Abstract
The outcome for adolescents and young adults (AYA) with acute lymphoblastic leukemia (ALL) has improved, mostly based on the use of pediatric-inspired intensive protocols. Due to increasing disease resistance and treatment-related toxicity with age, further improvements are now expected from the expanding knowledge of ALL biology, more accurate risk stratification, and the early introduction of targeted small molecules and immunotherapy. In the last decade, the rate of AYA with B-cell precursor ALL with undetermined genetic drivers (“B-other”) has shrunk from 40% to fewer than 10%. The high-risk subgroup of Philadelphia-like ALL is the most frequent entity diagnosed in this age range, offering a multitude of potentially actionable targets. The timely and accurate identification of these targets remains challenging, however. Early minimal residual disease (MRD) monitoring has become a standard of care for the risk stratification and identification of patients likely to benefit from an allogeneic hematopoietic stem cell transplantation. Recently approved immunotherapies are moving frontline to eradicate MRD, to improve the outcome of high-risk patients, and, eventually, to reduce treatment burden. Comprehensive care programs dedicated to AYA with cancer aim at improving inclusion in specific clinical trials and at giving access to appropriate psychosocial support, fertility preservation, and survivorship programs.
Learning Objectives
Understand the specific profile of BCP-ALL biology in AYA
Understand how MRD monitoring improves treatment decision-making
Understand how immunotherapies are becoming frontline therapies in BCP-ALL
Introduction
Acute lymphoblastic leukemia (ALL) is paradigmatic of the different issues raised by the care of adolescents and young adults (AYA) with cancer, including a specific disease biology profile, the benefit of age-adapted treatment strategies, and the need for dedicated programs that take into account the psychosocial impact of intensive and long-lasting therapy and the access to fertility preservation and survivorship programs.1-3
CLINICAL CASE
A 22-year-old college student is referred to the emergency department for fatigue and bruising. Her initial white blood cell count is 29 × 109/L with 74% lymphoblasts, and her platelet count is 8 × 109/L. The initial workup concludes the diagnosis of pre-pre-B-ALL with a normal karyotype and CRLF2 rearrangement. After a 4-drug induction, the patient reaches complete remission (CR). MRD after induction and consolidation is 5x10−3 and 1x10−4, respectively.
Frontline therapy
In the last 2 decades, the outcome for AYA with Philadelphia (Ph)-negative ALL has improved, mostly based on more intensive approaches using either full pediatric or pediatric- inspired protocols. This attitude spread after many comparisons of the outcome of adolescents aged 15 to 20 years contemporarily treated in pediatric and adult trials showed the clear benefit of pediatric strategies.1,2 The wider use of nonmyelosuppressive drugs such as asparaginase, high-dose methotrexate, or antimetabolite-based maintenance therapy was the main identified reason to explain this difference. However, many other parameters may have played a role, including differences in supportive care or compliance with treatment schedule.4 In recent reports for AYA with Ph- negative ALL, CR rates are 85% to 95%, and 5-year overall survival (OS) estimates range from 60% to 78%. Despite a strong reduction in the survival gap observed between children and adults in the protocols from the 2000s, the outcome for AYA remains inferior to pediatric patients, even when treated according to common protocols.5,6 This difference is mostly due not only to an increased risk of ALL resistance but also to nonrelapse mortality with age.5-7 It is commonly admitted that the benefit of chemotherapy intensification in AYA has reached a ceiling.
Further improvements in the field of AYA ALL have recently emerged from
a better knowledge of ALL biology to understand disease resistance and to identify potential therapeutic targets,
the endorsement of early MRD response as a major prognostic factor and tool for transplant decision-making, and
the development of new frontline therapies to decrease resistance and improve treatment tolerance.
ALL biology in AYA
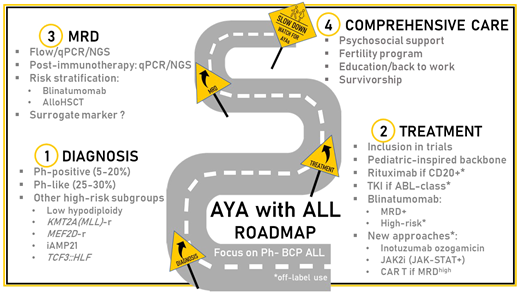
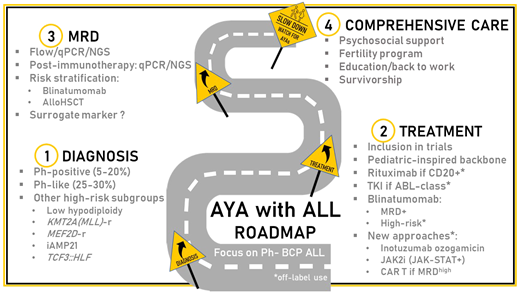
A transition in ALL biology occurs during the second and third decades of life and contributes to the overall increasing resistance to chemotherapy with age.3 In the subgroup of B-cell precursor (BCP)-ALL and apart from the lowest frequency of hyperdiploid and ETV6::RUNX1 ALL and the increasing incidence of Ph-positive, KMT2A-r, and low hypodiploid ALL in adults, many biological entities recently identified contribute to the understanding of disease resistance in AYA (Table 1).
Among them, Ph-like ALL is a heterogeneous subgroup of high-risk diseases characterized by a gene expression profile similar to Ph-positive ALL, a high frequency of IKZF1 gene alteration, and a higher frequency in the AYA population (25%-30%).8 Whatever the age at diagnosis, Ph-like ALL is associated with early resistance to treatment and overall poor outcomes. Ph-like ALL may be divided into 3 subgroups: (1) ALL with Janus kinase (Jak)/STAT pathway activation, a majority of cases being diagnosed with a CRLF2 gene rearrangement; (2) ALL with ABL-class fusions (ABL1, ABL2, PDGFRA/B, CSFR1) involving different partner genes; (3) ALL with other gene abnormalities (NTRK3, FGFR1, FLT3, TYK2); or pathway activation.9 The identification of activating lesions has opened the door to targeted interventions, especially with already approved kinase inhibitors, including Jak-, ABL-, FLT3-, or NTRK3 inhibitors. Besides promising preclinical studies in vitro or in patient-derived xenograft models, several case series have suggested the activity of tyrosine kinase inhibitors in ALL with ABL-class fusions.10 Many frontline strategies have also included an evaluation of the Jak inhibitor ruxolitinib in patients with Jak/STAT pathway activation, whatever the underlying mechanism involved (mostly abnormalities of CRLF2, IL7R, JAK2, EPOR).11 The results of these combinations in prospective studies are pending.
Other new BCP-ALL subtypes more frequent in AYA have recently been described (MEF2D-r, ZNF384-r/ZNF362-r, DUX4/ERG, CDX2/UBTF::ATXN7L3), some associated with high-risk features (Table 1).3,9,12 The best workup strategy to rapidly identify these genetic lesions and the capacity to evaluate not only the prognosis but also the benefit of targeted strategies in rare entities remain challenging.13 Different predictive algorithms have been proposed based on the expression profiling of selected genes (low-density polymerase chain reaction [PCR] array, quantitative PCR [qPCR]) to recognize Ph-like ALL, followed by the integration of flow cytometry, fluorescence in situ hybridization, and various molecular biology techniques (qPCR, multiplex PCR, next generation sequencing, RNA sequencing) to identify the underlying lesions. The choice of a “one-size-fits-all” inhibitor to combine with chemotherapy is pragmatic but may offer limited efficacy in some small subgroups of patients. In the near future, the integration of ex vivo drug screening with these complex algorithms should improve individual decision-making.14
Minimal residual disease
Postinduction MRD monitoring is considered to be the most important prognostic factor and risk-stratification tool in ALL. Many techniques and targets are used with an overall good correlation but with differences in sensitivity and specificity that are modulated by treatment strategy.15
Both the United Kingdom Acute Lymphoblastic Leukaemia (UKALL) 2003 and the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL 2008 trials showed higher postinduction MRD levels in AYA when compared to teenagers and younger children, mirroring the difference in ALL biology between these age subgroups.5,6 In the AYA included in the UKALL-2003 trial, MRD risk levels based on day 29, and postconsolidation assessments were correlated to cytogenetic risk.5 More interestingly, further analyses of the prognostic value of MRD in this trial demonstrated that the relapse risk predicted by a defined MRD level differs according to underlying cytogenetics or risk group.16 Relapse prediction could thus be improved by considering risk stratification based on genetic subtype-specific MRD values.
In Ph-negative ALL management, early MRD assessment is mostly used (1) to modulate the postconsolidation schedule and dose intensity, (2) to indicate eligibility for an allogeneic stem cell transplant (allo-HSCT), and (3), more recently, to introduce new therapeutic strategies such as immunotherapy. In full pediatric or pediatric-inspired trials designed for AYA, chemotherapy backbones tended to be maximized, with limited options to further increase dose intensity based on suboptimal MRD response. The Group for Research in Adult ALL (GRAALL) demonstrated that early MRD response was a good predictor of the benefit of allo-HSCT during the first CR.17 Nowadays, early MRD levels have been widely adopted to select young adult candidates for allo-HSCT.18 Differences in terms of MRD timing and thresholds for decision-making persist. In general, thresholds for indicating allo-HSCT for AYA treated in age-extended pediatric trials tend to be higher (1%-5% after induction, 0.05%-0.1% after consolidation) than those used in pediatric-inspired trials for adults only (0.1% after induction, 0.01% after consolidation).6,18,19 These discrepancies further highlight the need to incorporate age and underlying disease characteristics to define more appropriate decision thresholds for AYA.
The recent development of immunotherapy has confirmed the interest in MRD monitoring to predict patient outcome. Even in the relapsed/refractory (R/R) setting, MRD levels in patients who reach a CR after inotuzumab, blinatumomab, or chimeric antigen receptor (CAR) T-cell therapy are usually lower than after frontline chemotherapy.20-23 As detailed below, the sensitivity of the technique used to measure MRD in this context recently appeared to be of importance in identifying bad responders and in discussing further interventions.
CLINICAL CASE (Continued)
Per protocol, it is proposed that the patient receive blinatumomab as a bridge to allo-HSCT. Postblinatumomab MRD is undetectable. Ovarian tissue cryopreservation is performed just before allo-HSCT. After a conditioning regimen with total body irradiation and cyclophosphamide, the patient receives an HLA-matched sibling allograft. A college examination and interview are organized during an inpatient stay.
Moving immunotherapy frontline
Inotuzumab-ozogamicin
The antibody-drug conjugate inotuzumab-ozogamicin (INO), an anti-CD22 antibody linked to calicheamicin, was approved for adults with R/R BCP-ALL based on the results of the phase 3 INO-VATE study, which randomized 326 adult patients to receive either INO or the standard of care (SOC).20 The CR rate was higher in the INO arm (81% vs 29%; P < .001). Among the patients in CR, a complete MRD response was observed in 78% after INO and 28% after SOC (P < .001). The median OS was 7.7 months after INO vs 6.7 months after SOC (hazard ratio [HR], 0.77; P = .04). The most frequent grade 3 adverse events after INO were liver related, including 13% of patients who experienced veno- occlusive disease (VOD).24 Among the patients who received allo-HSCT after INO, 22% had VOD. Factors correlated to the risk of VOD during allo-HSCT after INO were (1) the use of 2 alkylating agents in the transplant-conditioning regimen, (2) pretransplant liver test abnormalities, and (3) a history of liver disease/hepatitis.24 A post hoc analysis showed that younger patients received a greater benefit from INO (HR, 0.53, 0.76, and 0.90 in patients aged 18-29, 30-54, and >55 years old, respectively). Younger patients who proceeded to allo-HSCT after INO were also less likely to develop VOD (7% of patients aged 18-29 years, 17% of patients <55 years, 41% of patients ≥55 years).25
The frontline development of INO in the AYA population is ongoing and takes into account the specific safety profile of the drug (Table 2). Academic phase 3 trials involving AYA with or without children or older adults raise the question of INO in the postinduction setting (NCT03150693, NCT03959085, NCT04307576, GRAALL-2022). Most of these studies randomize patients without indication to allo-HSCT. The role of INO in MRD eradication is also being explored (NCT03610438, NCT03441061, NCT03104491).
Blinatumomab
Blinatumomab is an anti-CD3 and CD19 bispecific antibody that aims to redirect T-cell toxicity against B lymphoid cells. The drug was first approved in adults with R/R Ph-negative BCP-ALL based on the results of the phase 3 TOWER study that randomized (2:1) blinatumomab vs SOC in 405 adult patients.21 The overall response rate after blinatumomab was 44% vs 25% after SOC (P < .001). Among patients with an overall response, a complete MRD response was achieved in 76% after blinatumomab and 48% after SOC (P < .001). The median OS was 7.7 months in the blinatumomab group vs 4.0 months in the chemotherapy group (HR, 0.71; P = .01). The benefit of blinatumomab over SOC in terms of overall response and median OS did not differ between AYA (<35 years) and older patients. In an exposure-adjusted analysis, cytopenias and infections of grade 3 or higher were less frequent after blinatumomab, whereas the occurrence of cytokine release syndrome (CRS) was more frequent.26 The drug was further approved for patients with Ph-positive ALL or with persistent or reappearing MRD.27,28 Recent observations have suggested that the preblinatumomab tumor burden correlates with patient early response and outcome.21,29,30 Whereas the role of debulking before blinatumomab has not been prospectively assessed, the results of 2 phase 3 trials performed not only in children but also AYA (up to 30 years of age) in a second CR of Ph-negative BCP-ALL encourage the use of blinatumomab in consolidation rather than as a salvage strategy.31,32
Frontline developments with blinatumomab in AYA with Ph-negative BCP-ALL are ongoing (Table 3). The results of the phase 3 trial by the National Cancer Institute/Eastern Cooperative Oncology Group-ACRIN in patients 30 to 70 years old are pending (NCT02003222). Many phase 2 studies assessing the role of blinatumomab in frontline consolidation for all patients who are MRD-positive or high risk are ongoing, or the results are pending (NCT02877303, NCT03109093, NCT03709719, NCT03367299, NCT04556084, NCT04334993). The role of blinatumomab as the first therapy before induction is also being explored (NCT03541083, NCT04554485). Other trials aim at improving the outcome after allo-HSCT (NCT04746209, NCT03114865, NCT03982992). Of interest, MRD is now widely used as a primary end point, whereas its role as a surrogate marker remains debated.
CD19 CAR T cells
Two autologous CD19 CAR T cells have been approved by the US Food and Drug Administration for AYA with R/R BCP-ALL. The ELIANA pivotal study for tisagenlecleucel (tisacel) involved pediatric and AYA patients with no prior exposure to blinatumomab who were refractory, in their first relapse after allo-HSCT, or in their second relapse.22 The overall remission rate was 81%, with a negative MRD response according to flow cytometry observed in all the patients who responded. The event-free survival (EFS) and OS estimates at 12 months were 50% and 76%, respectively. CRS and immune effector cell–associated neurotoxicity syndrome of grade 3 or higher occurred in 46% and 13% of patients, respectively. In real-world experiences, the safety profile was very similar in AYA when compared with children.33 However, the outcome of AYA tended to be inferior to children, with a trend for higher disease burden and more prior exposure to blinatumomab.33,34 The persistence of MRD as measured by qPCR or next generation sequencing after tisacel is a strong predictor of subsequent relapse.34,35 In this context, the potential emergence of a CD19− clone should be considered to decide further intervention. Prior exposure to blinatumomab and a high tumor burden before lymphodepletion were associated with a higher risk of CD19− relapse.34,36 Inversely, the loss of CAR T persistence is correlated with a higher risk of CD19+ relapse, and the optimal persistence time is still being debated.34 The ZUMA-3 pivotal study for brexucabtagene-autoleucel included adult patients (median age, 40 years) with R/R BCP-ALL.23 The overall response rate was 71%, with 97% complete MRD negativity by flow cytometry among responders. The overall response rate in patients aged 18 to 39 years, 40 to 64 years, and 65 years or older was 62%, 71%, and 100%, respectively. The median survival was 18 months. CRS and ICANS of grade 3 or higher occurred in 24% and 26% of patients, respectively.
The development of CAR T cells in ALL is mostly oriented toward safer and more persistent strategies that limit the risk of escape through the loss of the target antigen. Several frontline trials involving AYA that aim to control high postinduction MRD are also ongoing, with or without a bridge to allo-HSCT (NCT03876769).
Small molecules
Besides the use and evaluation of TK and Jak inhibitors in Ph-like ALL, other promising small molecules are being developed in both BCP and T-cell ALL. In a phase 1 study, BCL2 pathway inhibition with venetoclax (a BCL2 inhibitor) and navitoclax (a BCL2/BCLXL inhibitor) has shown promising results in R/R ALL and lymphoblastic lymphoma.37 The CR rate was 60%, with 17% of patients proceeding to allo-HSCT. The median survival was 7.8 months. Menin inhibitors prevent the formation of the menin-KMT2A complex. A phase 1 study of SNDX-5613 menin inhibitor in R/R acute myeloid leukemia and ALL showed an overall response rate of 61% among patients with KMT2A-r ALL and acute myeloid leukemia, a subgroup of leukemias with a dismal prognosis.38
Comprehensive care
In recent years, the need to develop multidisciplinary and holistic approaches to take care of AYA with cancer has been widely recognized. In AYA with ALL, specific challenges are linked to the complexity and duration of treatment, which have an impact on medical adherence; to the physical and psychological consequences of exposure to steroids; and to the use of allo-HSCT, which is the main cause of long-term side effects.39,40 Supporting the psychosocial health (including accessibility to treatment and education, family life, and sexuality) of AYA survivors is crucial during and following treatment.
Fertility preservation remains variably available and covered by health insurance. In AYA with ALL, infertility is almost exclusively due to allo-HSCT and is a major source of concern.41 Whereas oocyte preservation is usually not proposed/possible at diagnosis, ovarian tissue cryopreservation is feasible before transplant. Due to the risk of leukemic contamination, instances of ovarian tissue transplantation to restore endocrine function and/or fertility have so far been limited.42 In the future the quantification of ovarian infiltration using MRD tools may help to guide a decision regarding ovarian tissue transplantation,43 whereas the in vitro growth and maturation of immature oocytes increases the opportunity to restore fertility.44 In AYA female patients exposed to chemotherapy alone, fertility counseling after treatment completion is increasingly proposed to anticipate premature ovarian failure.
Similarly, while long-term sequelae are mostly observed after allo-HSCT,39 attention should be paid to the occurrence of specific adverse events, including avascular osteonecrosis or metabolic troubles, in patients who receive chemotherapy alone.45 Survivorship is being positively modified by the increasing use of new therapeutics. Specific concerns emerge from the use of B-cell-targeted immunotherapy that exposes patients to prolonged B-cell aplasia and hypogammaglobulinemia with an increased risk of late infection and a low response to vaccination, a real issue during the recent pandemic.
Conclusion
After 2 decades of improvement in the outcome of AYA with ALL, mostly supported by treatment intensification, new developments are based on better knowledge of ALL biology, the optimal use of MRD for risk-stratification, and the incorporation of immunotherapy and targeted small molecules in frontline therapy. As with other cancers diagnosed at this age, the key to patient management remains inclusion in clinical trials and comprehensive support by multidisciplinary teams during and after treatment.
Conflict-of-interest disclosure
Nicolas Boissel: honoraria: Amgen, Ariad, Bristol Myers Squibb, Celgene, Incyte, Jazz, Novartis, Pfizer, Sanofi, Servier, Shire; research funding: Amgen, Bristol Myers Squibb, Jazz, Novartis.
Off-label drug use
Nicolas Boissel: the off-label use of blinatumomab, inotuzumab ozogamicin, tisagenlecleucel, venetoclax, and navitoclax is discussed.