment raised the count from a level associated with a high risk of infection or bleeding to a safe level. Furthermore, only sustained responses, not peak counts achieved, correlate with prolonged clinical benefit. Reliable end-points of major clinical significance are independence from transfusion of blood and platelets and a recovery of neutrophils from below 500 to > 1000/mm3.
Immunosuppressive Treatments
Prednisone
Bagby et al reported in 1980 that prednisone alone improved low blood counts in a minority of patients with MDS and that the response could be predicted in vitro by enhancement of granulocyte macrophage colony-forming units (CFU-GM) growth.31 However, although steroids have frequently been used in treatment, low response rates and increased risk fo infection make corticosteroids unattractive agents in MDS.
Antithymocyte globulin
Because of the similarity of hypoplastic MDS to SAA, antithymocyte globulin (ATG) has been used occasionally to treat hypoplastic MDS with severe cytopenia. A summary of case reports and small series reveals hematological responses in 8/13 hypoplastic MDS patients.23,24 Based on these reports, unpublished observations, and the hypothesis that a T cell-mediated process may cause pancytopenia, we evaluated ATG as immunosuppressive treatment to improve marrow function in MDS.25 This study, now completed, involved 61 patients. Study entry criteria were red cell or platelet transfusion-dependence and no concurrent treatment with immunosuppressives, chemotherapy or growth factors. Thirty-seven had refractory anemia (RA), 12 had RA with excess of blasts (RAEB) and 10 RA with ring-sideroblasts (RARS). Marrow cellularity varied from hypo- to hyper-cellular. Most patients (75%) had failed previous treatment with single or multiple agents, including cyclosporine, amifostine, and growth factors. The interval between diagnosis and entry to the study was 3-300 months, with a median of 24 months. Twenty-three cases had hypoplastic MDS, seven had the PNH abnormality by flow cytometry, and 23 had karyotypic abnormalities characteristic of MDS. Patients received ATG at 40 mg/kg/day for 4 days and were periodically evaluated for 38 (range 20-58) months. The main criterion for response was independence from the requirement for red cell transfusions. Twenty-one (33%) patients became red cell transfusion-independent within 8 months of treatment (median 75 days). Transfusion-independence was maintained in 76% of responding patients for a median of 32 months (range 20-58). Twenty-three of 41 (56%) severely thrombocytopenic patients had sustained platelet count increases between 25,000-290,000/μl and 18/41 (44%) severely neutropenic patients achieved sustained neutrophil counts > 1000/μl. At last follow-up 39 patients were alive with an actuarial survival of 64% at a median of 34 (range 18-72) months. Of the 21 responders, 20 survived and one died following progression to AML (Figure 4). In the others no significant alteration in the bone marrow appearance or cellularity was observed and cytogenetic abnormalities, present in four, persisted after treatment. Three relapsed with transfusion-dependence but one regained red cell transfusion-independence after a second course of ATG. Of the 40 non-responders 21 died, 15 from cytopenia and 7 from progression to AML.
Survival of 61 patients with myelodysplastic syndrome given antithymocyte globulin (ATG) treatment.
Survival of 61 patients with myelodysplastic syndrome given antithymocyte globulin (ATG) treatment.
Factors Predicting a Response to Immunosuppression
In a multivariate analysis of 82 RA, RARS or RAEB patients treated with either ATG alone or cyclosporine alone, younger age, shorter duration of red cell transfusion dependence and the presence of HLA DRB1 15 predicted a response to immunosuppression. In other words, to maximize the probability of response to immunosuppression, patients should be treated sooner rather than later and this strategy appears to be particularly appropriate and effective in RA or RAEB patients who are young and/or have HLA DRB1 15. Pretreatment variables associated with a response to immunosuppression are summarized in Table 3. Responses were associated with a significant survival benefit at 4 years (95% versus 38% for non-responders, p = 0.006). In the subset of 41/61 patients with INT-1 IPSS given ATG, responders had 100% survival at 3 years and no disease progression versus 45% survival (p < 0.0004) and 51% probability of disease progression in non-responders (p = 0.02). Since we previously found correlation between response to ATG treatment and normalization of the T cell repertoire, we studied 15 patients with MDS who received immunosuppression for biological markers of response. Abnormalities in the TCR Vβ repertoire were common and occurred both in responders and non-responders, rendering Vβ analysis of no prognostic value.
Pretreatment variables correlating with a response to immunosuppression.
| Favorable Factors . | Significant Correlation in Univariate Analysis (p < 0.05) . | Significant Correlation in Multivariate Analysis (p < 0.05) . | Odds in Multivariate Predictive Model . |
|---|---|---|---|
| Younger age | yes | yes | 2X less likely to respond with each 5 year increase in age |
| Shorter duration of red cell transfusion dependence (RCTD) | yes | yes | 1.5X less likely to respond with each 3 month increase in RCTD |
| Positive for HLA DRB1 15 | yes | yes | 9.9X more likely to respond if HLA DRB1 15 positive |
| Decreased cellularity of bone marrow | yes | no | |
| Increased number of cytopenias | yes | no | |
| < 5% marrow blasts | yes | no | |
| Normal cytogenetics | yes | no | |
| PNH present by flow | yes | no | |
| Low IPSS score | no | no |
| Favorable Factors . | Significant Correlation in Univariate Analysis (p < 0.05) . | Significant Correlation in Multivariate Analysis (p < 0.05) . | Odds in Multivariate Predictive Model . |
|---|---|---|---|
| Younger age | yes | yes | 2X less likely to respond with each 5 year increase in age |
| Shorter duration of red cell transfusion dependence (RCTD) | yes | yes | 1.5X less likely to respond with each 3 month increase in RCTD |
| Positive for HLA DRB1 15 | yes | yes | 9.9X more likely to respond if HLA DRB1 15 positive |
| Decreased cellularity of bone marrow | yes | no | |
| Increased number of cytopenias | yes | no | |
| < 5% marrow blasts | yes | no | |
| Normal cytogenetics | yes | no | |
| PNH present by flow | yes | no | |
| Low IPSS score | no | no |
Cyclosporine
Jonasova and co-workers reported a high rate of hematological responses following cyclosporine treatment in MDS. Sixteen patients with RA and one with RAEB were given standard doses of cyclosporine for 5-31 months. Substantial hematological responses were observed,32 mostly occurring around 3 months. Transfusion independence was achieved in all 12 patients requiring red cells before cyclosporine administration, and significant increases in leucocyte and platelet counts also occurred. Responses occurred in RAEB as well as RA patients and in hyper- or normocellular MDS as well as in hypoplastic MDS (6/8 and 7/9 responders respectively). Of six patients with abnormal karyotype, three responded (all were 5q-). These clinical studies provide further strong evidence to support an immune mechanism of marrow suppression in patients with MDS.
New trials of immunosuppressive agents in MDS
Based on these preliminary findings, several trials evaluating immunosuppressive treatment for MDS are underway in the US and in Europe (Table 4). Collectively these studies should better define the potential benefit for immunosuppression by comparing ATG treatment with a control arm of conventionally supported patients. Prospective randomized trials will evaluate the relative effects of different ATG types and different immunosuppressive combinations in improving marrow function and prolonging survival.
Ongoing trials of immunosuppression in myelodysplastic syndromes.
| Site . | Type of Immunosuppression . | Study Design . |
|---|---|---|
| Hanover, Germany | rATG | single arm (n > 20, ongoing) |
| Basel, Switzerland | rATG +CSA | randomized, multicenter (new) |
| London, United Kingdom | rATG | single arm (n = 30, stopped) |
| Houston, USA | hATG, vs. hATG+CSA vs. hATG+fludarabine | randomized (n = 21, ongoing) |
| Scandinavian MDS Group | hATG +CSA | single arm (n = 13, ongoing) |
| Multicenter, USA | rATG, supportive care | randomized, multicenter (new) |
| Bethesda, USA | hATG +CSA | single arm (new) |
| Abbreviations: rATG, rabbit antithymocyte globuline; hATG, horse antithymocyte globulin | ||
| Site . | Type of Immunosuppression . | Study Design . |
|---|---|---|
| Hanover, Germany | rATG | single arm (n > 20, ongoing) |
| Basel, Switzerland | rATG +CSA | randomized, multicenter (new) |
| London, United Kingdom | rATG | single arm (n = 30, stopped) |
| Houston, USA | hATG, vs. hATG+CSA vs. hATG+fludarabine | randomized (n = 21, ongoing) |
| Scandinavian MDS Group | hATG +CSA | single arm (n = 13, ongoing) |
| Multicenter, USA | rATG, supportive care | randomized, multicenter (new) |
| Bethesda, USA | hATG +CSA | single arm (new) |
| Abbreviations: rATG, rabbit antithymocyte globuline; hATG, horse antithymocyte globulin | ||
Cytokine inhibition
Amifostine is a phosphorylated organic thiol that is metabolized to intermediates with antioxidant activity. The drug has two actions: (1) It protects cells from oxidative stress after exposure to cytokines including TNF-α; (2) it suppresses inflammatory cytokine release. Since amifostine reduces apoptotic marrow cell death following chemotherapy it may also reduce apoptosis in MDS.33 Indeed, incubation of MDS marrow cells with amifostine was found to improve colony growth.34 In a study reported by List et al,35 15 of 18 patients with MDS given amifostine had single or multilineage hematological responses. These results were recently updated for 75 patients; there were 40% platelet responses, 24% ANC responses and 20% with > 50% reduction in need for transfusions.36 There are ongoing, not yet reported studies on the use of amifostine in combination with growth factors and chemotherapeutic agents that should provide more information about the usefulness of amifostine.
Pentoxifylline
The observation that the three pro-inflammatory cytokines TNF-α, TGF-β and IL-1β are implicated in the increased apoptosis of MDS4 led Raza et al to treat MDS patients with pentoxifylline (PTX), a xanthine derivative known to interfere with the lipid-signalling pathway used by these cytokines.37 Ciprofloxacin was added because it reduced the hepatic degradation of PTX, and dexamethasone was used to further downregulate TNF-α production by reducing translation of TNF-α mRNA. Eighteen of 43 patients had hematopoietic responses that correlated with a reduction in blood levels of TNF-α. In a subsequent study a four-drug combination of amifostine, pentoxyfylline, ciprofloxacin and dexamethasone was used to optimize the anti-cytokine effect.38 Of 29 evaluable patients given this combination (evaluable because they survived 12 or more weeks), 22 (76%) showed partial responses, 19 with improvement in neutrophils, 11 with a reduction in transfusion requirement or a rise in hemoglobin, and 7 with improvements in platelet counts. However, these improvements were not sustained or statistically significant at 24 weeks and are similar to those reported for amifostine alone. There were no complete responses or conversions to transfusion independence. In contrast, in a study of 14 patients given PTX and ciproflovacin, Neumatis et al found a trend to lower TNF-α levels with treatment but failed to demonstrate a hematological response in any patient.39
Soluble TNF-α receptor (Enbrel)
Excessive amounts of soluble receptor can effectively block the function of TNF-α by competitive binding. In a recent report the soluble receptor Enbrel was reported to be well tolerated and to reduce plasma TNF-α concentrations. However, clinical responses were modest.40
Thalidomide
Thalidomide is an immunosuppressive drug. It switches T helper cells from a Th1 to a Th2 cytokine profile, inhibiting production of TNF-α.41 It selectively inhibits TNF-α production by monocytes.42 The drug has also been found to strongly inhibit angiogenesis in animal experiments and in in vitro cultures of human cells.43,44 Thalidomide has produced surprisingly substantial responses in patients with myeloma, possibly because of its effects on marrow angiogenesis. Both the immunosuppression and the angiogenesis inhibition of thalidomide might be beneficial in MDS. Raza and colleagues have recently completed a study of thalidomide in 83 patients with MDS.45 The drug was started at a dose of 100 mg daily and increased to a maximum, when tolerated, of 400 mg daily. Despite this approach, 26 patients stopped treatment within 12 weeks because of intolerance. Twenty-one of the 57 patients who continued treatment responded, 15 showing an erythroid, 13 a platelet and 7 a neutrophil response, with a median time to response of 10 weeks. By intention-to-treat analysis, 37% of patients became responders. Of note, eight patients of the erythroid responders became transfusion-independent. There was no evidence that thalidomide favorably or unfavorably affected disease progression, but follow-up was short. Patients most likely to respond to thalidomide had fewer blast cells in the marrow. These findings suggest that thalidomide can improve marrow function in some patients with MDS.
Future directions
We still know very little about the interaction of the immune system, the marrow microenvironment and the MDS stem cell. The antigens evoking the T cell response are unknown and the mechanism of T cell-mediated myelosuppression is not defined. The etiology of MDS remains unclear. Similarities between MDS and AA, including the response to immunosuppressive treatment, raise questions about the relationship between AA and MD.20 Are they different diseases sharing a common autoimmune pathology or are they two ends of a spectrum of a marrow failure syndrome differing only in their tendency to evolve to acute leukemia? Provocatively, it has been proposed that the abnormal cytokine milieu in AA is the initiator of genetic instability in the aplastic stem cell leading to clonal evolution and leukemia.46 Also, this abnormal milieu may act as selective pressure in favor of the abnormal MDS clone. In these scenarios immune modulation at an early stage of clonal evolution might be expected to maintain disease stability. Finally the relationship between the MDS stem cell and the marrow microenvironment deserves further study. In this regard it will be important to determine whether thalidomide exerts its beneficial effect through inhibition of angiogenesis as well as through immunosuppression.
III. Therapeutic Advances in MDS
Eva Hellström-Lindberg, M.D., Ph.D.*
Department of Medicine, Division of Hematology, Karolinska Institutet, Huddinge University Hospital, S- 141 86 Stockholm, Sweden
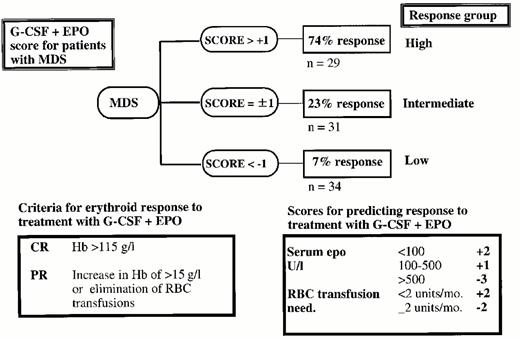
Increased biological understanding of different subtypes of MDS has resulted in new therapeutic alternatives for groups of patients in whom, hitherto, only conservative treatment was available. Achievements in the techniques for stem cell transplantation (SCT) have made it possible to cure an increasing, but still small proportion of the patients. Allogeneic SCT results for patients in older age groups are constantly improving, which will increase the proportion of MDS patients eligible for this treatment. A large phase II trial suggests that a proportion of patients with high-risk MDS with complete remission after highdose chemotherapy may benefit from consolidation with autologous stem cell transplantation. Growth factors may support hemoglobin levels and neutrophil counts in subgroups of patients, but is an ineffective and expensive treatment for others, which encourages the development of decision models for this type of treatment. While erythropoietin (Epo) as monotherapy has shown efficacy mainly in RA patients with low serum Epo-levels, the combination of G-CSF + Epo may induce long-lasting normalization of hemoglobin levels, in particular in patients with RARS. For RA, again, new therapeutic approaches, such as ATG (reviewed in Section II), seem promising. Low-dose chemotherapy may improve peripheral blood counts, but has previously been shown not to improve long-term outcome. Two DNA-hypomethylating agents, 5-azacytidine and 2,5-deoxycytidine may however change the role of low-dose chemotherapy, since 5-azacytidine has been shown to prolong time to leukemic transformation or death, compared to untreated patients.
The IPSS scoring system is at present the most useful tool to predict long-term outcome in cohorts of untreated patients and should be included as a variable in studies describing the effects of different treatment approaches.1 A new MDS classification has recently been proposed, but its capacity to improve the clinical characterization and management of MDS patients is under debate.2
Allogeneic Transplantation
Allogeneic bone marrow transplantation (allo-BMT) is a curative therapeutic option for younger patients with MDS (Table 5). The outcome of treatment is highly dependent on the selection of patients, and it is therefore difficult to evaluate the effect of different conditioning regimens and other treatment approaches. In a recent review from Fred Hutchinson Cancer Research Center, the results of allogeneic BMT in 251 patients with MDS were reported.3 The overall median disease-free survival (DFS) was 40% after a median follow-up of 6 years. Important predictors for long-term survival were age, morphology and cytogenetics. While patients below 20 years of age (i.e. pediatric MDS and young secondary MDS) showed a DFS of almost 60%, DFS in patients > 50 years of age was below 20%, mostly due to high transplant-related mortality. Increasing disease duration before transplant significantly increased the risk for non-relapse mortality but did not influence disease-free survival. A Canadian study reported the outcome of 60 adult patients with MDS.4 The 7-year event-free survival was 29% for all patients, > 60% for patients with RA/RARS, 20% for patients with ≥ 5% blasts, and 6% for patients in the poor cytogenetic subgroup. An update of the EBMT experience of SCT in 1378 patients with MDS has recently been published.5 Estimated DFS and relapse risk at 3 years were both 36% for 885 patients transplanted with stem cells from matched siblings. DFS and relapse rate in RA/RARS was 55% and 13%, respectively, while the corresponding figures for more advanced MDS was 28% and 43%. DFS in patients with advanced MDS treated to CR was 44%. This analysis did not indicate a significantly better DFS in patients transplanted < 1 year from diagnosis.
Large or new allogeneic stem cell transplantation studies in myelodysplastic syndromes.
| Author (Year)Ref. No. pts (period) . | Age M (range) . | Donor Related/VUD . | Outcome . | Comments . | |
|---|---|---|---|---|---|
. | . | . | DFS . | Relapse . | . |
| Appelbaum (1998)3 251 (1981-96) | 38 (1-66) | 59% / 28% | 40% at 6y (60% < 20y, 20% > 50y) | 18% | Age, IPSS highly predictive for DFS |
| Nevill (1998)4 60 (1986-96) | 40 (15-55) | 38/22 | 29% at 7y | 42% | 6% DFS in poor cytogenetic risk group |
| de Witte (2000)5 1378 (1997) | not given | 885/198 | 36% at 3 y36% 25% (VUD) | (all) | Relapse rate in patients with >5% blasts 42-43%, in MDS-AML 49% |
| Deeg (2000)6 50 (1989-98)* | 59 (55-66) | 36/6 | 42% at 3y | 19% | DFS in RA, 53%. In high cytogenetic risk group 21% |
| Abbreviations: DFS, disease-free survival; RA, refractory anemia; VUD, volunteer unrelated donor; IPSS, International Scoring System for Evaluating Prognosis | |||||
| Author (Year)Ref. No. pts (period) . | Age M (range) . | Donor Related/VUD . | Outcome . | Comments . | |
|---|---|---|---|---|---|
. | . | . | DFS . | Relapse . | . |
| Appelbaum (1998)3 251 (1981-96) | 38 (1-66) | 59% / 28% | 40% at 6y (60% < 20y, 20% > 50y) | 18% | Age, IPSS highly predictive for DFS |
| Nevill (1998)4 60 (1986-96) | 40 (15-55) | 38/22 | 29% at 7y | 42% | 6% DFS in poor cytogenetic risk group |
| de Witte (2000)5 1378 (1997) | not given | 885/198 | 36% at 3 y36% 25% (VUD) | (all) | Relapse rate in patients with >5% blasts 42-43%, in MDS-AML 49% |
| Deeg (2000)6 50 (1989-98)* | 59 (55-66) | 36/6 | 42% at 3y | 19% | DFS in RA, 53%. In high cytogenetic risk group 21% |
| Abbreviations: DFS, disease-free survival; RA, refractory anemia; VUD, volunteer unrelated donor; IPSS, International Scoring System for Evaluating Prognosis | |||||
Two patients transplanted 1978 and 1983
Recently, Deeg et al reported results on 50 MDS patients, aged 55-66, receiving allogeneic BMT with stem cells from matched siblings (36), unrelated volunteers (6), HLA-nonidentical family members (4), and identical twins (4).6 The Kaplan-Meier (KM) estimate of relapse-free survival at 3 years was 39% for all patients and 47% for patients with primary MDS transplanted with stem cells from an HLA-identical sibling. As in previous studies, cytogenetic risk group and IPSS score were highly predictive for the outcome of treatment. Moreover, conditioning regimen with cyclophosphamide + targeted busulfan showed an advantage compared to other conditioning regimens. The study shows that results in selected groups of older patients are beginning to improve.
Although the results from allogeneic SCT for MDS seem to improve over time, the outcome for patients with MDS is still worse than for those with other myeloid diagnoses, mostly due to a high transplant-related mortality and relapse rate. Future studies will need to focus on optimizing pre-treatment schedules, conditioning regimens and post-transplant immune-modulation. Case reports have shown an effect of donor lymphocyte infusion also in MDS.7 Slavin reported one patient with MDS who entered complete remission after non-myeloablative stem cell transplantation,8 and approximately 60 patients with MDS and secondary leukemia have been included in the protocols from Jerusalem, Seattle and NIH (Shimon Slavin, Rainer Storb, Ghulam Mufti, and John Barrett, personal communication), and at the Huddinge transplantation unit. Complete remissions lasting for 1-2 years have been observed, which encourages further investigations.
Autologous Stem Cell Transplantation for MDS
Conventional high-dose chemotherapy for MDS may lead to complete remission, but only to cure in extremely rare cases. For patients without a suitable donor, or with age and medical conditions making allogeneic stem cell transplantation unsuitable, alternative methods to maintain CR and obtain cure need to be developed. The presence of polyclonal peripheral blood stem cells after high-dose chemotherapy constitutes the theoretical basis for autologous stem cell transplantation,9,10 and several European groups have evaluated the effect of ABMT/APSCT in high-risk MDS.11,12 Encouraged by early findings that DFS seemed to enter a plateau phase approximately 3 years after transplantation, more studies have followed. The EBMT and EORTC working groups have conducted a large phase II trial, in which 184 evaluable patients with high-risk MDS and AML following MDS were given induction therapy to obtain CR. In CR, patients with a histocompatible sibling donor were candidates for allogeneic transplantation, while the remaining patients were planned for autologous stem cell transplantation after consolidation therapy and stem cell harvest (Th. de Witte et al, submitted). The complete remission rate was 54%, with a median remission duration of 12 months, and DFS was 29% at 4 years. Thirty-five of 57 patients without a donor received APSCT in first CR, and 13 of these (37%) were in continuous complete remission at follow-up. The benefit of APSCT compared to conventional high-dose chemotherapy was also supported by a study in which 184 patients from the EORTC-EBMT trials were compared with 216 patients from M.D. Anderson Cancer Research Center receiving only combined chemotherapy.13 Preliminary results indicate a possible benefit of using autologous transplantation as post-remission consolidation, since the survival curve of the transplanted patients showed a plateau, while that of the chemotherapy-treated patients continued to decline. An ongoing study within the EORTC-EBMT working group compares the effect of APSCT with the effect of a second consolidation with high-dose cytosine arabinoside. Careful studies of residual clonality in responding patients will serve as a tool to predict the usefulness of this treatment in individual patients. Although the relapse rate after APSCT is higher than for de novo AML, the results suggest that there is a subset of patients with MDS that may be cured by intensive chemotherapy in combination with stem cell support.
Intensive Chemotherapy
The results of AML-type induction regimens in high-risk MDS have improved during recent years, but are still poorer than for de novo AML. Estey et al compared 158 patients with MDS with a cohort of patients with AML.14 The CR rates were comparable in the MDS and AML groups (62-66%), but patients with RAEB showed significantly shorter event-free survival (EFS) than both those with RAEB-t and AML. Poor prognostic karyotype, length of MDS phase and age predicted for a poorer outcome to treatment. A French group compared highdose chemotherapy given with or without quinine.15 In-patients expressing P-glycoprotein (PGP) the response rate to chemotherapy + quinine was 52% compared to only 18% in those treated with chemotherapy only, indicating that one important reason for the lower CR rates in MDS is functional drug resistance. Bernasconi et al randomized 105 patients to chemotherapy, with or without G-CSF.16 The G-CSF-treated group showed a better response rate (74% vs 52%, p < 0.05), but no effect on long-term survival could be observed unless an allo-BMT was given. In another study, 112 patients with RAEB-t, MDS-AML and secondary AML (median age 58 years, range 22-75) were treated with intensive chemotherapy in combination with G-CSF.17 Overall response rate was 62%, but median duration of response was only 8 months. DFS was 15% at 28 months. The CR rate was higher in patients < 60 years (68% vs 55%), and patients who obtained CR showed a DFS of 25% at 36 months. Outcome was better compared with a previous trial in which patients were treated with the same chemotherapy, but without G-CSF.
Topotecan, a topoisomerase I inhibitor, has been used as single therapy in high-risk MDS. In a cohort of 47 patients with RAEB/RAEB-t and CMML, the CR rate was 28%, with a response rate in previously untreated patients of 31-38%.18 Toxicity, however, was significant with 19% of patients dying during induction treatment. In a recent review by Estey, the effects of topotecan, alone and in combination with cytosine arabinoside (ara-C), was compared with the effect of ara-C in other combinations, such as the FLAG regimen.19 In RAEB/RAEB-t, but not in CMML, ara-C seemed to add to the effect of topotecan as single therapy. Ara-C + topotecan did not show any overall benefit compared to high-dose ara-C alone, but in patients with high-risk (5/7) chromosomal aberrations the combination of ara-C + topotecan showed better results than both topotecan alone, high-dose ara-C, and the FLAG regimen. None of these differences translated into a difference in survival.
Thus, high-dose results are slowly improving over time but long-term survival is still very poor for the majority of patients. It is, however, important to continue to aim for better response rates, since CR is the starting point for curative approaches.
Low-Dose Chemotherapy
Non-curative treatments must aim at improving quality of life and reducing morbidity, but they may also carry hope for improved survival and reduced risk of leukemic transformation. Low-dose chemotherapy may improve peripheral blood values and reduce blast counts, and can be an effective and inexpensive alternative for certain patients with MDS.
The drug that has been most extensively used in low doses is ara-C. Low-dose ara-C may induce complete or partial remissions in approximately 30% of patients with RAEB, RAEB-t and MDS-AML.20,21 However, the only prospective randomized phase III trial comparing low-dose ara-C with supportive care failed to show a difference in survival between the two alternatives.22 Moreover, side effects (mainly bone marrow hypoplasia and pancytopenia) can be pronounced, with therapy-related deaths having a frequency of 7-19%.20 The addition of growth factors, mostly GM-CSF, to ara-C has not been successful.23 Based upon a large phase II trial, pretreatment bone marrow cellularity, chromosome aberrations and ringed sideroblasts were used to formulate a predictive model for the use of low-dose ara-C in MDS and MDS-AML.21 This model identified patient groups with a > 50% probability of response and those with no response to treatment.
Melphalan
Two recently published studies have reported the effects of low-dose melphalan (2 mg/day until progression/toxicity or response) in patients with high-risk MDS. In the first study, 8 of 21 patients with RAEB or RAEB-t (38%) achieved a complete (7) or partial (1) response with a median survival of 27 months for CR patients and 6.5 months for the rest.24 No severe side effects were observed in any patients. Recently, these results were confirmed by a European group,25 who reported a response rate of 40% and suggested a better response in patients with hypocellular MDS. The difference from other low-dose regimens seems to be that melphalan relatively frequently causes improvement without preceding cytopenia. Other advantages are the extremely low cost of the drug and its simple administration.
5-azacytidine and 5-aza-2′ -deoxycytidine
Several trials have investigated the effect of low doses of two DNA hypomethylating pyrimidine analogues, 5-azacytidine (5-aza) and 5-aza-2′ -deoxycytidine (DAC), in high-risk MDS. In spite of the similar structure of these drugs, their clinical effects differ in several ways. In phase II studies, treatment with 5-aza led to improved peripheral blood values and reduction of bone marrow blasts in 49-54% of the patients.26 Inspired by this relatively high response rate and moderate toxicity, 5-aza was evaluated by CALGB in a large randomised phase III study in which 5-aza, given subcutaneously 7/28 days, was studied against observation in a cross-over model.27 The overall response rate was 63% (6% CR, 10% PR, 47% improved). This is not better than for other chemotherapeutic compounds, but the median time to leukemic transformation or death was 22 months in the 5-aza arm versus 12 months for those on supportive care (p = 0.003). Median survival for 5-aza and observation was 18 and 14 months, respectively (p = 0.1). Moreover, treatment-related mortality was low, quality of life was enhanced in responding patients, and toxic side effects few.28 This promising study suggests that 5-aza may alter the natural course of MDS. However, confirmatory studies are warranted.
DAC, given intravenously, has been evaluated in two pilot trials and a large recently published multi-center phase II trial, in which primary endpoints were response rate and toxicity.29 The overall response rate was 49%, with 64% response in patients with an IPSS high-risk score. The actuarial median survival time from start of therapy was 15 months. Myelosuppression was common, and 7% therapy-related deaths were reported. Noticeable was the rapid and distinct platelet response, which occurred after the first course in the responding patients. Compared to 5-aza, DAC seems to be more efficient in inducing CR, it has more severe toxicity, and has not yet been investigated in a trial with survival as endpoint. It is, however, likely that both 5-aza and DAC will add to the arsenal of clinically useful therapies for MDS.
Growth factor treatment
The bone marrow of patients with RA and RARS is mainly characterized by ineffective hematopoiesis. This is paralleled by reduced clonogenic growth in vitro, and the presence of an increased proportion of apoptotic bone marrow precursors.30,31,32 Recent studies suggest that the underlying mechanisms behind apoptosis in MDS may differ between the subtypes of MDS.33,34 Colony culture studies of myelodysplastic bone marrow have shown that the impaired erythroid growth may respond to Epo alone, but that it may be further improved if Epo is combined with other growth factors.35 The ineffective hematopoiesis and cytopenia in RA, RARS and RAEB with < 10% blasts is considered to respond poorly to chemotherapy, and a number of clinical trials have therefore addressed the use of growth factors in these subtypes (Table 6).
Larger studies on erythropoietin (Epo) alone or Epo in combination with G-CSF.
| Author . | Year . | Treatment . | No. Pts. . | Inclusion Criteria . | Erythroid Response . | Favorable Variables/Comments . |
|---|---|---|---|---|---|---|
| Hellstrom-Lindberg40 meta-analysis | 1995 | Epo | 205 | Various, all studies since 1995 | 16%* (8% RARS) | S-Epo < 200 U/L RA/RAEB, no transfusions |
| Rose41 phase II | 1995 | Epo | 116 | Hb < 80 g/L, S-Epo < 500 U/L | 28%** (18% red. transf) | S-Epo < 100 U/L, RA |
| Italian Study Group42 phase III | 1998 | Epo / placebo | 87 | Hb < 90 g/L Blasts < 10% | 37% vs 11%** (p = 0.007) | Response significant in RA and in non-transfused pts |
| Negrin44 phase II | 1996 | Epo + G-CSF | 55 | Hb < 100 g/L | 48%**, duration median 10 mo. | S-Epo |
| Hellstrom-Lindberg45 phase II | 1998 | Epo + G-CSF | 71 | Hb < 100 g/L | 38%*, duration 24 months | S-Epo, transfusion need |
| Remacha47 phase II | 1999 | Epo + G-CSF | 32 | Hb < 100 g/L S-Epo < 250 U/L | 50%** | Predictive model (Figure 5) |
| Mantovani49 | 2000 | Epo + G-CSF | 33 | Blasts < 20% | 61%* (12 weeks) 80% (36 weeks) | 50% response after 2 years |
| Abbreviations: S-Epo, serum Epo level; G-CSF, granulocyte colony-stimulating factor | ||||||
| Author . | Year . | Treatment . | No. Pts. . | Inclusion Criteria . | Erythroid Response . | Favorable Variables/Comments . |
|---|---|---|---|---|---|---|
| Hellstrom-Lindberg40 meta-analysis | 1995 | Epo | 205 | Various, all studies since 1995 | 16%* (8% RARS) | S-Epo < 200 U/L RA/RAEB, no transfusions |
| Rose41 phase II | 1995 | Epo | 116 | Hb < 80 g/L, S-Epo < 500 U/L | 28%** (18% red. transf) | S-Epo < 100 U/L, RA |
| Italian Study Group42 phase III | 1998 | Epo / placebo | 87 | Hb < 90 g/L Blasts < 10% | 37% vs 11%** (p = 0.007) | Response significant in RA and in non-transfused pts |
| Negrin44 phase II | 1996 | Epo + G-CSF | 55 | Hb < 100 g/L | 48%**, duration median 10 mo. | S-Epo |
| Hellstrom-Lindberg45 phase II | 1998 | Epo + G-CSF | 71 | Hb < 100 g/L | 38%*, duration 24 months | S-Epo, transfusion need |
| Remacha47 phase II | 1999 | Epo + G-CSF | 32 | Hb < 100 g/L S-Epo < 250 U/L | 50%** | Predictive model (Figure 5) |
| Mantovani49 | 2000 | Epo + G-CSF | 33 | Blasts < 20% | 61%* (12 weeks) 80% (36 weeks) | 50% response after 2 years |
| Abbreviations: S-Epo, serum Epo level; G-CSF, granulocyte colony-stimulating factor | ||||||
The definition of response required a total stop in transfusion need in transfusion-dependent patients.
The definition of response required a 50% reduction in transfusion need in transfusion-dependent patients.
GM-CSF and G-CSF
GM-CSF and G-CSF are both effective in increasing peripheral neutrophil counts in MDS. In a preliminary reported clinical phase III trial, patients were randomised to either GM-CSF or observation.36,37 Granulocyte counts were significantly increased, while hemoglobin levels, survival, and the incidence of leukemic transformation were not affected. G-CSF, used in phase II studies, showed similar effects on neutrophil counts, and favorable longterm results with maintained response for up to 16 months were reported.38 A randomized study between G-CSF and observation showed a significant G-CSF-induced increase in neutrophil counts but no effect on hemoglobin levels.39 After correction for risk factors, survival or evolution to AML was not significantly influenced by treatment. Both these randomised studies showed that G-CSF, as well as GM-CSF, had an overall negative effect on platelet counts. Thus, there is at present no evidence that supports the use of G-CSF and GM-CSF as single therapy in MDS.
Erythropoietin
Erythropoietin (Epo) may improve the anemia in MDS. All 17 studies (205 patients) published before 1995 were included in a meta-analysis, which showed an overall response rate of 16%.40 Information about FAB group, transfusion need and serum Epo levels (S-Epo) were useful to characterize groups of patients with different outcomes to treatment. Patients with RARS responded significantly less well to treatment than the other subgroups (8% versus 21% in non-RARS). The response rates in patients with S-Epo below or above 200 U/l, were 21% and 10%, respectively, and 10% in patients with pretreatment transfusion need. Rose et al treated 116 patients with Epo.41 The response rate was 28%, S-Epo levels < 100 U/l predicited for a better response, and patients with RA had a better response than those with RARS (39% versus 17.5%). The Italian cooperative group performed a placebo-controlled randomized study, which showed that Epo significantly improved hemoglobin levels in MDS. However, the response was significant only in patients with RA and in patients who did not need transfusion before treatment.42 Finally, Hast et al reported a series of 18 patients responding to Epo, who continued on maintenance treatment and showed a median response duration of 15 months. The dose of Epo could be safely reduced or withdrawn, since the majority of patients who then relapsed with anemia responded well to reintroduction of Epo or to an increase of the dose.43
Erythropoietin in combination with other growth factors
Several clinical trials have studied the combination of Epo and G-CSF, GM-CSF or IL-3. The response rate to Epo + G-CSF has been shown to be around 40% in MDS patients with < 10% bone marrow blasts, i.e. twice as much as for Epo alone. A large US phase II study and the trials within the Scandinavian MDS Group have demonstrated evidence of a clear synergistic effect between the drugs in vivo.44,45 The US study showed that 50% of the patients with a response lost it when G-CSF was withdrawn and regained it when G-CSF was reintroduced, and the Scandinavian study showed that addition of G-CSF could induce erythroid responses in Epo-resistant patients. The synergistic effect was most pronounced in patients with RARS, since this subgroup responded poorly to Epo alone and showed the best response to the combination (around 50%). A study of predictive factors for a response to treatment identified S-Epo and the level of pre-treatment transfusion need as significant variables and these were combined in a predictive model for treatment with G-CSF + Epo (Figure 5).46 The value of this model was later confirmed in independent patient material from a Spanish study,47 and in a third prospective study within the Scandinavian MDS Group (data not reported). This study included 52 evaluable patients from the high (n = 31) and the intermediate (n = 21) predictive groups (for explanation, see Figure 5). The response rate was 61.3% in group 1 and 14.3% in group 2 (p = 0.004), and no other pre-treatment variables improved the model. Quality of life (QLQ 30) improved in patients with a response to treatment (p<0.05 for fatigue and global QOL). A simplified and clinically useful decision model will be developed based on these results. While waiting for the results of ongoing prospective randomized studies, we designed a case-control study in which 135 patients treated with G-CSF + Epo were compared with control cases from the data base generated by the International MDS Risk Analysis Workshop (also used to create the IPSS score). The median duration of response to G-CSF + Epo (defined as transfusion independence or normal hemoglobin levels) was 10 months (range 0-72+) in the whole group and 25 months in patients with RARS. Lastly, a recently published study showed a response rate of 61% in a cohort of 33 patients with MDS.48 A significant number of patients showed late responses (12-36 weeks after start of treatment).
Predictive model for treatment with G-CSF + erythropoietin for the anemia of myelodysplastic syndrome (MDS).
Predictive model for treatment with G-CSF + erythropoietin for the anemia of myelodysplastic syndrome (MDS).
These studies indicate a central role for G-CSF in the treatment of RARS, but the mechanism has not yet been clarified. Histopathological studies have shown that patients who respond to treatment have a significant reduction of apoptotic cells in the bone marrow. Thus, it seems likely that growth factors affect one of the major pathogenetic mechanisms in MDS, ineffective hematopoiesis.32 Recent laboratory studies49 have shown that the erythroid apoptosis in RARS is initiated at the CD34+level, is mediated by increase caspase activity, but not by endogenous stimulation of the Fas receptor, and can be significantly reduced by G-CSF incubation of CD34+ cells.
GM-CSF and EPO have also been used in combination as treatment for MDS.50,51,52 One pilot study showed that the combination could induce erythroid responses in patients not responding to Epo alone. A phase II trial using GM-CSF + Epo resulted in an erythroid response rate of 34.6%, but the authors questioned whether a synergistic effect in vivo was obvious. Finally, a randomized phase II trial showed that GM-CSF + Epo was more effective in reducing transfusion need than GM-CSF + placebo, in patients with S-Epo ≤ 500 U/l. Thus, there is some evidence that the combination of GM-CSF + Epo may also offer an advantage over Epo alone, but the experience is more limited than with to G-CSF + Epo, and GM-CSF has more side effects. Lastly, Epo has been combined with IL-3, but in spite of promising in vitro data, clinical results have not encouraged a continued development of this treatment.53
Concluding Remarks
To improve treatment for patients with MDS, better tools will be required to define the underlying pathogenetic mechanisms in individual patients. It is thus important to estimate the risk for leukemic transformation, the degree of ineffective hematopoiesis, signs of immunemediated myelosuppression, and overall prognosis including age and performance status, before making a therapeutic decision. Recent therapeutic advances have made it clear that new biological information in combination with statistically developed predictive models may help us to better select patients for different therapeutic options and to develop new treatment modalities. The future thus lies in developing new insights into the biology of MDS, in new therapeutic approaches and well-conducted clinical trials, and in the search for better tools to use existing therapeutic options.