Abstract
Despite the maximum intensification of chemotherapy and the increased use of hematopoietic stem cell transplantation (HCT) in pediatric patients with acute myeloid leukemia (AML), nearly 40% of patients still experience relapse, and cure in this setting remains a significant challenge. Recent improvements in AML characterization, including advances in flow cytometry and comprehensive genomic sequencing, have led to a better understanding of AML biology and the identification of multiple potential therapeutic targets. Novel agents targeting genomic lesions, cell surface antigens, and other mechanisms that permit oncogenesis or immune escape are being incorporated into current treatment strategies or are under investigation in efforts to improve outcomes and decrease the toxicities and late effects associated with traditional intensive chemotherapeutic and HCT treatment. However, multiple challenges still exist, including the biologic and immunophenotypic heterogeneity of childhood AML, the differences in underlying biology as compared to adult AML, and the significant potential for on-target/off-tumor toxicity associated with therapies directed at targets common to myeloid cells, both leukemic and normal. This article reviews the current landscape of genomic and cell surface targets for children with AML with a focus on the currently available targeted therapeutic agents, those in active clinical investigation, and those still in development.
Learning Objectives
Identify the most common targets for therapeutic intervention in children with AML
Learn which mutations, oncogenic pathways, and cell surface antigens in pediatric AML have targeted therapeutics available or are an area of ongoing development
Recognize the need for targeted therapies in pediatric AML and the challenges associated with their development
CLINICAL CASE 1
A 14-year-old boy is diagnosed with acute myeloid leukemia (AML) after presenting with hyperleukocytosis, coagulopathy, and 3 weeks of progressive symptoms. He is started on induction chemotherapy with cytarabine, daunorubicin, etoposide, and gemtuzumab ozogamicin as per AAML0531 (NCT00372593). On day 7 of induction, genomic studies return demonstrating the presence of an FLT3-internal tandem duplication (ITD) mutation and the absence of an NPM1 or CEBPA mutation; he starts sorafenib on day 11 of induction chemotherapy. The patient and his family are counseled regarding the high-risk nature of this mutation and the benefit of an allogeneic hematopoietic stem cell transplant (HCT). He receives sorafenib in combination with chemotherapy as per AAML1031 (NCT01371981) for induction 1 and 2 and intensification 1 and proceeds to a human leukocyte antigen-matched unrelated donor transplant; on day 64 plus following HCT, he begins sorafenib maintenance.
Introduction
Despite the intensification of therapy and the increased use of up-front HCT, approximately 40% of children with de novo AML experience relapse, and cure following relapse remains a significant challenge in most cases.1-4 Large-scale genomic sequencing studies have improved understanding of the genomic heterogeneity of AML that contributes to variable patient responses and relapse rates and have identified potential therapeutic targets.5-7 Comprehensive sequencing efforts have illuminated the significant differences in the underlying biology between pediatric and adult AML.5,8 Thus, many of the targeted therapies developed for adult AML can have comparatively limited utility in children.
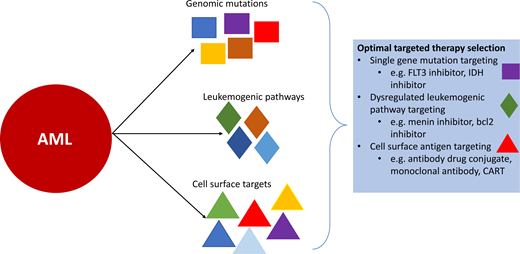
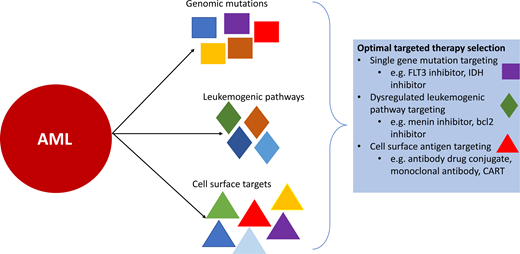
The diagnostic workup of pediatric AML ideally includes flow cytometry for immunophenotyping to define surface antigen expression, cytogenetic studies (karyotyping and fluorescence in situ hybridization), and comprehensive (DNA and RNA) sequencing to identify genomic lesions not captured by conventional diagnostic methods. Recognition of the leukemia's mutational profile is critical to informing prognostication and treatment. Multiple genomic aberrations have the potential to be targeted by directed therapeutic strategies (eg, FLT3, KMT2A, IDH1/2, KIT, and RAS); however, challenges to the development of effective agents in children include the ability to target driver mutations and low frequency of some alterations. FLT3 inhibitors directed against FLT3-mutated AML, particularly FLT3-ITD, have been the first targeted small-molecular inhibitors in AML to show significant clinical benefit both as a single agent and when added to intensive chemotherapy and serve as a paradigm for other molecularly targeted therapies. Cell surface antigens represent another target for effective and directed therapeutic interventions, particularly CD33 with gemtuzumab ozogamicin, with efforts underway to develop additional immunotherapeutics for CD33 and other cell surface targets. Pathways that become altered either as the result of a specific genomic alteration (eg, transcriptional regulation via KMT2A rearrangements) or are more broadly dysregulated in AML (eg, bcl2 and antiapoptotic signaling) are other active areas of interest that show exciting potential for targeted therapies in pediatric AML.
FLT3-targeted therapy
FLT3 mutations are among the most common somatic mutations in AML, with an age-associated increase in prevalence ranging from 10% to 25% in younger children and adolescents and young adults.5 FLT3 mutations generally occur as ITD or missense mutations in the tyrosine kinase domain (TKD), both resulting in aberrant and constituent kinase activity that results in increased cellular proliferation.9 FLT3-ITD mutations are associated with increased relapse risk and inferior outcomes, while FLT3-TKD mutations are a neutral prognostic factor.10,11 Multiple FLT3 inhibitors have been developed for use in FLT3-mutant AML and can be classified based on receptor binding and thus which mutations they are active against (FLT3-ITD and FLT3-TKD vs FLT3-ITD only) (Table 1).12 Sorafenib and midostaurin have shown efficacy in the de novo and relapsed/refractory (R/R) settings. In newly diagnosed FLT3-mutant adult patients, the addition of midostaurin to chemotherapy compared to placebo in the RATIFY trial (NCT00651261) resulted in a 21.6% decreased risk of an event, along with more modest increases in event-free survival (EFS) and overall survival (OS); the majority of patients (77%) had FLT3-ITD mutations.13,14 The Children's Oncology Group trial AAML1031 (NCT01371981) evaluated sorafenib in combination with chemotherapy for children with a high allelic ratio (> 0.4) FLT3-ITD who also received HCT in first complete remission (CR1). Compared to a nonrandomized control group that did not receive sorafenib, the sorafenib-treated cohort had significant improvements in EFS (55.9% vs 31.9%; P = .001) with lower relapse risk (17.6% vs 44.1%; P = .012).15 When sorafenib was administered sequentially following chemotherapy, there were no significant differences in targeted toxicities in the sorafenib treated vs not exposed groups.15 FLT3-ITD mutations confer a proliferative advantage to AML cells but may not be a leukemia-initiating event; thus their ability to serve as a potent therapeutic target may be limited in some FLT3-ITD AML according to the driving and cooperating genomic events.
AAML1031 also demonstrated that post-HSCT sorafenib as maintenance therapy for 1 year was feasible, which built on prior studies in pediatrics and adults.16,17 The randomized placebo- controlled SORMAIN trial (DRKS00000591) in adults evaluating post-HCT sorafenib administered for 2 years found that the sorafenib-treated group had significantly improved relapse-free survival compared to placebo (85.0% vs 53.3%; P = .002).18 Another randomized trial of post-HCT sorafenib in adults (NCT02474290) demonstrated significantly lower relapse rates of 7.0% for the sorafenib-treated group vs 24.5% in the untreated group (P = .001) and significantly improved leukemia-free survival and OS, as well as tolerability without increased rates of graft-versus-host disease.19 Multiple trials have shown that FLT3 inhibitors can be safely initiated in the first 30 to 60 days post HCT.19,20 Sorafenib and midostaurin are considered first- generation FLT3 inhibitors with broader kinase activity that can result in off-target effects, and thus there is significant interest in newer-generation inhibitors, such as gilteritinib, targeted more specifically against FLT3. Gilteritinib has shown single-agent efficacy as well as feasibility in the post-HCT setting, and its efficacy is currently being evaluated as 2-year post-HCT maintenance in adults (NCT02997202).21,22 This agent is currently being evaluated in the up-front phase 3 Children's Oncology Group trial AAML1831 (NCT04293562) and is administered to all FLT3-ITD patients before and after HCT for a 1-year maintenance. Quizartinib has shown significant activity with prolonged survival in adults, with an ongoing trial in pediatric patients with relapsed FLT3-ITD AML (NCT03793478) (Table 1).23
Although FLT3-TKD mutations do not have an impact on prognosis, they are a viable target for therapy. Studies in adults utilizing type 1 FLT3 inhibitors (eg, midostaurin, gilteritinib) have included smaller cohorts of FLT3-TKD patients, with subanalyses showing similar trends of improved responses in this group.13,21 A subsequent RATIFY trial analysis in FLT3-TKD patients showed longer EFS in the midostaurin-treated patients vs placebo (45.2% vs 30.1%; P = .044).24 Efforts to understand the impact of FLT3 inhibitors in pediatric patients are underway, with AAML1831 administering gilteritinib with chemotherapy and as maintenance following chemotherapy or HCT to all pediatric patients with FLT3-TKD and other non–ITD-activating mutations.
Gemtuzumab ozogamicin and CD33-targeted therapy
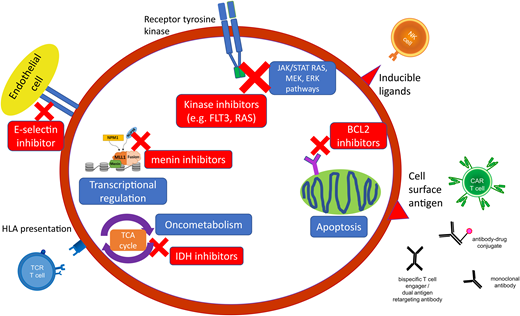
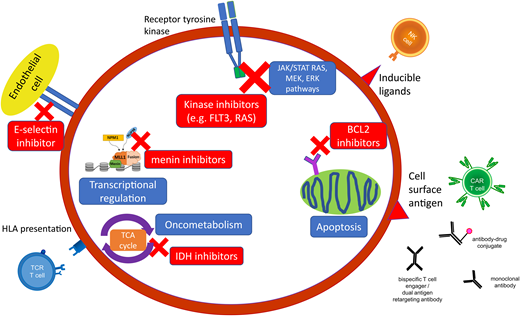
The cell surface antigen CD33 is expressed on myeloid cells, including the majority of AML, and is the first antigen to be successfully targeted with immunotherapeutic strategies with the antibody-drug conjugate (ADC) gemtuzumab ozogamicin (Figure 1). Gemtuzumab ozogamicin has demonstrated single-agent efficacy in R/R disease and has been successfully combined with conventional chemotherapy in the up-front setting.25 The addition of up-front gemtuzumab ozogamicin has been found to result in a relatively modest benefit on outcomes in pediatric AML and thus has been incorporated as standard induction therapy on the current AAML1831 trial.4 There have been efforts to determine which groups of patients benefit the most, with studies showing that pediatric patients harboring FLT3-ITD and KMT2A rearrangements (KMT2Ar) have significant improvements in EFS and reduced relapse rates when treated with gemtuzumab ozogamicin in induction compared to non-gemtuzumab ozogamicin groups, especially in the context of HCT consolidation.26,27 Additionally, the Myechild01 study (NCT02724163) is investigating the optimal dosing strategy of gemtuzumab ozogamicin based on studies in adults that suggested a higher benefit with fractionated dosing.28 Further, patients whose blasts express high levels of CD33 or the CD33 isoform that permits optimal gemtuzumab ozogamicin binding have been shown to derive a more significant benefit.29-31 Ongoing gemtuzumab ozogamicin studies may further clarify which patients derive benefit from this therapy and those for which alternative agents directed against CD33 or other more suitable targets are necessary.
Targeted agents in AML encompass small-molecular inhibitors directed against specific genomic alterations and leukemogenic pathways as well as cell surface antigens, which can be targeted by a variety of immunotherapeutic modalities. HLA, human leukocyte antigen; NK, natural killer; TCA, tricarboxylic acid; TCR, T-cell receptor.
Targeted agents in AML encompass small-molecular inhibitors directed against specific genomic alterations and leukemogenic pathways as well as cell surface antigens, which can be targeted by a variety of immunotherapeutic modalities. HLA, human leukocyte antigen; NK, natural killer; TCA, tricarboxylic acid; TCR, T-cell receptor.
CLINICAL CASE 2
A 7-year-old girl was diagnosed with AML with t(9;11)/KMT2A- MLLT3 fusion. She was measurable residual disease (MRD) negative by flow cytometry after the first induction and received 5 cycles of chemotherapy. She relapsed 10 months after diagnosis, and cytomolecular studies again demonstrated KMT2A-MLLT3 and identified an NRAS mutation. She underwent reinduction with CPX-351 and gemtuzumab ozogamicin, after which she had 12% residual disease. She received a course of high-dose cytarabine and venetoclax and was MRD negative and proceeded to allogeneic HCT in CR2. She unfortunately experienced relapse 5 months post HCT and was subsequently enrolled in a clinical trial receiving monotherapy with a menin inhibitor.
KMT2A rearrangements
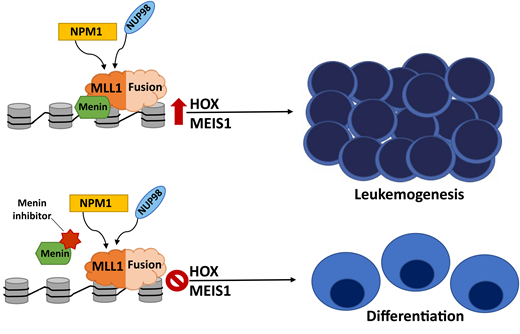
Translocations at chromosome 11q23 involving histone-lysine N-methyltransferase 2A (KMT2A) are one of the most common cytogenetic abnormalities in pediatric AML, occurring in 15% to 20% of cases and with a variety of translocation partners conferring variable prognostic significance.32 KMT2Ar alters the recruitment of transcriptional activation complexes through a KMT2A-menin interaction, leading to upregulation of the HOXA9 and MEIS1 genes that contribute to leukemogenesis.33 In efforts to target this lesion, menin inhibitors have been developed that block the essential interaction between menin and the N-terminal portion of the MLL1 fusion protein, which disrupts the chromatin complex and induces differentiation, therefore inhibiting the aberrant leukemogenic transcription mediated by HOXA9/MEIS1 (Figure 2).34,35 Early results from the first menin inhibitor study with SNDX-5613 (NCT04065399) demonstrated an acceptable safety profile and promising antileukemic activity in heavily pretreated adult patients with R/R KMT2Ar and NPM1-mutated (mNPM1) acute leukemias with a composite complete response (CR) rate of 44%.36 The phase 2 expansion portion of the study is ongoing, with its successor study investigating menin inhibitors in combination with chemotherapy in adult and pediatric patients with R/R KMT2Ar or mNPM1 acute leukemia. Multiple phase 1/2 clinical trials evaluating menin inhibitors are underway in adults, with pediatric trials ongoing with SNDX-5613 and in development with other agents (Table 1).
Menin inhibitors block the essential menin-MLL1 interaction, which disrupts the chromatin complex, inhibits HOXA9/MEIS1-mediated leukemogenesis, and induces differentiation. This pathway is critical in KMT2Ar AML. NPM1-mutated and NUP98- rearranged leukemias are also dependent on menin binding and aberrant HOXA9/MEIS1 expression and sensitive to this therapeutic strategy as well.
Menin inhibitors block the essential menin-MLL1 interaction, which disrupts the chromatin complex, inhibits HOXA9/MEIS1-mediated leukemogenesis, and induces differentiation. This pathway is critical in KMT2Ar AML. NPM1-mutated and NUP98- rearranged leukemias are also dependent on menin binding and aberrant HOXA9/MEIS1 expression and sensitive to this therapeutic strategy as well.
NPM1 mutations and NUP98 rearrangements
NPM1 mutations occur in about 8% of pediatric AML cases and confer a favorable prognostic impact.5 NPM1 is an intracellular chaperone protein associated with a number of cellular processes, including proliferation, ribosome and nucleosome assembly, and DNA repair.37 mNPM1 AML is associated with aberrant HOX gene expression and is dependent on the menin-binding site in MLL1, thus rendering this mutation sensitive to menin inhibition.35 Rearrangements in the NUP98 gene occur in about 4% of pediatric AML cases with multiple fusion partners, most commonly NSD1 and KDM5A.5 NUP98 rearrangements are associated with a poor prognosis and often co-occur with other high-risk mutations (ie, FLT3-ITD and WT1).5 Similarly, NUP98 rearrangements promote leukemogenesis through interactions with MLL1-chromatin complexes and alter HOXA9 and MEIS1 expression, with preclinical data suggesting that menin-MLL1 inhibition is an effective target for NUP98-rearranged AML.38 Due to shared mechanisms driving leukemogenesis, pediatric patients with R/R NUP98 fusion and mNPM1 AML will be included in some of the phase 1 clinical trials investigating menin inhibitors (Table 1 and Figure 2).
BCL2 expression
The overexpression of antiapoptotic B-cell lymphoma-2 (BCL2) family proteins (BCL2, BCL-XL, MCL-1) promotes cancer cell survival and is associated with tumor initiation, disease progression, and increased resistance to chemotherapy in multiple cancers, including AML.39 BCL2 expression inhibits programmed cell death by protecting cancer cells from a wide variety of proapoptotic stimuli, including cytotoxic drugs, cytokine withdrawal, irradiation, heat, and deregulated oncogenes.40 BCL-2 inhibitors, including venetoclax, that antagonize these pro-apoptotic proteins and can enhance response to chemotherapeutic agents and trigger apoptosis directly are active in AML (Figure 1).41
Venetoclax as a single agent and in combination with chemotherapy has shown early promising results in adult AML. The VIALE-A trial (NCT03069352) demonstrated improved responses with azacitidine and venetoclax compared to azacitidine alone (CR, 66.4% vs 28.3%, respectively; OS, 14.7 months vs 9.6 months, respectively; P < .001 for both).42 Studies in adults have demonstrated that venetoclax can be safely combined with intensive induction chemotherapy for newly diagnosed AML and results in high CR rates, MRD negativity, and excellent OS.43 Based on these encouraging results, a phase 1 study (NCT03194932) of venetoclax in combination with cytarabine with or without idarubicin in R/R pediatric AML demonstrated CR/CR with incomplete count recovery of 70% with high rates of MRD negativity in those treated at the 360-mg/m2 dose of venetoclax.44 Ongoing studies investigating the use of venetoclax in children with R/R AML include combinations with intensive chemotherapy, hypomethylating agents, and epigenetic modifiers, as well as selinexor, an inhibitor of exportin 1 that results in blocking the nuclear transport of several key proteins and leads to apoptosis (Table 1). Additional BCL-2 inhibitors are currently undergoing investigation in adults.
Additional targets
IDH mutations
Mutations in the IDH genes (IDH1, IDH2) are rare in pediatric AML compared to adults, but their role in leukemogenesis through production of the 2-HG oncometabolite makes them an attractive target for patients who harbor IDH mutations (Figure 1).45 IDH inhibitors (IDH1: ivosidenib and IDH2: enasidinib) have been shown to be effective targeted therapies in adults when used as single agents and in combination with chemotherapy and have provided a rationale for evaluation in pediatric patients.46,47 Enasidinib is currently being evaluated in R/R IDH2-mutant pediatric AML (NCT04203316) (Table 1).
RAS mutations
RAS mutations in pediatric AML most commonly occur in NRAS and KRAS and lead to constitutive activation of the RAS pathway.48 These mutations are found in approximately 20% of pediatric AML patients and are not considered prognostic.5 RAS pathway mutations are especially important in juvenile myelomonocytic leukemia (JMML), where they occur in 90% of patients and involve several genes (ie, NRAS, KRAS, PTPNP11, CBL, NF1).49 Thus, targeting this pathway with multiple downstream effector inhibitors, including MEK and ERK inhibitors, is a compelling therapeutic strategy.48 Despite encouraging preclinical data in RAS-mutant leukemias, these agents have not shown compelling efficacy in adults with RAS-mutated R/R myeloid malignancies.50 Trametinib is currently being studied in combination with azacitidine and venetoclax in adults with R/R AML or high-risk myelodysplastic syndrome and as a single agent in pediatric patients with R/R JMML (NCT03190915) (Table 1). Trametinib will be utilized as an up-front treatment in the upcoming JMML trial through the Therapeutic Advances in Childhood Leukemia and Lymphoma consortium and combined with azacitidine, intensive chemotherapy, and allogeneic HCT for high-risk patients. Additional MEK/ERK inhibitors (eg, selumetinib) are under investigation or approved by the US Food and Drug Administration for pediatric malignancies with RAS pathway mutations.
Cell surface targets
CD33 has already been shown to be an effective therapeutic target with gemtuzumab ozogamicin, and attempts to optimize the efficacy of CD33 targeting are underway and include bispecific antibodies and chimeric antigen receptor T cells (CAR T), with particular attention to the risk of significant and prolonged myelosuppression that may necessitate HCT following CAR T therapy. CD123, or interleukin 3 receptor alpha chain, is highly expressed in nearly all cases of AML. The CD123-targeting agents, IMGN632, an ADC coupled to the DNA-alkylating payload indolinobenzodiazepine pseudodimer (IGN), and tagraxofusp (SL-401), a CD123-directed cytotoxin consisting of recombinant human IL-3 fused to a truncated diphtheria toxin, are both tolerable with promising efficacy in adults with myeloproliferative neoplasms and blastic plasmacytoid dendritic cell neoplasm.51,52 Both agents are being studied in upcoming trials in pediatric relapsed acute leukemia in combination with chemotherapy, while additional strategies include CD3xCD123 bispecific antibody and CAR T (Figure 1 and Table 2).
Cell surface targets that have shown promise in preclinical evaluations for CAR T cells with ongoing investigation in early-phase clinical trials include CLL-1, NKG2D ligand, CD7, and CD135 (FLT3). Some cell surface antigens are present only on leukemic blasts (eg, PRAME) or are generally restricted to specific genomic subtypes of AML (eg, FOLR1 in CBF2T3-GLIS2 rearrangements, mesothelin in KMT2Ar), and thus immunotherapeutic strategies against these targets are not expected to result in myelosuppression that limits strategies for common myeloid antigens (eg, CD33, CD123). Therapeutic strategies that target key components of the innate immune system (eg, CD47 with the macrophage immune checkpoint inhibitor magrolimab, natural killer cell therapies) are also an active area of research with early-phase clinical trials evaluating these agents in adult AML. While these therapies are almost always initially evaluated in adults, hopefully, any promising agents will move quickly into trials for children (Figure 1 and Table 2).
Conclusions
The identification and development of effective targeted treatments for pediatric AML have remained challenging due to disease heterogeneity, the overall rarity of pediatric mutations that are often distinct from adults, and the shared expression of AML antigens with normal hematopoietic precursors. With encouraging efforts over the last decade, multiple agents have emerged, demonstrating the potential of targeted agents to improve outcome and decrease the toxicities associated with current chemotherapeutic treatment regimens in pediatric AML.
Conflict-of-interest disclosure
Lauren Pommert: no competing financial interests to declare.
Katherine Tarlock: no competing financial interests to declare.
Off-label drug use
Lauren Pommert: sorafenib, midostaurin, gilteritinib, trametinib, enasidenib, ivosidenib, venetoclax, and selinexor are discussed.
Katherine Tarlock: sorafenib, midostaurin, gilteritinib, trametinib, enasidenib, ivosidenib, venetoclax, and selinexor are discussed.