Abstract
Patients with multiple myeloma (MM) have up to a 20-fold increased risk of venous thromboembolism (VTE) compared with the general population, with most events occurring within the first 6 months of diagnosis. Treatment with immunomodulatory drugs (IMiDs) is a strong risk factor for VTE in MM. In a meta-analysis of 2 large, randomized trials comparing anticoagulant thromboprophylaxis vs placebo in ambulatory patients with cancer at high risk of VTE based on a validated risk score, the risk of VTE decreased without increasing the risk of major bleeding. However, few patients with MM participated in these trials (1.1%). Initial guidance for risk-stratifying patients with MM resulted in persistent rates of VTE >10% and highlighted the need for improved VTE risk stratification in patients with MM. Three validated risk scores are now available to quantify risk of VTE in patients with MM: SAVED, IMPEDE VTE, and PRISM scores. Using best available data, thromboprophylaxis should be strongly considered in patients with MM assessed as high risk for VTE, especially newly diagnosed patients receiving IMiD-based combination therapies. However, prospective studies are needed to further validate available models and identify the optimal thromboprophylactic agent for each VTE risk category.
Learning Objectives
Quantify the risk of venous thromboembolism in patients with multiple myeloma starting immunomodulatory-based therapy
Understand thromboprophylaxis strategies for venous thromboembolism in patients with multiple myeloma treated with immunomodulatory drugs
CLINICAL CASE
A 60-year-old Caucasian man with medical history of obesity (body mass index 32 kg/m2) and diabetes mellitus was recently diagnosed with multiple myeloma. Imaging studies did not show any bone lesions. Cytogenetic analysis shows standard-risk disease. Induction regimen with bortezomib, lenalidomide, and low-dose dexamethasone is planned. Should this patient receive primary thromboprophylaxis to reduce the risk of venous thromboembolism (VTE)?
Introduction
Multiple myeloma (MM) is a clonal plasma cell neoplasm and second most common hematologic malignancy. Development of novel therapeutic agents, such as immunomodulatory drugs (IMiDs), has shifted the treatment paradigm and led to significant improvements in overall survival. Patients with MM have up to a 20-fold increased risk of VTE compared with the general population, with most events occurring within the first 6 months of diagnosis.1 While there was no association between VTE and survival in 1 randomized MM treatment trial,2 a large cohort study of patients with newly diagnosed MM found a 2-fold increased risk of death in patients with MM and VTE at 6 months.3
A pooled analysis of 2 large, randomized trials of ambulatory patients with cancer at high risk of VTE (Khorana score ≥2) starting chemotherapy found a reduction in risk of VTE in patients who received primary thromboprophylaxis with a direct oral anticoagulant (DOAC) vs placebo.4 Of the cohort of 1415 patients included in these trials, only 15 (1.1%) had a diagnosis of MM. Thus, while a similar approach in MM has the potential to improve outcomes, extrapolation to patients with MM should be cautioned.5,6 In addition, in 1 study, the Khorana score had low discriminatory performance for identifying patients with MM at high risk of VTE (6-month concordance statistic, 0.53; 95% confidence interval, 0.50-0.57).7 This may be related to the multifactorial etiology of VTE in MM, which can be categorized into patient-, MM-, and treatment-related risk factors. Risk factors, including history of VTE, obesity, immobility, hyperviscosity, and elevated cytokine levels, are examples of patient- and MM-related risk factors. The use of cytotoxic chemotherapy, high-dose dexamethasone (>160 mg per month), and IMiDs also increases the risk of VTE in MM and may have a role in identifying high-risk patients for receipt of primary thromboprophylaxis.
IMiDs
Thalidomide, lenalidomide, and pomalidomide are the 3 IMiDs approved by the US Food and Drug Administration for treatment of patients with MM. Attention to the association between IMiDs and VTE was first noted in randomized trials that combined thalidomide with cytotoxic chemotherapy (eg, anthracyclines) and/or dexamethasone, especially high dose. Similar findings were seen with lenalidomide use, especially when coupled with high-dose dexamethasone. In a pooled analysis of patients with newly diagnosed MM treated on clinical trials, the risk of VTE with thalidomide was 14% when combined with dexamethasone and increased to 24% when combined with doxorubicin in the absence of thromboprophylaxis.8 In the same study, the risk of VTE was also 14% in patients with newly diagnosed MM treated with combination lenalidomide plus dexamethasone in the absence of thromboprophylaxis. Rates with pomalidomide appear to be as high as those with thalidomide and lenalidomide; however, fewer data are available regarding risk. In an initial phase 1 study of single-agent pomalidomide for the treatment of relapsed/refractory MM, the rate of VTE was 17%, with none of the enrolled patients receiving primary thromboprophylaxis.9 The risk of VTE was highest within the first 6 to 12 months of treatment initiation in the clinical trials, with median follow-up of at least a year in most of them.
Guidelines and risk assessment models
International Myeloma Working Group guidelines
Given the high rates of VTE, in 2008, the International Myeloma Working Group published thromboprophylaxis guidelines for patients with MM based on expert opinion. Patients were defined as high risk in the presence of 2 or more risk factors; otherwise, they were considered low risk. The risk factors included universal individual VTE risk factors (eg, history of VTE, thrombophilia) as well as myeloma-specific risk factors (ie, treatment with IMiDs). Prophylactic-dose low-molecular-weight heparin or dose- adjusted warfarin (international normalized ratio 2-3) was recommended for patients at high risk while patients categorized as low risk were recommended aspirin. Subsequently, the Myeloma XI trial enrolled 4358 newly diagnosed transplant-eligible and transplant-ineligible patients with MM randomized to treatment with thalidomide- vs lenalidomide-containing regimens. The protocol required primary thromboprophylaxis as recommended by the International Myeloma Working Group criteria, for which there was 80.5% prescription compliance (thromboprophylaxis consisted of aspirin 33.7%, low-molecular-weight heparin 60.3%, warfarin 4%, other 2%).2 Despite the high rates of thromboprophylaxis, the rate of VTE was >10% in all study arms at 6 months, highlighting the need for improved risk stratification and thromboprophylaxis strategies in MM.
SAVED score
Published in 2019, the SAVED score was developed using Surveillance, Epidemiology, and End Results–Medicare data on 2397 newly diagnosed patients with MM starting IMiD therapy (Table 1).10 Of the variables assessed, 5 remained in the final model and included surgery within 90 days prior to MM diagnosis, Asian race, history of VTE, age eighty or older, and dexamethasone dose. Patients with a high-risk score (≥2 points) had a 12% incidence of VTE within the first 6 months of MM therapy, representing a 1.85-fold increased risk (P < .01) compared with low-risk patients (7% incidence of VTE). Using data from US Veterans, the score was externally validated with a c-statistic (discrimination) of 0.60.
IMPEDE VTE score
Also published in 2019, the IMPEDE VTE score was developed from a cohort of 4446 patients with newly diagnosed MM starting treatment within the Veterans Health Administration (Table 1).11 This model stratifies patients into high-risk (≥8 points), intermediate- risk (4-7 points), and low-risk (≤3 points) groups. The model contains 11 variables, including preexisting antithrombotic or antiplatelet use. The retained variables include IMiD use; body mass index 25 kg/m2 or greater; pelvic, hip, or femur fracture within 30 days prior to MM diagnosis; use of erythropoiesis- stimulating agents, doxorubicin or dexamethasone; Asian/Pacific Islander ethnicity/race; history of VTE; presence of a tunneled line/central venous catheter; and existing thromboprophylaxis at the start of chemotherapy. The cumulative incidence of VTE at 6 months was 3.3%, 8.3%, and 15.2% in the low-, intermediate-, and high-risk groups, respectively. The model has been externally validated in several cohorts with c-statistics ranging from 0.64 to 0.68.
PRISM score
The PRISM score, published in 2022, was developed from a single- institution retrospective cohort of 783 patients with newly diagnosed MM starting treatment.12 The final model includes 5 variables: history of VTE, black race, IMiD use, surgery within 90 days, and abnormal metaphase cytogenetics (Table 1). The cumulative incidence of VTE at 12 months was 2.7%, 10.8%, and 36.5% in the low-risk (0 points), intermediate-risk (1-6 points), and high-risk (≥7 points) groups, respectively. The model was externally validated in 257 patients with a c-statistic of 0.59.
Comparison of models
The available models have similarities and differences that should be considered when selecting a model for use. There is overlap in risk variables included, with history of VTE the strongest predictor in all 3 models, placing patients at an intermediate to high risk of developing VTE. Additional variables present in at least 2 of the models include race, surgery within 90 days before treatment start, dexamethasone, and use of IMiDs. It should be noted that the SAVED score is intended for use only in patients starting induction therapy with an IMiD, while the IMPEDE VTE and PRISM scores can be used in patients receiving induction with any myeloma-directed therapy.
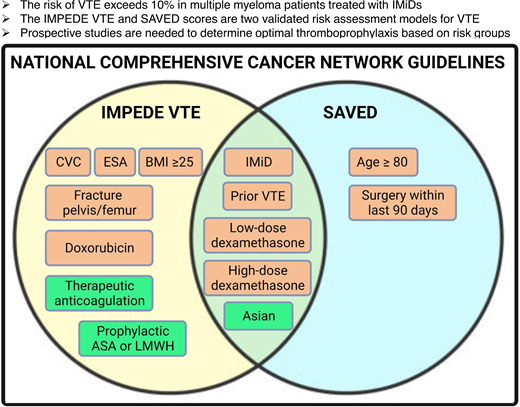
National Comprehensive Cancer Network Recommendations
Both the SAVED and IMPEDE VTE scores are recommended for quantification of VTE risk in patients with newly diagnosed MM starting chemotherapy by the National Comprehensive Cancer Network Multiple Myeloma Treatment Guidelines (version 5.2022). Recommended thromboprophylaxis strategies include the following: low-risk scores receive thromboprophylaxis with aspirin, while those with intermediate- or high-risk scores receive antithrombotic thromboprophylaxis (eg, low-dose DOAC, low-molecular-weight heparin).
Thromboprophylaxis strategies in MM
Initial randomized trials assessing the safety and efficacy of thromboprophylaxis focused on patients with MM starting an IMiD-based treatment and compared strategies including aspirin, low-molecular-weight heparin, and warfarin.13,14 However, these trials did not risk stratify patients enrolled and excluded patients from enrollment with some risk factors for VTE. More recently, the role of DOACs has been assessed in the prevention of cancer-associated thrombosis.5,6 In the AVERT trial,6 patients with a modified Khorana score (diagnosis of MM was given 1 point) of 2 or more were randomized to antithrombotic prophylaxis with apixaban (2.5 mg twice daily) vs placebo. Myeloma-specific outcomes are not available. The role of DOACs in risk-stratified patients with MM warrants study. Limited retrospective and single-arm prospective data are available using DOACs in MM, which are summarized in Table 2. In the interim analysis of the RithMM trial, a randomized controlled trial, 17 patients with MM received rivaroxaban for 6 months, and none of them had a thromboembolic event or major bleeding (Table 2).
Summary
In summary, patients with MM have an increased risk of VTE, with treatment with IMiDs representing a strong risk factor. Risk- assigned thromboprophylaxis with DOACs decreased the risk of VTE in two large, randomized trials of ambulatory patients with cancer,4 but only 1.1% of participants had a diagnosis of MM.5,6 Initial guidance for VTE risk assessment and thromboprophylaxis in MM resulted in rates of VTE >10% in newly diagnosed patients within the first 6 months of chemotherapy.2 Now, 3 MM-specific scores for VTE risk assessment are available.10,11 However, the optimal thromboprophylactic agent to be used in each score- assigned risk category is unknown. Prospective studies are needed to further validate these scores and identify the optimal thromboprophylactic agent for each VTE risk category.
Graded recommendations
Thromboprophylaxis should be strongly considered in patients with MM assessed as high risk for VTE, especially newly diagnosed patients receiving IMiD-based combination therapies. (Strong recommendation, moderate certainty in evidence about effects)
The SAVED, IMPEDE VTE, and PRISM scores are validated clinical tools that can quantify risk of VTE in newly diagnosed patients with MM. (Conditional recommendation, moderate certainty of evidence about effects)
If available, patients should be enrolled in clinical trials evaluating risk-assigned thromboprophylaxis strategies in patients with MM.
Conflict-of-interest disclosure
Fahrettin Covut externally validated the IMPEDE VTE score.
Kristen M. Sanfilippo has received research funds from AstraZeneca (paid to the institution), Astellas Pharma Global (paid to the institution), American Cancer Society Institutional Research Grant-18-158-61-04, and National Institute of Health 1K01HL136893-01; honoraria for consultancy from the Health Services Advisory Group; and honoraria for expert case review from Covington & Burling, all outside the submitted work. Kristen M. Sanfilippo was involved in the development and validation of the SAVED and IMPEDE VTE scores.
Off-label drug use
Fahrettin Covut: nothing to disclose.
Kristen M. Sanfilippo: nothing to disclose.