Abstract
The incorporation of BCR::ABL1 tyrosine kinase inhibitors (TKIs) to intensive chemotherapy significantly improved the outcomes of patients with Philadelphia chromosome (Ph)–positive acute lymphoblastic leukemia (ALL). This was first shown with the addition of the first-generation TKI imatinib, which allowed more patients to be bridged to an allogeneic stem cell transplant (SCT) and led to superior long-term outcomes compared with chemotherapy alone. The use of second-generation TKIs (eg, dasatinib and nilotinib) has led to further improvement in outcomes of patients with Ph- positive ALL, with a long-term survival of 40% to 60% in several studies. Ponatinib is a third-generation, more potent TKI that results in high rates of molecular response and promising long-term survival even when allogeneic SCT is not routinely performed. While randomized data to support the TKI selection in Ph-positive ALL are lacking, data from single-arm studies suggest deeper molecular responses and superior survival outcomes with each successive generation of TKI. More recently, chemotherapy-free regimens with blinatumomab and TKIs have shown excellent results in the frontline setting and may represent an emerging paradigm shift in the treatment of Ph-positive ALL.
Learning Objectives
Understand how to select different tyrosine kinase inhibitors (TKIs) for patients with Philadelphia chromosome (Ph)–positive acute lymphoblastic leukemia
Understand the use of TKI-based combinations and chemotherapy-free regimens for the management of newly diagnosed Ph-positive acute lymphoblastic leukemia
CLINICAL CASE
A 47-year-old healthy man with no significant comorbidities presents with a complaint of fatigue and easy bruising for the past 2 weeks. Peripheral blood examination shows anemia, leukopenia, and thrombocytopenia. A bone marrow biopsy is performed and shows B-cell acute lymphoblastic leukemia (ALL) with t(9;22) by conventional karyotyping. What is the optimal treatment regimen and tyrosine kinase inhibitor to use in this setting?
Introduction
Historically, the outcome of Philadelphia chromosome (Ph)–negative ALL was poor, with long-term survival of around 10% to 35% with induction chemotherapy followed by allogeneic stem cell transplantation (SCT). With the addition of BCR::ABL1 tyrosine kinase inhibitors (TKIs) to intensive chemotherapy, the outcomes of patients with Ph-positive ALL has significantly improved over the past 2 decades. Multiple TKIs for the treatment of Ph-positive ALL are currently available, including imatinib (first-generation TKI), dasatinib and nilotinib (second-generation TKIs), and ponatinib (third-generation TKI). Table 1 shows the results from studies with various TKI-based combinations in Ph-positive ALL.
Treatment regimens for newly diagnosed Ph-positive ALL
| Treatment regimens . | n . | Overall CMR rate, % . | Allogeneic SCT rate, % . | RFS rate, % . | OS rate, % . |
|---|---|---|---|---|---|
| Imatinib | |||||
| High-intensity regimens | |||||
| Lee et al,15 2005 | 20 | 45 | 85 | — | 33 (5-yr) |
| Daver et al,1 2015 | 54 | 45 | 30 | 43 (5-yr) | 43 (5-yr) |
| Yanada et al,16 2006 | 80 | 38 | 61 | 60 (1-yr) | 76 (1-yr) |
| Bassan et al,17 2010 | 59 | — | 72 | 39 (5-yr) | 38 (5-yr) |
| Fielding et al,2 2014 | 175 | — | 71 | 50 (4-yr) | 38 (4-yr) |
| de Labarthe et al,18 2006 | 45 | 29 | 49 | 51 (1.5-yr) | 65 (1.5-yr) |
| Tanguy-Schmidt et al,19 2013 | 45 | 61 | 76 | 44 (4-yr) | 52 (4-yr) |
| Lim et al,20 2015 | 87 | 89 | 64 | 39 (5-yr) | 33 (5-yr) |
| Chalandon et al,21 2015 | 133 | 23 | 65 | 32 (5-yr) | 43 (5-yr) |
| Low-intensity regimens | |||||
| Vignetti et al,22 2007 | 29 | 4 | — | 48 (1-yr) | 74 (1-yr) |
| Chalandon et al,21 2015 | 135 | 28 | 62 | 37 (5-yr) | 46 (5-yr) |
| Dasatinib | |||||
| High-intensity regimens | |||||
| Ravandi et al,3 2015 | 72 | 60 | 17 | 44 (5-yr) | 46 (5-yr) |
| Ravandi et al,4 2016 | 97 | — | 42 | 62 (3-yr) | 69 (3-yr) |
| Low-intensity regimens | |||||
| Foà et al,23 2011 | 53 | 23 | 34 | 51 (1.7-yr) | 69 (1.7-yr) |
| Rousselot et al,24 2016 | 71 | 24 | 10 | 28 (5-yr) | 36 (5-yr) |
| Chiaretti et al,25 2015 | 60 | 19 | 42 | 49 (2.5-yr) | 58 (3-yr) |
| Nilotinib | |||||
| High-intensity regimens | |||||
| Kim et al,26 2015 | 90 | 86 | 70 | 72 (2-yr) | 72 (2-yr) |
| Liu et al,27 2019 | 30 | 83 | 53 | 45 (4-yr) | 45 (4-yr) |
| Low-intensity regimens | |||||
| Ottmann et al,28 2018 | 79 | 58 | 33 | 42 (4-yr) | 47 (4-yr) |
| Chalandon et al,29 2018 | 60 | — | 52 | 85 (1-yr) | 96 (1-yr) |
| Rousselot et al,30 2021 | 156 | — | 58 | 57 (3-yr) | 74 (3-yr) |
| Ponatinib | |||||
| High-intensity regimens | |||||
| Jabbour et al,31 2019 | 86 | 84 | 21 | 68 (5-yr) | 73 (5-yr) |
| Ribera et al,32 2021 | 30 | 47 | 87 | 72 (3-yr) | 97 (3-yr) |
| Low-intensity regimens | |||||
| Martinelli et al,33 2022 | 44 | 41 | 14 | 32 (2-yr) | 66 (2-yr) |
| Blinatumomab-based regimens | |||||
| In combination with dasatinib | |||||
| Foà et al,8 2020 | 63 | 60 | 50 | 71 (3-yr) | 80 (3-yr) |
| In combination with ponatinib | |||||
| Short et al,34 2022 | 35 | 85 | 3 | 93 (2-yr) | 93 (2-yr) |
| Treatment regimens . | n . | Overall CMR rate, % . | Allogeneic SCT rate, % . | RFS rate, % . | OS rate, % . |
|---|---|---|---|---|---|
| Imatinib | |||||
| High-intensity regimens | |||||
| Lee et al,15 2005 | 20 | 45 | 85 | — | 33 (5-yr) |
| Daver et al,1 2015 | 54 | 45 | 30 | 43 (5-yr) | 43 (5-yr) |
| Yanada et al,16 2006 | 80 | 38 | 61 | 60 (1-yr) | 76 (1-yr) |
| Bassan et al,17 2010 | 59 | — | 72 | 39 (5-yr) | 38 (5-yr) |
| Fielding et al,2 2014 | 175 | — | 71 | 50 (4-yr) | 38 (4-yr) |
| de Labarthe et al,18 2006 | 45 | 29 | 49 | 51 (1.5-yr) | 65 (1.5-yr) |
| Tanguy-Schmidt et al,19 2013 | 45 | 61 | 76 | 44 (4-yr) | 52 (4-yr) |
| Lim et al,20 2015 | 87 | 89 | 64 | 39 (5-yr) | 33 (5-yr) |
| Chalandon et al,21 2015 | 133 | 23 | 65 | 32 (5-yr) | 43 (5-yr) |
| Low-intensity regimens | |||||
| Vignetti et al,22 2007 | 29 | 4 | — | 48 (1-yr) | 74 (1-yr) |
| Chalandon et al,21 2015 | 135 | 28 | 62 | 37 (5-yr) | 46 (5-yr) |
| Dasatinib | |||||
| High-intensity regimens | |||||
| Ravandi et al,3 2015 | 72 | 60 | 17 | 44 (5-yr) | 46 (5-yr) |
| Ravandi et al,4 2016 | 97 | — | 42 | 62 (3-yr) | 69 (3-yr) |
| Low-intensity regimens | |||||
| Foà et al,23 2011 | 53 | 23 | 34 | 51 (1.7-yr) | 69 (1.7-yr) |
| Rousselot et al,24 2016 | 71 | 24 | 10 | 28 (5-yr) | 36 (5-yr) |
| Chiaretti et al,25 2015 | 60 | 19 | 42 | 49 (2.5-yr) | 58 (3-yr) |
| Nilotinib | |||||
| High-intensity regimens | |||||
| Kim et al,26 2015 | 90 | 86 | 70 | 72 (2-yr) | 72 (2-yr) |
| Liu et al,27 2019 | 30 | 83 | 53 | 45 (4-yr) | 45 (4-yr) |
| Low-intensity regimens | |||||
| Ottmann et al,28 2018 | 79 | 58 | 33 | 42 (4-yr) | 47 (4-yr) |
| Chalandon et al,29 2018 | 60 | — | 52 | 85 (1-yr) | 96 (1-yr) |
| Rousselot et al,30 2021 | 156 | — | 58 | 57 (3-yr) | 74 (3-yr) |
| Ponatinib | |||||
| High-intensity regimens | |||||
| Jabbour et al,31 2019 | 86 | 84 | 21 | 68 (5-yr) | 73 (5-yr) |
| Ribera et al,32 2021 | 30 | 47 | 87 | 72 (3-yr) | 97 (3-yr) |
| Low-intensity regimens | |||||
| Martinelli et al,33 2022 | 44 | 41 | 14 | 32 (2-yr) | 66 (2-yr) |
| Blinatumomab-based regimens | |||||
| In combination with dasatinib | |||||
| Foà et al,8 2020 | 63 | 60 | 50 | 71 (3-yr) | 80 (3-yr) |
| In combination with ponatinib | |||||
| Short et al,34 2022 | 35 | 85 | 3 | 93 (2-yr) | 93 (2-yr) |
RFS, relapse-free survival.
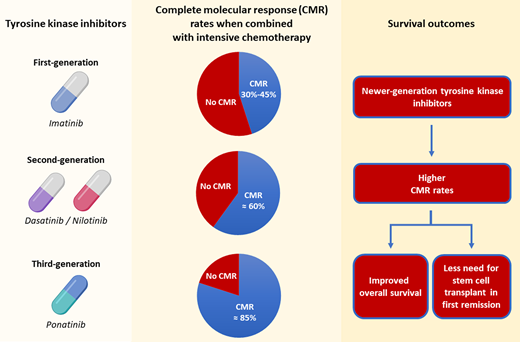
First-generation TKIs (imatinib)
Imatinib was the first TKI to be added to chemotherapy regimens for Ph-positive ALL. In a study of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine (hyper-CVAD) plus imatinib in 54 patients with newly diagnosed Ph-positive ALL, the complete remission (CR) rate was 93% and 16 patients (30%) underwent allogeneic SCT.1 This translated to a 5-year overall survival (OS) rate of 43%. The ECOG 2993/UKALLXII trial also evaluated the addition of imatinib to intensive chemotherapy.2 The incorporation of imatinib resulted in higher CR rates (92% vs 82%; P = .004) and superior 4-year OS (38% vs 22%; P = .003), compared with the preimatinib cohort. More patients who received imatinib were able to proceed to an allogeneic SCT (46% vs 31%), and the outcomes of transplanted patients were superior to those who did not undergo SCT. These findings demonstrated that the addition of TKIs improved outcomes compared with chemotherapy alone and facilitated the bridging of patients to a potentially curative allogeneic SCT.
Second-generation TKIs (dasatinib and nilotinib)
The outcome of Ph-positive ALL further improved with the advent of broader-spectrum, second-generation TKIs, specifically dasatinib and nilotinib. Although neither agent is approved in the United States for the frontline management of Ph-positive ALL in adults and used “off-label” in this setting, several trials have examined their role, particularly dasatinib (less evidence available for nilotinib). In a single institution phase 2 trial, the combination of dasatinib with hyper-CVAD in 72 patients with newly diagnosed Ph-positive ALL resulted in a 96% CR rate, with 65% of patients achieving complete molecular remission (CMR), defined as undetectable BCR::ABL1 transcript levels by polymerase chain reaction.3 Only 12 patients (17%) underwent allogeneic SCT in first remission, and the estimated 5-year OS rate was 46%. The Southwest Oncology Group trial evaluated the same combination of hyper-CVAD plus dasatinib in 94 younger patients (age ≤60 years) with Ph-positive ALL.4 The CR rate was 88%, and all patients with available donor were offered allogeneic SCT. In a landmark analysis, patients who underwent allogeneic SCT had superior relapse-free survival (hazard ratio, 0.42; 95% confidence interval [CI], 0.18-0.97; P = .038) and OS (hazard ratio, 0.35; 95% CI, 0.12-0.97; P = .038).
Similar outcomes have been observed with nilotinib-based combinations. In a study of 30 patients who received intensive chemotherapy plus nilotinib, 83% achieved CMR and 53% of patients underwent allogeneic SCT in first remission.5 The 4-year OS rate with this combination was 45%. Notably, bosutinib-based chemotherapy combinations have not been systemically explored in Ph-positive ALL.
In the absence of randomized studies to guide TKI selection in adults with Ph-positive ALL, a recent pediatric study provides some guidance. In total, 189 patients aged ≤18 years were randomized to receive either dasatinib or imatinib in combination with chemotherapy.6 Patients who received dasatinib had improved 4-year event-free survival (EFS) (71% vs 49%; P = .005) and 4-year OS (88% vs 69%; P = .04). The superior outcomes with dasatinib were largely driven by a lower 4-year cumulative risk of central nervous system (CNS) relapse (2.7% vs 8.4%; P = .06) compared with those who received imatinib. This is consistent with preclinical data suggesting that dasatinib has superior penetration into the CNS. Due to a higher risk of CNS relapse compared with Ph-negative ALL, patients with Ph-positive ALL should ideally receive 12 doses of intrathecal chemotherapy (IT) as prophylaxis. In 1 retrospective study, administration of 12 ITs as compared with 8 ITs resulted in improved CNS relapse-free survival.7
Attenuated chemotherapy combined with a first- or second- generation TKI has proven safe and effective in several studies, although generally results in lower CMR rates as compared with higher-intensity regimens. More recently, the bispecific anti-CD3/CD19 T-cell engaging antibody blinatumomab has been combined with TKIs in the frontline setting. The D-ALBA trial examined the combination of dasatinib and corticosteroids for ~3 months, followed by up to 5 cycles of blinatumomab consolidation and 12 doses of intrathecal chemotherapy.8,9 Sixty-three patients with a median age of 54 years were treated. The rate of molecular response increased from 29% at the end of dasatinib induction to 60% after 2 cycles of blinatumomab. Twenty-nine patients received an allogeneic SCT in first remission. After a median follow-up of 29 months, the 3-year disease-free survival rate was 71% and the 3-year OS rate was 80%. Nine relapses were observed with this regimen, including 4 relapses in the CNS.
Third-generation TKIs (ponatinib)
The 5-year survival rate of patients with Ph-positive ALL treated with first- or second-generation TKIs in combination is ~40% to 65%, with most patients eventually relapsing when allogeneic SCT is not performed in first remission. Relapses in patients treated with first- and second-generation TKIs are driven by 2 major factors: (1) a relatively low CMR rate (~20% with low-intensity combinations and 50% with high-intensity combinations) and (2) the emergence of T315I mutations in ABL1, detected in up to 75% of patients at the time of relapse.
Ponatinib is a pan-BCR::ABL1 TKI with activity against T315I mutations. It was evaluated in a phase 2 trial in combination with hyper-CVAD among 86 patients with newly diagnosed Ph-positive ALL.10 All patients achieved CR after induction therapy, and there were no early deaths. The overall CMR rate was 84%, and the estimated 5-year OS was 73%. Nineteen patients (22%) underwent allogeneic SCT, and there was a trend toward better 5-year OS for nontransplanted patients (86% vs 69%; P = .08), although it is important to note that patients who proceeded to SCT were generally higher risk (eg, had persistently detectable BCR::ABL1 transcripts). These encouraging results observed in nontransplanted patients suggested that allogeneic SCT may not be needed in most patients when treated with a ponatinib- based regimen, particularly those who achieve a CMR.
Notably, ponatinib is associated with a dose-dependent risk of arterial and venous thrombosis, pancreatitis, and hepatoxicity. In the initial study design, ponatinib was given at a dose of 45 mg daily continuously, and 2 patients died of myocardial infarction attributed to ponatinib therapy. The protocol was later amended so that patients received response-adapted dosing of ponatinib (30 mg once in CR and 15 mg once in CMR), which mitigated the risk of adverse events with no additional ponatinib-related deaths reported.10
In the PONALFIL trial, the combination of ponatinib with semi-intensive chemotherapy followed by allogeneic SCT (in 87% of the cases) also demonstrated very good results, with a CMR rate of 71% and a 3-year EFS and OS rates of 70% and 96%, respectively.11 In patients aged ≥60 years and those unfit for chemotherapy, the chemotherapy-free combination of ponatinib (45 mg daily) and steroids induced a CMR rate of 41% and a 2-year survival of 66%.12 However, many patients had to discontinue ponatinib due to adverse events; use of a lower dose of ponatinib could potentially mitigate these toxicities.
In the absence of randomized data comparing ponatinib with other TKIs, a propensity score–matched analysis was conducted to compare ponatinib vs dasatinib in combination with hyper-CVAD. The combination of hyper-CVAD plus ponatinib was superior to hyper-CVAD plus dasatinib with 3-month CMR rates of 84% and 63% (P = .25) and 3-year OS rates of 83% and 56%, respectively (P = .03).13
Ponatinib was also evaluated in a phase 2 trial in combination with blinatumomab.14 Ponatinib and blinatumomab were started concomitantly in the first cycle, rather than sequentially as in the D-ALBA trial. Blinatumomab was administered for 5 cycles along with ponatinib 30 mg daily in cycle 1, followed by a decrease to 15 mg daily once CMR is achieved. Patients received 12 doses of prophylactic intrathecal chemotherapy. Thirty-five patients with newly diagnosed Ph-positive ALL (median age of 57 years) have been treated. The rates of CMR after 1 cycle and overall were 64% and 85%, respectively. Only 1 patient underwent an allogeneic SCT and no relapses have been observed. The estimated 2-year EFS and OS rates were 93%. These encouraging results suggest that the combination of ponatinib and blinatumomab may represent an effective chemotherapy-free and SCT-sparing approach in patients with Ph-positive ALL.
Conclusion
Significant progress has been made in the management of Ph-positive ALL with the combination of chemotherapy plus TKIs. The rates of CMR and long-term survival have been improving with each newer generation of TKIs. The chemotherapy-free regimens of blinatumomab plus a TKI demonstrated excellent results in the frontline setting and may represent an emerging paradigm shift in the treatment of Ph-positive ALL.
Recommendations
A BCR::ABL1 TKI should be incorporated into the induction regimens of Ph-positive ALL (Grade 1A).
Second- and third-generation TKIs are preferred over imatinib for patients with Ph-positive ALL (Grade 1C).
In patients without a contraindication, ponatinib is preferred over first- and second-generation TKIs (Grade 2B).
Conflict-of-interest disclosure
Fadi G. Haddad: no competing financial interests to declare.
Nicholas J. Short has served as a consultant for Jazz Pharmaceuticals and Pfizer; reports receiving research grants from Takeda Oncology, Astellas Pharma Inc., Stemline Therapeutics Inc., and Xensor; and has received honoraria from Amgen, Astellas Pharma Inc., Novartis, and Pfizer.
Off-label drug use
No BCR::ABL1 TKI is FDA-approved for frontline use in adults with Ph-positive ALL; therefore, use of any TKI in this setting is considered off-label. The use of blinatumomab in the frontline setting also constitutes off-label use.