Abstract
Globally, patients living with sickle cell disease are now surviving to reproductive age, with life expectancy approaching 50 years in most countries. Thus, reproductive options are now essential for patients living with the condition. However, it can be associated with maternal, delivery, and fetal complications. Outcomes may vary depending on the level of expertise and resources. In this piece we provide an optional guideline for managing sickle cell disease in pregnancy. The therapeutic option of serial exchange prophylactic transfusion has been offered in the context of a clinical trial (TAPS2).
Learning Objectives
Understand the maternal delivery and fetal complications associated with SCD and pregnancy
Learn about the management options available for SCD and pregnancy
Review the evidence and the research approaches to addressing transfusion management in pregnancy
CLINICAL CASE
A 28-year-old woman of African background, known to have sickle cell disease (SCD), presented to the clinic during her second pregnancy at 8 weeks' gestation. Her husband was a carrier of SCD, and thus this pregnancy was conceived through preimplantation genetic diagnosis (PGD) to avoid the 50% chance of having offspring with SCD. Her first pregnancy was complicated by preeclampsia, necessitating delivery at 36 weeks by cesarean. During that pregnancy she had an acute painful episode close to delivery and, as a result, had an exchange blood transfusion before the cesarean. The baby weighed 2.34 kg. During the second pregnancy, she was started on aspirin at 150 mg/d, folic acid, penicillin at 250 mg twice daily, and thromboprophylaxis in the form of dalteparin from 28 weeks onward. She declined to go on any trials, including the TAPS2 trial (transfusion for pregnant women with SCD). The pregnancy progressed well with normal serial growth scans and minimal manifestation of SCD. Therefore, we recommended delivery at 38 weeks' gestation. Still, a day before the delivery date she had a severe acute painful episode with significant anemia that required a top-up transfusion. After she was stabilized, a decision was made for a planned cesarean due to her previous cesarean. The baby weighed 2.20 kg. The mother and her baby did well post delivery without further concerns.
Literature review
This review was written in accordance with the American Society of Hematology processes. The level of evidence was based on the grade nomenclature—ie, grading of recommendations, assessment, development, and evaluation (http://www.gradeworkinggroup.org). It was also based on a guideline by the British Society of Haematology and updated according to a Royal College of Obstetricians and Gynaecologists Green Top guideline.1,2
A series of databases were searched, including Medline, Embase, the Cochrane Library, and others for randomized controlled trials, systematic reviews, and meta-analyses between 2000 and 2021. In all, over 220 papers were identified. The search terms included “sickle cell,” “antenatal,” “pregnancy,” “intrapartum,” “hydroxycarbamide,” “penicillin prophylaxis,” “risk factors,” “preconception,” and “sickle cell crises.”1
This article describes how SCD is managed during pregnancy in the Western World, particularly in the United Kingdom. It aims to cover preconception screening and antenatal, intrapartum, and postnatal management of women with SCD. It does not cover the management of women who are sickle cell carriers. Instead, it reviews comprehensive information on preconception screening, medications, PGD, thromboprophylaxis, aspirin and vitamin D, and serial ultrasound scans.
Definition
SCD consists of a group of conditions caused by the inheritance of an abnormal sickle hemoglobin (HbS) gene. The most common is homozygous SCD, but there are other compound heterozygous types, one of the unusual being HbS—for example, HbSC (Table 1). SCD is the most common inherited condition worldwide, with well over 300 000 children born with the disease each year, 75% of whom are born in Africa.1 In the UK and France, there are estimated to be 12 000 to 15 000 affected individuals,3 and in the UK, approximately 260 children are born with the condition each year. The pathophysiology of SCD is a result of HbS in low oxygen conditions giving rise to rigid and fragile sickle-shaped red cells.3 This leads to an increase in the breakdown of these cells, resulting in anemia and the sickle-shaped red cells polymerizing and causing the clinical features of acute pain, significant anemia, shortness of breath, and fatigue. The polymerization/vaso-occlusion can result in end-organ damage, such as acute chest syndrome, pulmonary hypertension, stroke, renal dysfunction, retinal disease, and leg ulcers. These manifestations result in reduced life expectancy. In Western countries and some developing countries, patients live up to their mid-50s. Still, as most are living to reproductive age, management of this condition in pregnancy becomes more relevant. Pregnancy in women with the condition is associated with a high risk of mortality, estimated to be up to 2%, and morbidity.4 Pregnancy-related conditions such as hypertension and venous thromboembolism are significantly increased, as confirmed in other observational studies.4,5 During pregnancy, patients are more likely to require blood transfusions and admission to the critical care unit. The babies of mothers with SCD are more likely to be born preterm, before 37 weeks' gestation, and thus there is a high need for neonatal intensive care admission, as well as an increased risk of reduced birth weight and stillbirth. These risks may be more pronounced in HbSS than in HbSC. Although even rarer, HbS beta-zero also behaves like HbSS.
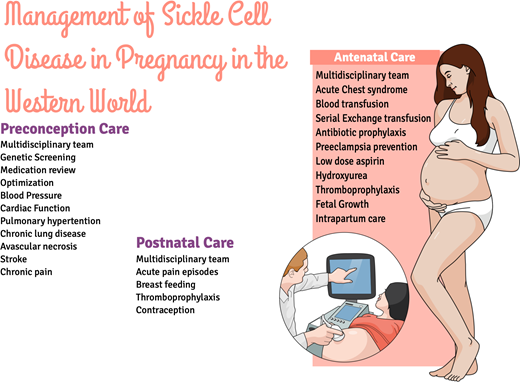
Preconception care
Discussions about pregnancy and conception care for women with SCD should take place between the health care provider and the patient. This should be part of a patient's comprehensive annual review and include fertility planning, which should begin in pediatrics services where appropriate. Reproductive options, partner screening, the optimization of health before conception, the use of preconception folic acid supplementation, and any potentially teratogenic medications should be discussed. Preconception clinics should be available and easily accessible, ensuring that vaccination regimes are up to date.3
Genetic screening
When a woman has SCD and the father of the baby is a carrier of the Hb beta chain variant, the risk of offspring having SCD is 50%; thus, women with SCD should receive counseling about their reproductive choices and the likelihood of having a child with SCD as well as the option of PGD. This is so important in the United Kingdom, where it is paid for, particularly for mothers without an unaffected offspring. Also, the choices regarding prenatal diagnosis and future noninvasive prenatal testing need to be discussed.6,7
To a large extent, most women are unaware of the possibility of PGD and hence do not have the opportunity to consider it.8 For example, a review conducted in a London tertiary referral center showed that 60 at-risk couples over 5 years had 74 cycles of PGD, and the take-home baby rate was 63% per couple, a significant increase in the take-home baby rate compared to 5 years earlier. Therefore, the national guidelines recommend that as part of the annual review, all women with SCD should be encouraged to engage in preconception partner testing.1
Particularly relevant preconception investigations should incorporate renal function, echocardiography, and liver function, as well as medications and therapies. Blood pressure and urinary protein should be monitored in the form of albumin creatinine ratio or protein creatinine ratio. Abnormal renal and liver function should be investigated, excluding nonsickle causes. Hypertensive medications should be considered in women with blood pressure higher than 130/80 mm Hg, and appropriate antihypertensives should be prescribed based on the National Institute for Health and Care Excellence guidelines (eg, nifedipine, labetalol, or methyldopa). Pulmonary hypertension, ventricular ectopics, and early cardiac deaths are increased in SCD; hence, echocardiography is very pertinent in the preconception period.8
A raised tricuspid regurgitation velocity is associated with increased mortality. Hence, women planning a pregnancy should have echocardiography performed at least within a year of trying to conceive, and pregnancy is contraindicated in those with raised pulmonary hypertension or tricuspid regurgitation velocity.1,9
Other complications of SCD that merit screening and optimization before embarking on pregnancy are summarized in Table 2. We recommend that a specialist review women before conception to optimize health and screening for disease complications and that preconception clinics are available and accessible.3
Preconception medication review
After a thorough review of medications prior to conception, folic acid supplementation should be 5 mg/d, as recommended, particularly if preparing for pregnancy, as this significantly improves or prevents neural tube defects. Vitamin D deficiency is common in pregnancy, and hence we suggest at least 400 IU/d and no more than 4000 IU/d.10 Because of the hyposplenism associated with SCD, it is important to highlight vaccinations for meningococcal, pneumococcal, and Haemophilus influenzae, as well as penicillin prophylaxis to reduce the frequency of pneumonia and bacterial infections.1,11
The optimal analgesia during pregnancy should be discussed and the appropriate medications prescribed. Paracetamol and codeine-containing analgesics can be offered during pregnancy. If these are not ablating the pain, then nonsteroidal anti- inflammatory drugs in the form of ibuprofen can be used between 12 and 30 weeks but should be considered with caution before 12 weeks and avoided after 30 weeks.12.13 Opioid intake should be assessed, with referral to the chronic pain team if needed.1,13,14 SCD pregnant patients are at risk of developing opioid related disorders, such as opioid type dependence, non-dependent opioid abuse, and accidental poisoning by opioids, four times more than their non-SCD counterparts.15
Medications that may be teratogenic, such as angiotensin-converting enzyme inhibitors and hydroxycarbamide, should be reviewed and discontinued prior to pregnancy, and this also goes for crizanlizumab. The teratogenic effects of these agents have been demonstrated in animal studies. It is crucial to advise that these medications be stopped since the evidence in human studies is negligible.
Iron chelators are often not recommended in pregnancy due to the lack of a safety profile, and they should be stopped. Iron overload should be appropriately assessed with liver and cardiac magnetic resonance imaging before conception to highlight those at high risk of iron-related complications. If there is evidence of iron overload, it should be treated before conception. Cardiac iron overload is unusual in SCD, but if present, it would be essential to regress the chelate before pregnancy. New medications such as voxelator, crizanlizumab, and glutamine are not approved in pregnancy and should be discontinued before conception. However, these medications may be approved at some point, and thus women may have been prescribed these from overseas.1
The recommendation is that folic acid be given before conception and throughout pregnancy. Women should also be given vitamin D as per national recommendations for all pregnant women.16 Daily antibiotic prophylaxis is recommended for the prevention of pneumococcal pneumonia,1,5,11 and vaccinations should be updated as per national recommendations for SCD. These should include the COVID-19 vaccine as well as the flu vaccination.
Antenatal care/antenatal hemoglobinopathy screening
Women whose partners are carriers or are affected by significant hemoglobinopathies must be aware of this and receive the appropriate counseling. In addition, partner status should be documented. The objective of the screening program is to ensure that screening tests for important prenatal diagnoses are offered between 8 and 10 weeks of pregnancy by the primary care or maternity services.
Prenatal diagnosis in the form of chorionic villus sampling or amniocentesis should be offered as early as possible in pregnancy to ensure early access to termination of an affected pregnancy if the woman chooses.
Prenatal diagnosis can be performed from 11 weeks, with the risk of miscarriage quoted to be approximately 1%. Current studies are ongoing with regard to utilizing free fetal DNA and next generation sequencing.6
Maternal health
All of the actions outlined in the preconception section should be followed for women who become pregnant without preconception care, including vaccination advice and the assessment of end-organ damage, red cell antibodies, iron status, and transfusion status.
The challenge in the antenatal period of SCD is the prevention of general and SCD-specific complications.6,14,17-20 Preventing SCD-specific complications requires multidisciplinary care, with the involvement of obstetricians, midwives, and hematologists interested in SCD. Good communication is fundamental to patient safety, and hospital protocols for transfusion indications and the detection and management of SCD complications, including infection and acute pain, and the management of labor should be available.
At the antenatal appointments, information and education regarding acute pain episode prevention measures, such as the avoidance of infection, the need to rest, and the need to stay well hydrated, are crucial. In addition, women's housing and work circumstances should be reviewed and appropriate interventions made to reduce the potential of triggering an acute pain episode.
Medication compliance, as well as ensuring patients have the appropriate medications, is pertinent. Early pregnancy assessment for hyperemesis and instruction about the importance of proper hydration may prevent sickle cell acute pain episodes and avoid hospital admission. Low-dose aspirin should be taken at 150 mg/d, from 12 weeks onward until 36 weeks, to prevent preeclampsia and hypertension in pregnancy.1,21 The available evidence suggests that low-dose aspirin reduces the incidence of preeclampsia by 50% to 80%.9 Identifying high blood pressure and proteinuria allows for timely intervention to save lives. The need for thromboprophylaxis to start at 28 weeks and continue for 6 weeks postnatally for those whose risk factor is only SCD and at booking for those with additional risk factors is very important.1
Serial growth scans every 4 weeks are recommended, and those with extremely low levels of pregnancy-associated plasma protein (under 0.5) will require 2 weekly scans from 20 weeks onward, as the risks in those with low pregnancy- associated plasma protein are even more heightened, especially for those with SCD.1-3,21
Blood transfusion during pregnancy
Transfusions are needed to correct severe anemia, reduce sickle cell complications, reduce the extent of sickling, and maintain oxygen supply to the fetus.22,23 However, this must be weighed against the side effects of transfusion, including the risk of alloimmunization and delayed hemolytic reaction.
The type of blood selected for the transfusion of women with SCD should be cytomegalovirus negative, HbS negative, and a standard Rh cell matched line as per previous British Society of Haematology recommendations. In addition, a full transfusion history should be taken prior to transfusion and communicated with the transfusion laboratory and the national transfusion database to ensure no historical autoantibodies are present.22
Patients with antibodies need to be referred for fetal red cell antibody titers to prevent hemolytic disease in the newborn. There is a fundamental question about whether to offer exchange blood transfusions prophylactically throughout pregnancy, but there is currently insufficient evidence to recommend serial prophylactic exchange blood transfusions.22
A phase 2 clinical trial is currently ongoing (ClinicalTrials.gov Identifier: NCT03975894), and so far, 34 patients have been recruited out of the 40 women needed for the feasibility study. The transfusions commence between 6 and 18 weeks 0 days' gestation. The transfusions commence between 6 and 18 weeks of gestation and are repeated every 6 to 10 weeks until the end of pregnancy, aiming to maintain HbS% or combined HbS/HbC% below 30%. They are continued throughout pregnancy and are to be stopped at the end of pregnancy.24 A previous randomized controlled trial over 20 years ago showed a significant reduction in vaso-occlusive crises in the prophylactic transfusion arm. There was no clear difference in other outcomes, but the small numbers may explain this. The Cochrane Review concluded that there were no clear clinical benefits of prophylactic exchange blood transfusions over standard care and that appropriate randomized controlled trials are needed.22,24
A recent systematic review by Malinowski et al demonstrated that prophylactic transfusion was associated with a reduction in maternal mortality, vaso-occlusive crises, pulmonary complications, pyelonephritis, perinatal mortality, neonatal death, and preterm birth.25
There was no difference in rates of pulmonary infection, acute chest syndrome, or preeclampsia, and they concluded that prophylactic transfusion might have a positive impact on severe maternal and neonatal outcomes. Still, the evidence comes from a very small sample size, and a further randomized controlled trial is needed. Clearly, the risks and benefits of prophylactic transfusion should be discussed with patients, hematologists, and obstetricians in early pregnancy. Factors to consider would include genotype, phenotype, previous obstetric history before pregnancy, and alloimmunization.22
We recommend that women who are already on long-term serial exchange transfusion continue it during pregnancy. Those on hydroxycarbamide (hydroxyurea) should be advised to stop and be considered for prophylactic transfusion if they experience worsening sickle symptoms afterward.
A recent study demonstrated that the use of hydroxyurea up to the time of conception does not seem to have an adverse effect on the fetus and may be considered safe. Women who continue taking hydroxyurea during pregnancy may be at an increased risk of fetal demise or their fetus being small for gestational age. There was no increase in birth defects when hydroxyurea was used during pregnancy.26
A recent report from the Boston Birth Cohort showed that children exposed to maternal SCD are at risk of attention deficit hyperactivity disorder, even though the authors stated that more studies are needed to confirm these results. In addition, maternal anemia and opioid use constitute risks, and regular red cell transfusions can be used to prevent fetal exposure to maternal anemia as well as reduce pain and limit opioid use.27
The standard of care is to give a transfusion only if the patient is symptomatic. There is no evidence of optimal Hb levels or HbS percentages before delivery by cesarean.
Pain management
All pregnant women with SCD should have a prospective pain management plan that has been developed and discussed with the multidisciplinary team.6 There should be a low threshold to refer the woman to a tertiary unit or secondary care, particularly if simple analgesics in the form of paracetamol and dihydrocodeine are not working, and women who are febrile should be referred to secondary or tertiary care in a timely fashion. When admitted to the hospital with pain after trying dihydrocodeine or paracetamol, they may require admission, and either the oral, subcutaneous, or intravenous forms of morphine or diamorphine can be considered, depending on the woman's preference and the local expertise. Pethidine should be avoided because of the risk of associated seizures or toxicity.1
Women with an acute pain episode at presentation should be assessed rapidly, and any precipitating factors should be treated quickly. A complete set of observations using the modified obstetric early warning chart, including systolic and diastolic blood pressure, respiratory rate, temperature, oxygen saturation, and pain score, should be documented and repeated every 1 to 2 hours.
Women should be cared for in units with experience in SCD and high-risk pregnancy. Senior obstetricians, hematologists, obstetric anesthetists, obstetric physicians, specialist nurses, and midwives should meet as a multidisciplinary team. Care should be considered and planned by the group and discussed with the woman and her family.
Fluid balance and fluid status should be carefully documented, and dehydration should be simultaneously avoided, ensuring fluid overload. The oxygen saturation should be above 94%. When it is below 94%, blood gasses should be performed, and acute chest syndrome, pulmonary hypertension, and pulmonary embolus should be excluded.1
Suppose the oxygen saturation is not recovering with facial oxygen? In that case there should be early recourse to transfer to intensive care if satisfactory oxygen saturation cannot be maintained using a face mask or nasal cannula. The woman should be assessed for infection, including urine culture and microscopy. A chest x-ray should be indicated if the woman has abnormalities on the chest examination or significant hypoxia.
Antibiotics should be prescribed appropriately.1 In addition, thromboprophylaxis should be prescribed for women with SCD, as alluded to above.
National Institute for Health and Care Excellence guidelines on the management of acute painful episodes should be followed. Nonsteroidal anti-inflammatory drugs may be used cautiously in the first trimester and avoided after 30 weeks' gestation. Between 12 and 30 weeks, it is appropriate to use a nonsteroidal.13
Management of acute chest syndrome.
Acute chest syndrome is a significant complication of SCD characterized by fever and/or respiratory symptoms and new pulmonary infiltrate shown on chest x-ray. Patients should be observed closely. Essential investigations for diagnosis and prognosis should ensure a timely chest x-ray and full blood count, as well as arterial blood gas analysis. Low oxygen saturation or severe hypoxia mandate a timely transfer to the intensive care unit, as this may save lives. A differential diagnosis in the form of pulmonary embolus and severe pneumonia and in this current environment, COVID-19 pneumonitis, should be excluded by chest computed tomography.20,28-30 Prompt pain relief and treatment of bacterial and viral infections save lives. Assessment for either exchange blood transfusion or top-up transfusion may improve the acute situation. Involving the critical care team early when a diagnosis of acute chest syndrome is made avoids deterioration of the patient, as the timely use of noninvasive or invasive ventilation with transfusion may be protective for the patient.
The management of both thrombotic and hemorrhagic stroke could be necessary in SCD in pregnancy, and timely diagnosis saves lives.1,14,17-19 Patients who present with acute neurological impairment or the suspicion of acute stroke require urgent brain imaging, with a referral to a stroke unit, a neurologist, and a hematologist. Rapid exchange blood transfusion can decrease long-term neurological damage, and the role of thrombolysis should be discussed with a specialist neurologist and the obstetrician.17
Acute anemia with SCD may be attributable to parvovirus B19, and a rapid top-up transfusion significantly saves lives. However, all causes of anemia should be appropriately investigated because they sometimes may not be SCD related but are due to bleeding, be it antenatally, intrapartum, or postnatally. Sometimes, either obstetric related or indeed in countries where malaria is endemic, they could also be due to malaria.1,18
Intrapartum care
With increased perinatal mortality, particularly during the latter stages of pregnancy in patients with SCD and given the risk of abruption and unexplained stillbirth due to placental abnormalities, we recommend the need to offer induction of labor between 38 and 40 weeks. Thus, appropriate delivery planning, including the time, mode, and place of delivery with multidisciplinary input, could make a difference in improving outcomes. The mode of delivery depends on obstetric complications; otherwise, supporting a vaginal delivery or cesarean incorporating the patient's choice is very pertinent.1,14,18
Optimal intrapartum care in the form of good analgesia, the avoidance of dehydration, regular monitoring of oxygen saturation, and the avoidance of protracted labor is recommended. An antenatal review by the anesthetist to provide the appropriate planning to avoid a general anesthetic is pertinent to the care of patients with SCD. In addition, continuous electronic fetal monitoring is needed, given the increased risk of unexplained stillbirth or adverse perinatal outcomes. Epidural analgesia is safe and effective and should be available for women with SCD.
Postpartum care
It is important for clinicians to remain vigilant because the risk of a sickle cell acute pain episode increases during this time, with almost 25% of women having an acute pain episode post delivery.1,14 Ensuring hydration and oxygenation with early mobilization helps minimize acute pain episodes and complications. The use of nonsteroidal anti-inflammatory drugs, as well as paracetamol and codeine sulfate, can be used. Infants should be monitored for sedation, breathing difficulties, and weight gain. Breastfeeding should be encouraged. Antithrombotic stockings should be provided and low-molecular-weight heparin should be administered in women with SCD post delivery.1,14
Contraceptive advice should be given after delivery, but while breastfeeding, estrogen-based contraceptives are contraindicated. Otherwise, all forms of contraceptives are advisable. If oral, a progesterone-only pill such as Cerazette or injectable forms such as Depo-Provera or implants and intrauterine devices can be considered. Using copper devices may increase the risk of infection; thus, using prophylactic antibiotics around the time of insertion is very important.1
In conclusion, most women with SCD in developed countries have pregnancies with good maternal and infant outcomes. Patients who have reached reproductive age require preconception and pregnancy advice and a discussion about reproductive options. It is essential to be aware that pregnancy and delivery provide an added risk for the woman and the fetus; thus, the need for protocolized management plan from each unit incorporating the multidisciplinary team's advice is vital.
The multidisciplinary team should include an obstetrician with expertise in the management of high-risk pregnancies, a team of midwives, and a hematologist. If in an area of low SCD prevalence, a link to a specialist center or hemoglobinopathy coordinating center is pertinent.1,14,18,19 It is essential to highlight the need for well-designed clinical trials in this area to ascertain optimal pregnancy treatment options (Table 3).
Conflict-of-interest disclosure
Eugene Oteng-Ntim: no competing financial interests to declare.
Panicos Shangaris: no competing financial interests to declare.
Off-label drug use
Eugene Oteng-Ntim: nothing to disclose.
Panicos Shangaris: nothing to disclose.