Abstract
Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) carried a very poor prognosis prior to the advent of tyrosine kinase inhibitors (TKIs) that block the activity of the BCR-ABL1 oncoprotein. With improvements in TKI efficacy and allogeneic hematopoietic cell transplantation (HCT), survival has improved over the past 3 decades, and the role of chemotherapy and allogeneic HCT is now changing. Better risk stratification, the application of the third-generation TKI ponatinib, and the use of immunotherapy with the CD19-CD3 bifunctional T-cell engaging antibody blinatumomab in place of chemotherapy has made therapy for Ph+ ALL more tolerable and arguably more efficacious, especially for older patients who comprise most patients with Ph+ ALL.
Learning Objectives
Understand the biology and prognosis of Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL)
Appreciate the history and efficacy of the generations of BCR-ABL1 TKIs in Ph+ ALL
Understand the changing role of postremission remission therapies in Ph+ ALL
CLINICAL CASE
A 73-year-old man presenting with severe dyspnea on exertion is found to be anemic with circulating leukemic blasts. A bone marrow biopsy and aspirate are performed, which show a hypercellular marrow with 90% blasts expressing CD19, CD22, and CD79a without coexpression of myeloid markers. Cytogenetics shows t(9;22)(q34;q11) by karyotype, and fluorescence in situ hybridization reveals a BCR-ABL1 translocation. He is diagnosed with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). He is treated with dasatinib and prednisone. A bone marrow specimen at day 21 of therapy shows a complete hematologic and cytogenetic remission without a major molecular remission. He asks about future therapy now that he is in a morphologic remission.
Biology and prognosis
Ph+ ALL is defined by the presence of a translocation between chromosomes 9 and 22, with t(9;22)(q34;q11) being the most common chromosomal abnormality.1,2 The translocation leads to loss of the N-terminal negative regulatory domain of the ABL1 tyrosine kinase and production of the constitutively active BCR-ABL1 fusion oncoprotein. Three variants of the BCR-ABL1 gene have been described: p190, p210, and p230. In Ph+ ALL, the p190 gene variant is most common with the p210 gene variant, which can also express p190 through alternative splicing, present in 20% to 30% of cases.3,4 BCR-ABL1 activity leads to increased cell proliferation and resistance to apoptosis. In addition, differences in signaling downstream of p190 and p210 isoforms may account for the worse prognosis seen with the p210 isoform in some studies of therapy for adult Ph+ ALL.5-9 Ph+ ALL is frequently accompanied by additional recurrent karyotypic abnormalities, although the clinical significance of these has not been consistently demonstrated.10-13
Gene copy number alterations have been shown to have prognostic significance in Ph+ ALL. Loss of function of the lymphopoietic transcription factor Ikaros through gene deletion or mutation, dominant-negative interactions, or haploinsufficiency occurs in most adult and pediatric patients with Ph+ ALL and is associated with a worse prognosis than Ikaros wild-type Ph+ ALL.14-16 In a study of adults with Ph+ ALL, the presence of IKZF1 (Ikaros gene) deletions was associated with a significantly higher cumulative incidence of relapse (69% vs 40% at 24 months, P = .01) and reduced disease-free survival (31% vs 54% at 24 months, P = .02).10 Additional studies support this finding, with Ph+ ALL with IKZF1plus alterations (IKZF1 deletions with deletions in CDKN2A, CDKN2B, PAX5, or PAR1 in the absence of ERG deletion16 ) appearing to be particularly resistant to therapy.17-20 In addition, deletions in CDKN2A/2B have been associated with a poor prognosis after allogeneic hematopoietic cell transplantation (HCT) in first complete response (CR) due to a high relapse rate.21 Conversely, long deletions of MEF2C and K-Ras have been associated with improved CR rate and, in the case of MEF2C, improved disease-free survival.22
Relapse of Ph+ ALL treated with tyrosine kinase inhibitors (TKIs) is typically associated with mutations in the BCR-ABL1 kinase domain. Notably, kinase domain mutations conferring resistance to BCR-ABL1–targeted TKIs can be detected at diagnosis and may be associated with subsequent relapse, at least with imatinib-based therapy.23 In other studies, the significance of these low-level clones at diagnosis for relapse risk and TKI choice is unclear as the detected mutations may not be in clones that give rise to relapse.24,25 Kinase domain mutations that arise during TKI therapy, however, tend to be clones that lead to relapse in both the chemotherapy and HCT settings.25,26
During the course of therapy, failure to achieve a deep molecular response early in therapy has been associated with poor prognosis and high risk of relapse. In the MD Anderson experience, failure to achieve at least a major molecular response and measurable residual disease (MRD) negativity by flow cytometry at 3 to 4 months from start of therapy has been associated with a worse prognosis.27 Similarly, with the addition of nilotinib to pediatric-style chemotherapy, achieving at least a deep major molecular response was associated with a good prognosis.28 In both experiences, deep molecular remissions with TKI and chemotherapy seemed to abrogate any survival benefit with subsequent allogeneic HCT.28,29 However, as with Ph-negative ALL, patients with Ph+ ALL and persistent minimal residual disease, especially when combined with Ikaros/Ikarosplus abnormalities, have a poor prognosis and likely benefit from allogeneic HCT. In addition, recurrence or progression of molecular disease after a molecular response typically heralds impending relapse.
The incidence of Ph+ ALL increases with age. Ph+ ALL accounts for 1% to 2% of pediatric ALL but ~30% of adult ALL. In addition, Ph+ ALL accounts for ~45% of ALL above the age of 35 years.30 The older age of most patients with Ph+ ALL has generally equated to very poor survival given that this group generally does not tolerate intensive chemotherapy and is often ineligible for myeloablative allogeneic HCT.
Prior to the advent of targeted therapy for Ph+ ALL, the chromosomal abnormality was found to confer a poor prognosis with multiagent cytotoxic chemotherapy.31-34 Durable remissions were observed in 0% to 20% of patients with chemotherapy alone, with allogeneic HCT showing improved cure rates and survival outcomes in eligible patients.34 The advent of TKIs capable of inhibiting BCR-ABL1 function (eg, imatinib, dasatinib, ponatinib) has markedly improved outcomes for patients with Ph+ ALL, and novel therapies reducing or eliminating chemotherapy appear to be further improving outcomes for what was once a poor-risk category of ALL.30 In addition, these therapies have extended highly effective, highly tolerable therapies to older patients and those with comorbidities. Below, we outline current approaches to the treatment of adults with Ph+ ALL. All approaches integrate intrathecal chemotherapy into the cerebral spinal fluid to help prevent relapse in the central nervous system.
Intensive therapy
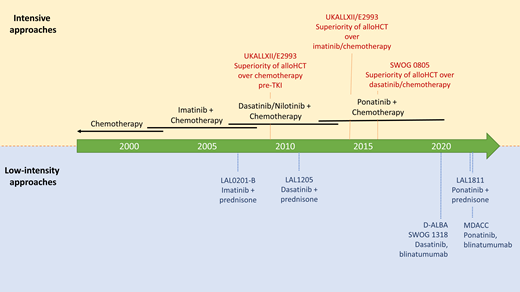
Prior to the addition of BCR-ABL1–targeted TKIs to frontline therapy for Ph+ ALL, complete remission rates were 70% to 90%, although durable remissions were rare.31-34 The addition of the first-generation TKI imatinib to frontline chemotherapy appeared to improve response rates and survival relative to historical data and bridge more patients to successful allogeneic HCT.35-39 In the UKALLII/E2993 trial, the addition of imatinib to chemotherapy improved CR rates (92% vs 82%, P = .004) and overall survival (OS; 4-year OS 38% vs 22%, P = .0003). The survival benefit was limited to those undergoing allogeneic HCT after a complete response to imatinib-containing therapy.35
Subsequently, the second-generation TKIs dasatinib and nilotinib were added to frontline chemotherapy with apparent improvement in survival outcomes compared with historical outcomes in adults with imatinib on similar intensive chemotherapy backbone regimens.28,40,41 In children, a randomized phase 3 study by the Chinese Children's Cancer Group (study CCCG ALL-2015) demonstrated superior event-free survival (EFS) with dasatinib compared with imatinib in combination with pediatric chemotherapy (4-year EFS 71% vs 49%, P = .005).42 In adults, a US Intergroup phase 2, single-arm study treated patients 18 to 60 years of age with Ph+ ALL with dasatinib and hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine (HyperCVAD), followed by allogeneic HCT, for those with a fully human leukocyte antigen–matched related or unrelated donor. Patients allocated to allogeneic HCT had a 12-month relapse-free survival (RFS) and OS after transplant of 71% and 87%, respectively. A landmark analysis showed significantly better outcomes with allogeneic HCT vs no allogeneic HCT (RFS, P = .038; OS, P = .037), giving continued supportive evidence for myeloablative allogeneic HCT in Ph+ ALL in first CR in eligible patients.41 The study did not report outcomes by molecular response and benefit, if any, of allogeneic HCT in patients with deep or complete molecular responses.
Resistance to second-generation TKIs through ABL1 kinase domain mutation, especially the T315I mutation6,7,18,43 targetable by ponatinib, led to study of the third-generation TKI ponatinib in the frontline in adults with Ph+ ALL. A phase 2, single-center study in 86 adult patients with newly diagnosed Ph+ ALL treated with ponatinib and HyperCVAD yielded a CR rate of 100%, no induction mortality, and a 3-year OS of 78%.44 In a subsequent propensity-matched comparison from the same group, ponatinib and HyperCVAD showed significantly superior responses, 3-year EFS, and 3-year OS compared with matched patients receiving dasatinib and HyperCVAD.45 See Table 1 for a summary of selected studies of intensive chemotherapy with BCR-ABL1-targeted TKIs.
Low-intensity induction approaches
The exquisite sensitivity of Ph+ ALL to BCR-ABL1–targeted TKIs, coupled with a high induction death rate in older patients receiving intensive chemotherapy, led investigators in multiple countries to explore low-intensity, minimally myelosuppressive induction with a TKI and corticosteroid with or without vincristine. The first-generation TKI imatinib, second-generation TKIs dasatinib and nilotinib, and the third-generation TKI ponatinib have all been tested in this setting.
In a rare, randomized study in Ph+ ALL, intensive induction with imatinib and HyperCVAD was compared with low-intensity induction with imatinib, vincristine, and dexamethasone. The CR rate was higher with the low-intensity approach (98% vs 91%, P = .006), with lower 60-day mortality (2.2% vs 9.0%, P = .017) and equivalent 5-year EFS supporting the low-intensity induction approach.39 Other studies have used a TKI in combination with only a corticosteroid. The approach has yielded complete remission rates of 96% to 100% regardless of the TKI used (imatinib, dasatinib, or ponatinib) with near-zero induction mortality.6,7,46,47
A TKI + corticosteroid ± vincristine appears to be an optimal induction approach, especially for older or less fit patients, given high complete response rates, early marrow recovery, and near-zero early mortality. Without effective postremission therapy, however, relapse is common, especially with the use of first- and second-generation TKIs in which kinase domain mutations conferring resistance to therapy appear to be more common. See Table 2 for a summary of studies.
Postremission therapy
Remission rates are now 95% to 100% with the use of BCR-ABL1 TKIs in induction, but effective postremission therapy is necessary to effect cure. For instance, in the LAL1205 study of dasatinib and prednisone remission induction, relapse occurred in 14 of 19 patients treated only with TKI, 2 of 2 with no additional treatment, 5 of 14 treated with a TKI plus chemotherapy and/or autologous HCT, and only 2 of 18 undergoing allogeneic HCT.7
Most Ph+ ALL is intrinsically resistant to traditional cytotoxic chemotherapy, limiting this approach to effect cure after achieving a CR to induction therapy. In studies using first- and second-generation TKIs with chemotherapy, intensive chemotherapy alone did not appear to achieve the best long-term disease-free and overall survival. Allogeneic and, to a variable extent, autologous HCT showed superior results to intensive chemotherapy alone in some studies.6,34,35,37,48 The UKALLXII/E2993 study used multiagent cytotoxic chemotherapy to consolidate response to imatinib and chemotherapy induction. Long-term survival and relapse were not different between a cohort not receiving imatinib and cohorts receiving imatinib, with only allogeneic HCT conferring a survival benefit in both groups.34,35 The SWOG-led US Intergroup study noted above showed that myeloablative allogeneic HCT was a superior postremission treatment strategy compared with dasatinib and chemotherapy alone,41 although reduced intensity allogeneic HCT may not offer a benefit over chemotherapy alone as postremission therapy.6 Further improvement in EFS appears to have been achieved by the addition of ponatinib to HyperCVAD (3-year EFS 70%).44,45 It remains to be examined if ponatinib and HyperCVAD is equivalent or superior to allogeneic HCT, especially for the uncommon patients not achieving a complete molecular remission (CMR) to ponatinib and HyperCVAD.
For older patients especially, there appears to be limited benefit to intensive postremission therapy due to high rates of treatment-related death, therapy reduction or discontinuation, and relapse.49 The benefit of allogeneic HCT with either myeloablative or reduced intensity conditioning in the older population is also unclear, and no studies have adequately addressed this question. Alternative approaches to limit toxicity and toxic death while achieving long-term OS and RFS have been reported. The high efficacy of blinatumomab in relapsed B-cell ALL led to its study in frontline treatment of adult patients with Ph+ ALL. Both the D-ALBA and SWOG 1318 studies treated adults with newly diagnosed Ph+ ALL with dasatinib and prednisone, followed by postremission therapy with dasatinib and blinatumomab, then dasatinib maintenance. Both studies reported excellent OS (D-ALBA, 18-month OS 95%; SWOG 1318, 3-year OS 85%) and disease-free survival (DFS) (D-ALBA, 18-month DFS 88%; SWOG 1318, 3-year DFS 80%).19,50 In the D-ALBA study, failure to achieve a CMR and presence of Ikarosplus abnormalities was associated with an inferior DFS, with the majority of relapse clones harboring the T315I mutation. A study from MD Anderson Cancer Center combined ponatinib and blinatumomab as induction and postremission therapy followed by ponatinib maintenance. In a recent report, the study had enrolled 35 adult patients (median age, 57 years; range, 22-83 years) with newly diagnosed Ph+ ALL. The composite CR rate was 96%, with a CMR rate of 85% at any time during therapy. With a median follow-up of 11 months (range, 1-46 months), the 2-year EFS and OS were 93% with no deaths related to ALL.51 See Table 3 for a summary of completed and ongoing studies of TKI + blinatumumab.
Ponatinib and corticosteroids alone were studied in the single-arm LAL 1811 trial. The approach may be sufficient therapy for some elderly patients given the excellent reported OS (median not reached) but with significant study dropout and disappointing EFS (median EFS, 14 months), possibly due to the high dose of ponatinib tested (45 mg daily).47 Reducing the dose of ponatinib based on hematologic and molecular response appears to be highly efficacious with lower toxicity.44,51
Risk-stratifying patients based on depth of molecular response and ALL mutational patterns has been explored, although using the approach to guide treatment decisions has not been prospectively studied. The GIMEMA LAL 1509 phase 2 study evaluated dasatinib with or without chemotherapy based on early response. Attainment of CMR at day 85 and lack of IKZF1plus deletions were associated with improved disease-free survival.18 Korean investigators found that among adult patients given nilotinib with pediatric-style chemotherapy, the achievement of a major molecular response or better was associated with a lack of benefit of allogeneic HCT relative to the chemotherapy approach.28 Investigators at MD Anderson Cancer Center compared their institutional outcomes with Hyper-CVAD plus a TKI (imatinib, 22%; dasatinib, 43%; ponatinib, 35%). For 3-month CMR patients, 5-year progression-free survival and OS rates were 68% without allogeneic HCT and 72% with allogeneic HCT.29 Current data support that patients with deep molecular responses to second-generation TKIs in combination with intensive chemotherapy may not derive additional benefit from allogeneic HCT.28,29
Maintenance therapy with a TKI with or without chemotherapy after intensive postremission therapy including HCT has been included in most regimens for adults with Ph+ ALL. No study has shown a definitive benefit of TKI-based maintenance therapy for Ph+ ALL in any setting, and this standard practice is primarily historical and based on consensus recommendations. Prior to the advent of TKIs targeting BCR-ABL1, maintenance chemotherapy for Ph+ ALL was the same as for Ph-negative ALL, commonly with 2 to 3 years of outpatient chemotherapy often containing mercaptopurine, vincristine, methotrexate, and/or a corticosteroid. With the advent of TKIs, a TKI was often added to this regimen, sometimes with reduction or elimination of traditional maintenance chemotherapy. As part of intensive regimens using this approach, outcomes appeared to have improved with improvements in TKI efficacy.28,29,35,46
After allogeneic HCT, preemptive therapy with the TKI imatinib appears to be as effective as a prophylactic maintenance strategy. Pfeifer et al52 randomized 55 patients treated with allogeneic HCT for Ph+ ALL to receive either prophylactic maintenance or MRD-triggered (by positive BCR-ABL1 quantitative reverse transcription polymerase chain reaction) therapy with imatinib. The remission duration, EFS, DFS, and OS were the same in both arms. As such, for imatinib, an MRD-triggered strategy would spare some patients treatment that is not beneficial and potentially harmful. Studies of second- and third- generation TKIs as prophylactic maintenance after HCT have been very small, heterogeneous, and not comparative, precluding conclusions about efficacy, although the drugs appear to be tolerated by most patients.53 In addition, if patients are not randomized, the impact of therapies and patient management prior to HCT must be taken into account when considering outcomes after HCT. Well-designed, randomized, prospective studies are needed to guide this approach. If a prophylactic approach is pursued after HCT, the optimal duration of maintenance TKI is not known. In CALGB 10701, a molecular response–based approach was studied with discontinuation of dasatinib after 1 year in patients with sustained complete molecular response for at least 3 months.6 The MD Anderson experience with TKI discontinuation (in patients not undergoing transplant) suggests that it can be done safely without frank relapse if molecular disease is followed closely. In addition, patients with molecular recurrence responded to retreatment with TKI in that report.54
The optimal tyrosine kinase inhibitor
In newly diagnosed Ph+ ALL, the dominant clone is highly sensitive to all BCR-ABL1–targeted TKIs in combination with corticosteroids with CR rates 90% to 100% regardless of the generation of TKI used. Durable remission rates are unacceptably low, however, with first- and second-generation TKIs without intensive consolidation with allogeneic HCT, autologous HCT, or chemotherapy. The primary mechanism of resistance is mutations in the ATP-binding domain of the BCR-ABL1 oncoprotein.6,7,19,43 Relapse in the central nervous system is also a risk, which is mitigated by the delivery of prophylactic intrathecal chemotherapy.
Unlike chronic myeloid leukemia, Ph+ ALL frequently develops relapse due to the presence at diagnosis or development of clones with TKI-resistant kinase domain mutations in BCR-ABL1 during therapy. Imatinib resistance is common, with numerous different kinase domain mutations conferring imatinib resistance observed at relapse.55 Studies with the second-generation TKI dasatinib hoped to overcome this limitation of imatinib, but outgrowth of clones with dasatinib-resistant kinase domain mutations, especially T315I seen in most relapses, is common without effective postremission therapies to eliminate these clones.6,7,19,43,55 Third-generation TKIs such as ponatinib overcome TKI resistance by targeting the T315I mutation and most other kinase domain mutations. In addition, ponatinib appears to be more effective at generating a complete molecular response. A recently published study from MD Anderson Cancer Center showed higher 3-month CMR rates with progressive generations of TKIs in combination with HyperCVAD (imatinib, 32%; dasatinib, 52%; ponatinib, 74%). By multivariable analysis, ponatinib was the only independent factor predicting progression (P = .028; hazard ratio, 0.388; 95% confidence interval, 0.166-0.904) and death (P = .042; hazard ratio, 0.379; 95% confidence interval, 0.149-0.966).29
Based on this and other comparisons, ponatinib appears to be the optimal commercially available TKI for the frontline treatment of Ph+ ALL, but cardiac and vascular toxicities need to be prevented and well managed to optimize patient outcomes. Unfortunately, a lack of randomized data in ALL has left many clinicians with difficulty getting the best drugs for their patients with Ph+ ALL. Current data support ponatinib as the optimal TKI for frontline treatment of adults with Ph+ ALL with improved molecular response rates and excellent long-term OS and DFS compared with historical outcomes. However, hospital formularies and insurance providers frequently do not approve its use, although this may be changing with recent publications. Recently published studies44,45,47,51,56 and an ongoing randomized study comparing imatinib to ponatinib (NCT03589326), both with a common chemotherapy backbone, may help change patterns of institutional and insurance authorization. Preventing and managing vascular side effects of BCR-ABL1–targeted TKIs remains an area of needed ongoing research, although dose reductions and holds appear effective at mediating toxicity in most patients.
Overcoming resistance to therapy
Relapse after CR still occurs even with the use of ponatinib in the frontline and highly effective postremission therapy. Understanding resistance mechanisms to ponatinib is an important area for researchers. Dual targeting of BCR-ABL1 by combining a TKI with an allosteric inhibitor of BCR-ABL1 (eg, asciminib, NCT03595917) may be one way to limit relapse while maintaining safety and ease of treatment. As above, postremission therapy with highly active, targeted immunotherapy (eg, blinatumomab, inotuzumab ozogamicin, chimeric antigen receptor T cells) may help eliminate resistant clones.19,50,51 Recently published data suggest that combining ponatinib with the BCL2 inhibitor venetoclax may be another low-toxicity oral approach to eliminating resistant clones,57 and venetoclax is being studied with dasatinib in the frontline (NCT04872790).
Conclusions
Given the rarity of the disease and wide age range affected by Ph+ ALL, randomized, controlled clinical study of treatment approaches has been difficult in Ph+ ALL. The advanced age of most patients has, until recently, also hindered improvements in therapy outside of the application of more effective BCR-ABL1–targeted TKIs. The application of novel, highly active targeted therapies such as blinatumomab in addition to second- or third-generation TKIs appears to be revolutionizing the care of Ph+ ALL with very high complete remission rates, complete molecular response rates, and long-term disease-free survival without allogeneic HCT. The role of HCT will need to be refined with these more effective regimens, with persistent molecular disease and Ikarosplus mutations being possible triggers for allogeneic HCT. Alternatively, reserving HCT for relapse may be the optimal way to promote optimal survival for patients. Other novel therapies, such as inotuzumab ozogamicin, chimeric antigen receptor–modified autologous or allogeneic T cells, novel TKIs, asciminib, and BH3 mimetics/Bcl family inhibitors may further improve cure rates for what was once a poor-risk leukemia.
Conflict-of-interest disclosure
Matthew J. Wieduwilt: advisory boards: Bristol-Myers Squibb, Jazz, Gilead/Kite; stock ownership: Reata; data monitoring committee member: Sorrento.
Off-label drug use
Matthew J. Wieduwilt: dasatinib, ponatinib, nilotinib, blinatumumab, asciminib, inotuzumumab ozogamicin, chimeric antigen receptor T-cells, and venetoclax are discussed.