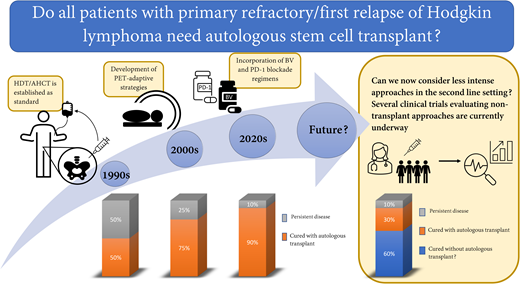
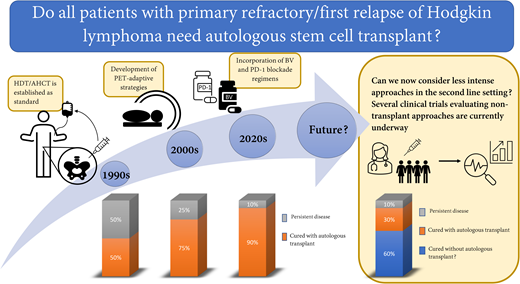
Abstract
The standard approach to treatment of primary refractory/first relapse of classical Hodgkin lymphoma (cHL) is administration of second-line therapy (SLT) followed by consolidation with high-dose therapy and autologous hematopoietic cell transplantation (HDT/AHCT). Historically, this approach cured about 50% of patients. Due to improvements in supportive care, positron emission tomography–adaptive strategies, and incorporation of novel agents into SLT, contemporary studies show that about 75% of patients with primary refractory or first relapse of cHL can be cured. Recent studies evaluating incorporation of PD-1 blockade in SLT appear to show even further improvement in remission rates and bring into question whether an aggressive approach that includes HDT/AHCT is needed for everyone. To address this question, several ongoing studies are beginning to explore the possibility of avoiding or delaying HDT/AHCT for patients with primary refractory or first relapse of cHL.
Learning Objectives
Understand the current standard of care for patients with primary refractory or first relapse of classical Hodgkin lymphoma (cHL)
Appreciate recent data showing improved efficacy of second-line therapy for cHL that may challenge the current standard of care
CLINICAL CASE
A 36-year-old woman was initially diagnosed with stage IIIB classical Hodgkin lymphoma (cHL) associated with a 12-cm mediastinal mass and international prognostic score of 2. She was treated with brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (BV-AVD) for 6 cycles. Her positron emission tomography (PET) scan was negative after 2 and 6 cycles of BV-AVD, but 1 month after completion of treatment, she developed recurrent pruritis, fatigue, shoulder pain, and dullness in her chest. A repeat PET performed about 2 months after completion of BV-AVD showed new and increasing hypermetabolic masses in the anterior mediastinum, with the largest mass measuring 5.6 × 4.9 cm, standardized uptake value (SUV) 17.5. Biopsy of the dominant mediastinal mass confirmed recurrent cHL.
Historical approach to relapsed and refractory cHL
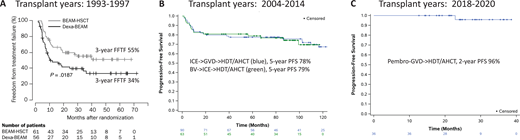
The first thing I would tell this patient is that we get 2 good chances to cure cHL. This statement was true even before brentuximab vedotin (BV) or programmed death 1 (PD-1) blockade became available. In fact, our expectation was that patients with relapsed or refractory disease following front-line therapy still had about a 50% chance of cure. This expectation was based on 2 randomized studies that established high-dose therapy and autologous hematopoietic cell transplantation (HDT/AHCT) as standard for patients with relapsed or refractory disease. The first, led by the British National Lymphoma Investigation, randomized 40 patients who failed MOPP (mechlorethamine, vincristine, procarbazine, prednisone) or similar chemotherapy to up to 3 cycles of mini carmustine, etoposide, cytarabine, and melphalan (BEAM) chemotherapy or high-dose BEAM and AHCT.1 This study showed improvement in 3-year event-free survival (53% vs 10%) but no improvement in overall survival. The second, led by the German Hodgkin Study Group and the European Group for Blood and Marrow Transplantation, enrolled 144 patients who received 2 courses of dexamethasone and BEAM (dexa-BEAM) followed by randomization of chemosensitive patients to 2 more cycles of dexa-BEAM or high-dose BEAM and AHCT.2 Among the chemosensitive patients, which represented 81% of patients, BEAM and AHCT was associated with improved 3-year freedom from treatment failure of 55% compared with 34% for dexa-BEAM alone (P = .019) (Figure 1A). Overall survival was again not significantly different between the 2 groups.
Three decades of improvement in posttransplant outcomes for primary refractory and first relapsed cHL. (A) Freedom from treatment failure (FFTF) following conventional chemotherapy with dexa-BEAM or high-dose chemotherapy with BEAM followed by autologous stem cell transplantation (BEAM-HSCT). (Reprinted from Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065-2071, with permission from Elsevier.) (B) Progression-free survival after PET-adapted therapy with ICE and GVD (green) or BV and ICE (blue) followed by AHCT (curves updated from references 12 and 13). (C) Progression-free survival after pembro-GVD followed by AHCT (curve updated from reference 16).
Three decades of improvement in posttransplant outcomes for primary refractory and first relapsed cHL. (A) Freedom from treatment failure (FFTF) following conventional chemotherapy with dexa-BEAM or high-dose chemotherapy with BEAM followed by autologous stem cell transplantation (BEAM-HSCT). (Reprinted from Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065-2071, with permission from Elsevier.) (B) Progression-free survival after PET-adapted therapy with ICE and GVD (green) or BV and ICE (blue) followed by AHCT (curves updated from references 12 and 13). (C) Progression-free survival after pembro-GVD followed by AHCT (curve updated from reference 16).
These studies provided the rationale for our current treatment standard of using HDT/AHCT in second remission as well as the basis for the expectation that 50% of patients failing frontline therapy can still be cured. But the studies are outdated. In fact, more recent phase 2 studies show considerably superior outcomes for patients with relapsed or refractory disease, with 2-year progression-free survival (PFS) of about 70% or better.3-5 Furthermore, a recent pooled analysis including 718 transplant-eligible patients treated on prospective clinical trials showed that use of modern second-line therapies (SLTs) is associated with a 3-year PFS of 74%.6 Two main factors account for the improved outcomes observed in recent studies: the establishment of pre-HDT/AHCT 18F-fluoro-deoxyglucose (FDG)-PET (PET) response as the single most important prognostic factor predicting post-HDT/AHCT outcome and more effective SLTs that include BV and PD-1 blockade.
Pretransplant PET in relapsed/refractory cHL
Numerous studies have shown that pretransplant PET is an important prognostic factor for patients with relapsed or refractory cHL.7-13 A meta-analysis of 11 studies comprising 745 patients with relapsed or refractory cHL showed that PFS ranged from 0% to 52% for pretransplant PET-positive patients and 55% to 85% for pretransplant PET-negative patients. PET-adapted studies evaluating SLT in transplant-eligible patients further demonstrated the importance of achieving PET normalization before HDT/AHCT. In 1 such study, patients with refractory or first relapsed cHL initially received ifosfamide, carboplatin, and etoposide (ICE)–based SLT; PET-negative patients proceeded to HDT/AHCT, while those with persistent abnormalities on PET after ICE received additional non-cross-resistant therapy with gemcitabine, vinorelbine, and liposomal doxorubicin (GVD) before HDT/AHCT. PET was repeated after GVD, and those with responding disease proceeded to HDT/AHCT. Of 98 patients enrolled on this study, 60% were PET negative after ICE-based chemotherapy and an additional 17% became PET negative following additional therapy with GVD. The 5-year PFS for patients undergoing transplantation on this study was 78%, and the outcomes were identical for patients proceeding to transplant in complete response (CR) after ICE alone or ICE followed by GVD (Figure 1B).14 These findings indicate that normalization of PET should be a goal of pre-HDT/AHCT salvage, and patients who achieve pretransplant PET normalization have excellent outcomes, even if greater than 1 salvage program is necessary.
Interpretation of PET has changed over the past 20 years and will continue to evolve with the use of immunotherapy. Earlier studies used the International Harmonization Project criteria that defined a negative PET as having residual sites with an SUV less than or equal to mediastinal blood pool.15 More recent studies use the Deauville score, where PET negative is typically defined as a Deauville score of 3 or better (residual sites must have an SUV less than or equal to liver).16 PD-1 blockade complicates PET interpretation due to the possibility of tumor flare or pseudo-progression that can be mistaken for residual disease or true progression. To aid in interpretation of PET imaging following PD-1 blockade, the LYRIC (lymphoma response to immunomodulatory therapy criteria) criteria were developed.17 LYRIC introduced the term indeterminate response to identify abnormal PET findings that could reflect flare or pseudo-progression. To verify the etiology of findings consistent with indeterminant response, biopsy or subsequent imaging (at a maximum of 12 weeks from initial imaging) is required. The prognostic significance of pretransplant PET after PD-1 blockade-containing therapy is not known, and therefore it is not clear whether PET normalization is the appropriate end point for studies evaluating PD-1 blockade- based SLT. Ongoing studies may identify more suitable end points, such as reduction in circulating tumor DNA or metabolic tumor volume. Due to the uncertainty regarding PET-positive findings after PD-1 blockade-based SLT, there is a low threshold to obtain biopsies to clarify findings of indeterminate response.
Incorporation of BV into second-line therapy for cHL
One of the first studies to evaluate BV as part of SLT was a phase 2 study evaluating PET-adapted therapy with BV and augmented ICE (augICE).18,19 On this study, patients initially received single-agent BV followed by PET; those who were PET negative after BV proceeded to HDT/AHCT, while those with residual PET avidity received augICE chemotherapy followed by consideration for HDT/AHCT. Among 65 patients enrolled, 27% achieved PET normalization after BV alone, and 83% achieved PET normalization after the whole treatment program. The 5-year PFS for transplanted patients (63 of 65) was 79% (Figure 1B). Once again, the importance of pretransplant PET negativity was demonstrated in this study as the outcomes for patients proceeding to transplant in CR after BV alone were identical to those in CR after BV and ICE. The major drawback of this treatment strategy was that the CR rate to BV alone was low (27%), and therefore most patients needed augICE to achieve PET normalization. An additional drawback of this study was the use of augICE, which is considerably more toxic than standard ICE. Subsequent studies evaluating BV concurrently with chemotherapy reported high PET-negative rates and similarly promising PFS rates (Table 1) yet enabled patients to proceed to HDT/AHCT sooner. For example, 3 cycles of BV combined with dexamethasone, cytarabine, and cisplatin produced a CR rate of 81% and 2-year PFS of 74%.3 Likewise, a median of 2 cycles of brentuximab plus bendamustine allowed 74% of patients to achieve CR, 73% to proceed to transplant, and 70% of transplanted patients to remain in remission at 2 years.5,20 Overall, the strategy of using BV-based SLT facilitates high PET-negative rates and high rates of HDT/AHCT consolidation, and it leads to durable remissions in nearly three-quarters of patients with relapsed/refractory disease. These results are quite satisfactory; however, the introduction of PD-1 blockade into SLT has resulted in even further improvements.
Incorporation of PD-1 blockade into second-line therapy for cHL
The 2 PD-1–based SLT regimens with longest follow-up are BV plus nivolumab (BV-nivo) and pembrolizumab plus GVD (pembro- GVD). BV-nivo was evaluated in a phase 1/2 study in which patients with relapsed or refractory cHL following 1 line of therapy received 4 cycles of the combination followed by consideration for HDT/AHCT.21 PET negativity was not required for patients proceeding to HDT/AHCT, and management of patients with residual PET avidity after BV-nivo was left up to the treating physician. Among 91 patients, 67% achieved CR, and 74% (84% in CR) proceeded directly to HDT/AHCT after BV-nivo. The 3-year PFS for the entire study was 77%, while the PFS for patients proceeding directly to HDT/AHCT after BV-nivo was 91%. Despite the modest CR rate with BV-nivo, the PFS rate remains impressive, suggesting that perhaps PD-1 blockade may contribute to more durable remissions in the relapsed/refractory setting.
Even higher efficacy was observed with pembro-GVD, which was evaluated in a phase 2 study in a similar patient population.22 The pembro-GVD study enrolled 39 patients who received up to 4 cycles of treatment; patients were assessed for response after 2 and 4 cycles, and those in CR after only 2 cycles could proceed directly to HDT/AHCT. Among 38 evaluable patients, all responded and 36 (95%) achieved CR. Most (92%) achieved CR after only 2 cycles, and a total of 36 patients (95%) proceeded to HDT/AHCT (including 30 after only 2 cycles). Currently, the median posttransplant follow-up for this study is 25 months and only 1 patient has relapsed; thus, the 2-year PFS is 96% (Figure 1C). The patients enrolled on the pembro-GVD study represented a high-risk patient population as 41% had primary refractory disease and 31% had extranodal disease at enrollment. These unprecedented results led us to begin to question the utility of HDT/AHCT in second remission.
Posttransplant maintenance with BV
The AETHERA study established the role of post-HDT/AHCT BV maintenance for relapsed and refractory cHL.23 AETHERA was a phase 3 study in which 329 BV-naive, high-risk, relapsed and refractory patients were randomized after HDT/AHCT to placebo or 16 cycles of BV. High risk was defined as the presence of primary refractory disease, relapse within the first year of initial therapy, or presence of extranodal disease. At 5-year follow-up, BV maintenance continued to demonstrate a 30% reduction in PFS events, and thus BV maintenance remains an important option for relapsed or refractory patients.24 The benefit of BV maintenance was most pronounced in patients with 2 or more risk factors (risk factors included primary refractory disease, relapse within 1 year of initial treatment, extranodal disease, B-symptoms, 2 or more salvage regimens before transplant, and less than complete response prior to transplant). Since BV is now used routinely before HDT/AHCT, the current role of BV maintenance is less clear; however, it is still reasonable to consider for patients previously exposed to BV, provided a durable response to prior BV-based therapy was achieved. Interestingly, the benefit of BV maintenance post-HDT/AHCT was diminished in patients PET negative before HDT/AHCT, further demonstrating the importance of achieving complete response to SLT.
Does every patient need a transplant?
For decades, HDT/AHCT in second remission has been our standard for relapsed and refractory cHL. With the addition of highly effective, targeted drugs to the armamentarium for cHL, we are now able to ask whether our current approach of using aggressive approaches such as HDT/AHCT is still necessary for all patients with relapsed or refractory cHL. Similar questions have been asked for frontline treatment of cHL. Historically, all patients with newly diagnosed cHL received highly aggressive combination chemotherapy and often radiation. With time, interim PET emerged as an important predictor of outcome, and we learned that treatment can be modified based upon interim PET response.25,26 This led to elimination of radiation for bulky advanced stage patients who achieve PET CR, reduced exposure to bleomycin for PET-2–negative advanced-stage patients, and reduced use of radiation for early-stage patients.26-28 Likewise, we learned that the length of treatment can be reduced to as little as 3 cycles for the most favorable early-stage patients who achieve PET CR.29 Statistically, some of these modifications are associated with reduced tumor control rates, but in many cases, this is justified by the fact that most individuals are successfully treated with less chemotherapy and/or without radiation, which allows for less treatment-related toxicity. HDT/AHCT has the potential to cause significant long-lasting toxicity such as infertility, cardiac dysfunction, pulmonary compromise, and secondary malignancies.30 Given the high efficacy of PD-1–based SLT, we now face similar questions previously asked for frontline therapy. Does every patient require aggressive therapy with HDT/AHCT? Can we now consider less intense approaches in the second-line setting and shift HDT/AHCT to the third-line setting for those who still need it? These questions are beginning to be explored through clinical trials.
Which patients may be most appropriate for nontransplant approaches? It is possible that known risk factors associated with less favorable outcomes following transplant may aid in selecting patients for transplant vs nontransplant approaches. These risk factors include remission duration less than 1 year from initial therapy, B-symptoms, extranodal disease, advanced stage, age, disease bulk, and anemia.19,31-33 Furthermore, emerging biomarkers for cHL, such as circulating tumor DNA and metabolic tumor volume, may eventually aid in selecting patients for transplant.19,34 For older patients with cHL, the role of HDT/AHCT for relapsed/refractory disease is unclear as older patients are not well represented on studies evaluating transplant approaches; therefore, it is possible that nontransplant approaches may be more appropriate for this group as well.35
Can long-term remission (and possibly cure?) be achieved without transplant? Reports of long-term remission for relapsed/refractory disease using nontransplant approaches are currently anecdotal, but there are clues from prior studies that select patients may not need transplant for cure. One clue comes from the pivotal phase 2 study with BV in which patients with relapsed or refractory disease after HDT/AHCT received up to 1 year of BV. Among 102 patients, 9 remained in remission at 5-year follow-up without receiving additional therapy, suggesting that they were potentially cured from single-agent BV.36 Furthermore, the study evaluating SLT with BV plus bendamustine (which included BV maintenance) included 5 patients who achieved complete response to BV plus bendamustine but did not proceed to transplant. Although the numbers are small, the duration of CR was similar among patients who did and did not undergo HDT/AHCT.20 These data suggest that select patients may achieve durable remissions without transplant, but our challenge is to determine how to select patients for nontransplant approaches and to identify treatment regimens that optimally balance efficacy and toxicity.
Is radiation alone appropriate for localized relapse?
Outside of clinical trials, for patients who relapse with localized disease, it is tempting to consider radiation alone; however, there are very little data to support this approach. Two of the largest retrospective analyses (including 81 and 100 patients, respectively) assessing radiation alone or combined-modality therapy for relapsed disease reported 5-year freedom from treatment failure rates of 36% and 28%, respectively.37,38 Most treatment failures occurred outside of the radiation field, suggesting that inclusion of systemic therapy is important for most patients. A factor predictive of treatment success was late relapse (defined as relapse >12 months from initial treatment). The limited data from these retrospective series indicate that even patients with localized relapse should be considered for treatment that includes systemic therapy and likely AHCT.
Studies evaluating nontransplant approaches for relapsed and refractory cHL
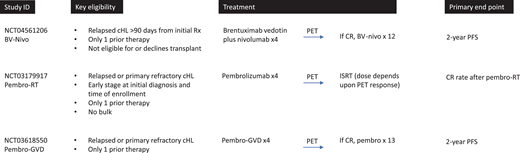
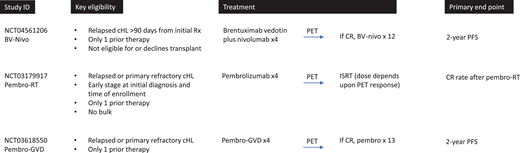
Several studies evaluating nontransplant approaches are currently under way (Figure 2). One approach evaluates a group of more “favorable” relapse/refractory patients. This study is enrolling patients with nonbulky, early-stage disease who are eligible for involved site radiation therapy (ISRT). On this study, patients receive 4 cycles of pembrolizumab followed by involved site radiation therapy (clinicaltrials.gov NCT03179917). This study is enrolling up to 22 patients; primary end point is complete response following pembrolizumab plus radiation, and a secondary end point is 2-year event-free survival. Two other studies focus on a broader population that is less selected for favorable features (with 1 study including non-ASCT-eligible patients). These trials represent a stepwise approach away from HDT/ASCT. One is led by investigators at City of Hope and is enrolling patients with relapsed cHL following 1 line of therapy who are not candidates for HDT/AHCT (due to comorbidities or patient preference) (clinicaltrials.gov NCT04561206). Patients are treated with BV plus nivolumab, and those with complete response after 4 cycles are eligible to continue BV-nivo for up to 16 cycles (without transplant). Thirty-one patients are planned, and the primary end point is 2-year PFS. For all other patients with first relapse or primary refractory cHL, a study at Memorial Sloan Kettering Cancer Center is exploring the possibility of avoiding or delaying transplant through use of pembro-GVD and maintenance pembrolizumab (clinicaltrials.gov NCT03618550). On this study, patients receive 4 cycles of pembro-GVD. Those with complete response continue with 13 doses of pembrolizumab maintenance (no transplant). This study is enrolling 23 patients, and the primary objective is 2-year PFS.
Clinical trials evaluating nontransplant approaches for primary refractory and first relapsed cHL. ISRT, involved site radiation therapy; Pembro-RT, pembrolizumab plus radiation therapy.
Clinical trials evaluating nontransplant approaches for primary refractory and first relapsed cHL. ISRT, involved site radiation therapy; Pembro-RT, pembrolizumab plus radiation therapy.
CLINICAL CASE (Continued)
We started with a case of a 36-year-old woman with advanced-stage cHL with biopsy-proven relapse 2 months after completion of 6 cycles of BV-AVD. Today, the standard of care is to treat with SLT followed by consolidation with HDT/AHCT. My current standard, particularly for a patient already exposed to BV, is to use pembro-GVD followed by HDT/AHCT. I use post-HDT/AHCT BV maintenance for patients with higher-risk disease (2 or more risk factors defined by the AETHERA study) who are either BV naive or with previous durable remission (12 months) to a BV-based regimen; therefore, this patient would not have received BV maintenance. This patient opted to enroll on our clinical trial evaluating a nontransplant approach. She received 4 cycles of pembro-GVD and achieved a complete response. She subsequently received 13 cycles of maintenance pembrolizumab and is currently in remission, under observation.
Conclusion—2 good chances to cure cHL . . . or maybe more?
While the current standard for patients with first relapse or primary refractory cHL includes HDT/AHCT, the landscape of cHL has dramatically changed, placing this standard into question. Studies are now asking whether a substantial portion of patients with relapsed or refractory disease can be cured with a nontransplant approach. Furthermore, for those who cannot be cured with a nontransplant approach, does moving HDT/AHCT to the third-line setting hurt their chance of cure? It has been clear for several decades that we have 2 good chances to cure cHL; however, we may learn that HDT/AHCT can safely be moved to the third-line setting (for those who need it) and that we therefore have at least 3 good chances for cure. It is possible, though, that HDT/AHCT may be less curative when used later in the disease course for cHL. Furthermore, delaying HDT/AHCT may ultimately expose patients to increased toxicity due to a requirement for additional lines of therapy. The current single-arm studies are beginning to tackle whether AHCT is needed for everyone and whether AHCT can be safely delayed for those who need it. If the results are promising, I look forward to the randomized study aimed to change this standard.
Acknowledgments
I thank Nivetha Ganeson, MPH, for help with data analysis and design of the figures and visual abstract. I am tremendously grateful for support from the Adam R. Spector Foundation. A.J.M. is a Scholar in Clinical Research of The Leukemia & Lymphoma Society.
Conflict-of-interest disclosure
Alison J. Moskowitz receives research support from ADC Therapeutics, Beigene, Miragen, Seattle Genetics, Merck, Bristol-Myers Squibb, Incyte, and SecuraBio. She has received honorarium from Affimed, Imbrium Therapeutics L.P./Purdue, Janpix Ltd., Merck, Seattle Genetics, and Takeda.
Off-label drug use
Alison J. Moskowitz: nothing to disclose.