Abstract
Both myeloproliferative neoplasms (MPNs) and coronavirus disease 2019 (COVID-19) are characterized by an intrinsic thrombotic risk. Little is known about the incidence and the outcome of thrombotic events in patients with MPN infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but common mechanisms of coagulation activation, typical of both disorders, suggest that these patients can be at particularly high risk. To define the best thromboprophylaxis and treatment regimens in both MPN and COVID-19, individual- and disease-specific thrombotic risk factors, bleeding risk, and concomitant specific treatments need to be considered. In this case-based review, an individualized approach is presented in a case of SARS-CoV-2 infection occurring in a man with polycythemia vera (PV). A primary anticoagulant thromboprophylaxis strategy and adjustment of his PV treatment were implemented. However, during the hospital stay, he experienced pulmonary embolism and therapeutic anticoagulation had to be set. Then his condition improved, and discharge was planned. Postdischarge decisions had to be made about the type and duration of venous thromboembolism treatment as well as the management of PV-specific drugs. The steps of our decisions and recommendations are presented.
Learning Objectives
To learn the current risk-adapted approaches to prevent VTE in patients with MPN, COVID-19, or both
To identify the appropriate strategies for initial, long-term, and extended treatment of VTE and for MPN-specific therapy management in patients with MPN, thrombosis, and COVID-19
Introduction
BCR/ABL-negative myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET), myelofibrosis (MF), and prefibrotic MF (pre-MF), carry a high risk of thrombosis, which affects morbidity and mortality. The disease management, particularly in PV and ET, remains highly dependent on the patient's thrombotic risk.1 Recently, the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread all over the world, causing millions of deaths. The severe form of COVID-19 is characterized by interstitial pneumonia, multiorgan dysfunction, and a high thrombotic incidence, especially venous thromboembolism (VTE).2
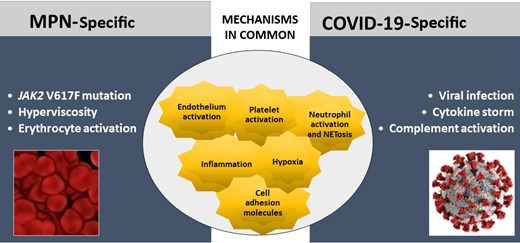
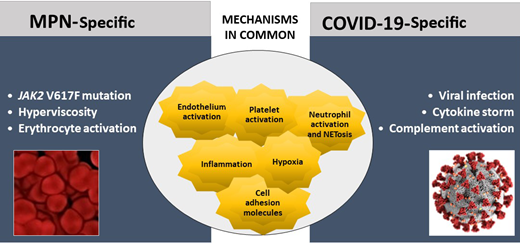
MPN and COVID-19 share common pathogenic pathways of hypercoagulability and thrombosis (Figure 1). In MPN, platelets, erythrocytes, and leukocytes stemming from the clonal proliferation of hematopoietic progenitor cells are constitutively activated.3 Furthermore, inflammatory mechanisms are highly involved and contribute to the overexpression of adhesion molecules by blood cells and endothelial cells (ECs), favoring cellular interactions and thrombosis. Moreover, JAK2 V617F has been detected in mature ECs in MPN, showing that JAK2-mutant ECs are prothrombotic by overexpressing P-selectin.4 In COVID-19, thromboinflammation, triggered by the viral infection, is a very important thrombogenic mechanism. An excessive immune response, named the “cytokine storm,” together with hypoxia and endothelial damage, is involved5,6 and precipitates the occurrence of thrombosis.
Specific and common mechanisms of hypercoagulability and thromboinflammation in MPN and COVID-19. Mechanisms of hypercoagulability in MPN and COVID-19 share common pathways. Major differences among the 2 diseases rely on the type of trigger of clotting activation, which in COVID-19 is due to the action of acute infection by SARS-CoV-2 on the prothrombotic characteristics of various hemostatic compartments. A maladaptive hyperactivation of innate immune systems in COVID-19 is also responsible for the activation of the complement cascade and endothelial dysfunction. Differently, in MPN, the systemic hypercoagulability results from the exposure to a long-term (chronic) subinflammatory condition, caused by the abnormal and clonal proliferation of JAK2-mutated myeloid cells.
Specific and common mechanisms of hypercoagulability and thromboinflammation in MPN and COVID-19. Mechanisms of hypercoagulability in MPN and COVID-19 share common pathways. Major differences among the 2 diseases rely on the type of trigger of clotting activation, which in COVID-19 is due to the action of acute infection by SARS-CoV-2 on the prothrombotic characteristics of various hemostatic compartments. A maladaptive hyperactivation of innate immune systems in COVID-19 is also responsible for the activation of the complement cascade and endothelial dysfunction. Differently, in MPN, the systemic hypercoagulability results from the exposure to a long-term (chronic) subinflammatory condition, caused by the abnormal and clonal proliferation of JAK2-mutated myeloid cells.
Data on thrombosis in patients with both MPN and COVID-19 are limited, but it is likely that patients with MPN who become infected are more susceptible to develop thrombotic complications.7 In this case-based review, my approach to prevent and treat VTE in a patient with PV and COVID-19 is presented.
CLINICAL CASE
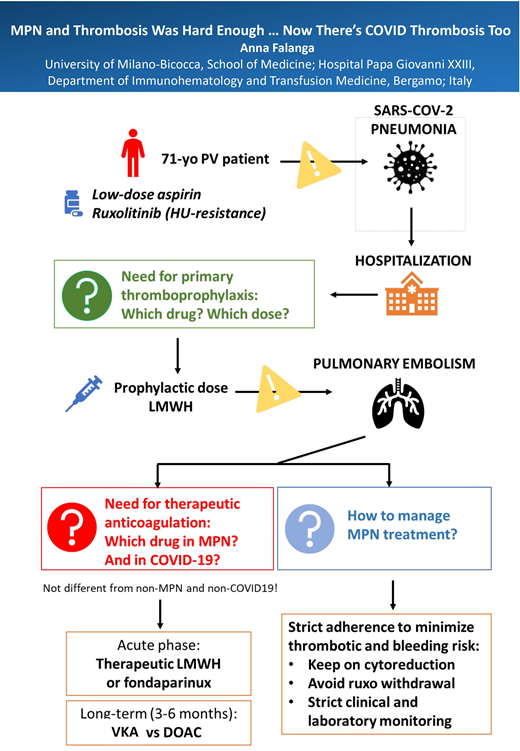
March 2020: a 71-year-old male patient presents to the emergency room with fever, generalized weakness, cough, lethargy, and dyspnea. His medical history was remarkable for PV from 2016, currently being treated with oral aspirin 100 mg daily and ruxolitinib 10 mg twice daily due to intolerance to hydroxyurea (HU), with good disease control; he had stopped requiring therapeutic phlebotomy for 2 years. He also had diabetes, hypertension, and history of transient ischemic attack. His body mass index was 27.2 kg/m2.
On admission to the emergency room, he was screened for SARS-CoV-2 by real-time reverse transcriptase–polymerase chain reaction assay on nasal swab, which proved to be positive. Blood pressure was 130/70 mm Hg, heart rate was 123 beats/min, and body temperature was 38.7°C. The patient's respiratory rate was 18 breaths/min and oxygen saturation was 75% on room oxygen.
A chest x-ray showed large bilateral multiple lung shadows. The laboratory findings included hemoglobin, 145 g/L; white blood cell, 13 × 109/L; and platelet count, 328 × 109/L.
Due to the severity of SARS-CoV-2 infection, he was hospitalized and commenced on oral hydroxychloroquine for 5 days and oral lopinavir/ritonavir, plus large-spectrum antibiotics, as this was the standard approach in March 2020.
We debated on whether and which VTE anticoagulant thromboprophylaxis to adopt and how to manage PV-specific treatment (aspirin and ruxolitinib).
Incidence of thrombosis, risk factors, and primary thromboprophylaxis in MPN and in COVID-19
MPN
Patients with MPN have a 3- and 10-fold higher risk of arterial and venous thrombosis, respectively, compared with controls.8 Arterial thromboses account for approximately two-thirds of total thrombotic events.9 Venous thrombosis represents the remaining one-third, including deep venous thrombosis (DVT) of the legs and/or pulmonary embolism (PE), plus a high rate of splanchnic and cerebral vein thrombosis.10 These patients also carry a high risk of bleeding,9 which is a very important element to consider when starting antithrombotic treatments. Older age and a history of thrombosis are well-established independent predictors of thrombosis in MPN,11,12 whereas JAK2 mutation is an independent risk factor only in ET.13 Hyperviscosity due to erythrocytosis correlates with greater thrombotic risk in PV, and a hematocrit less than 45% is protective against cardiovascular (CV) deaths.14 Leukocytosis is likely to be a risk factor, but data are not conclusive, and it is not formally included in prognostic scores.15 Risk factors for thrombosis in MPN are summarized in Table 1 (left panel).
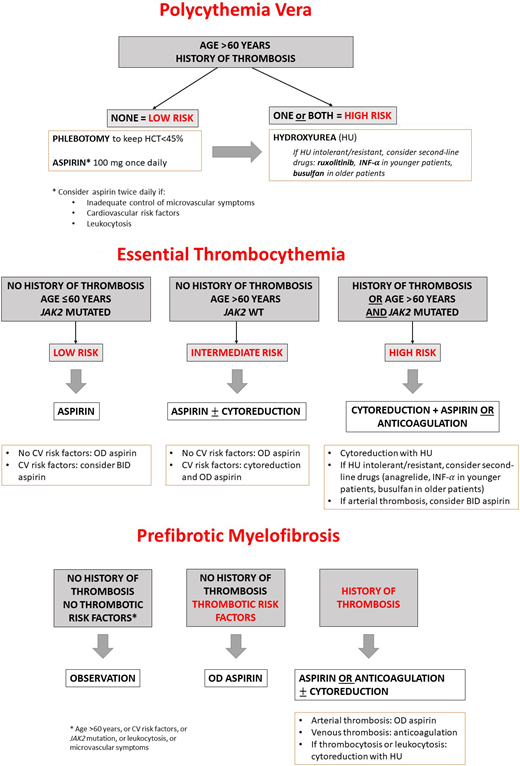
Treatment decisions are driven by risk stratification, based on the probability to develop a thrombotic event.16 As shown in Figure 2A, low-risk patients with PV should receive primary thromboprophylaxis with low-dose aspirin (75-100 mg) once daily. This indication originates from the European Collaboration on Low-Dose Aspirin in Polycythemia Vera study results, demonstrating that aspirin reduced the risk of combined end points of nonfatal myocardial infarction, nonfatal stroke, PE, major venous thrombosis, or death from CV causes.17 Of note, aspirin was not significantly protective of major CV and venous thrombotic events taken individually. In all patients with PV, phlebotomy is recommended to maintain hematocrit less than 45%.14 Finally, in high-risk patients with PV, cytoreduction must be given to minimize the thrombotic risk.16 To date, HU is the first-line drug of choice, with a starting dose of 500 mg 2 times daily. Interferon and peginterferons have also shown to prevent thrombotic complications in patients with PV and can be considered for younger patients or pregnant women requiring cytoreduction.18 Two randomized trials Randomized Study of Efficacy and Safety in Polycythemia Vera with JAK Inhibitor INCB018424 versus Best Supportive Care (RESPONSE and RESPONSE-2) in patients with PV resistant or intolerant to HU compared the JAK1/2 inhibitor ruxolitinib to best available therapy, showing a better control in hematocrit levels and symptoms in the ruxolitinib arm.19,20 A 5-year follow-up analysis of the RESPONSE trial showed that also thrombotic complications were lower in the ruxolitinib group.21 Currently, ruxolitinib is approved as second-line therapy in high-risk patients with PV in the United States and Europe.
(A) Thromboprophylaxis in PV according to risk stratification. (B) Thromboprophylaxis in ET according to risk stratification. (C) Thromboprophylaxis in pre-MF according to risk stratification. BID, twice daily; HCT, hematocrit; INF-α, interferon α; OD, once daily; WT, wild type.
(A) Thromboprophylaxis in PV according to risk stratification. (B) Thromboprophylaxis in ET according to risk stratification. (C) Thromboprophylaxis in pre-MF according to risk stratification. BID, twice daily; HCT, hematocrit; INF-α, interferon α; OD, once daily; WT, wild type.
In ET, primary thromboprophylaxis with low-dose aspirin is recommended in low- to high-risk patients, whereas very low-risk patients might not require any therapy unless in the presence of CV risk factors; cytoreduction is recommended in patients with intermediate-risk disease and CV risk factors, as well as in high-risk patients (Figure 2B). First-line drugs of choice in ET are HU and anagrelide,22 whereas interferons have proved effective in HU-intolerant/resistant patients and should be preferred in young or pregnant patients.18 In MF, the other major competing events (ie, acute leukemia transformation, infections, etc) may obscure the real incidence of thrombotic complications, and treatment is focused on symptom relief or disease eradication rather than thrombosis prevention. However, in pre-MF, in which the risk of vascular events in patients is similar to that of ET,23 a proposed pragmatic approach includes no treatment or low-dose aspirin in asymptomatic patients, aspirin or oral anticoagulation if having a positive history of arterial or venous thrombosis, and HU as first-line cytoreduction in case of thrombocytosis or leukocytosis24 (Figure 2C). Of interest, new drugs targeting specific mechanisms are becoming available, such as the antibody blocking the adhesion molecule P-selectin crizanlizumab, used to prevent vaso-occlusive crisis in sickle cell disease,25 which is under investigation in combination with ruxolitinib in MF (Platform Study of Novel Ruxolitinib Combinations in Myelofibrosis Patients, NCT04097821).
COVID-19
VTE has emerged as an important complication in hospitalized patients with COVID-19. High rates of VTE, particularly PE, have been demonstrated in both acutely ill patients, who require hospital admission without advanced clinical support, and critically ill patients, who develop respiratory or CV failure requiring advanced clinical support in the intensive care unit. In these 2 categories of patients, the incidence of VTE has been assessed to be 5% to 8% and 18% to 28%, respectively, regardless of pharmacologic thromboprophylaxis,26,27 whereas the pooled incidence of bleeding is 7.8%.27 Arterial thromboembolism, including stroke, also occurs but less frequently.28 Thrombotic complications in COVID-19 are associated with mortality.29
Hemostatic abnormalities (ie, elevation of D-dimer and fibrinogen), dysregulated immune response, and endothelial damage are the major features of a systemic thromboinflammation process.30 Autopsy findings have revealed widespread pulmonary microthrombosis and extensive pulmonary angiogenesis, in addition to frequent extrapulmonary microthrombosis and thromboemboli, consistent with disease-specific hypercoagulability.31 Among predictors of VTE in hospitalized patients with COVID-19, elevated D-dimer levels have been associated with clotting complications and poor outcome.32,33 Older age and the development of acute respiratory distress syndrome are among clinical risk factors for VTE29 (Table 1, right panel). However, risk stratification models have not been validated in the COVID-19 setting.
Primary anticoagulant prophylaxis is required, but the optimal strategy for patients hospitalized with COVID-19 is still a matter of debate. In general, in all acutely ill hospitalized medical and critical patients, the American College of Clinical Pharmacy (ACCP) and American Society of Hematology (ASH) guidelines recommend prophylactic (low-dose) low molecular weight heparin (LMWH) or fondaparinux once daily or unfractionated heparin (UFH) subcutaneously 2 or 3 times daily, unless there are contraindications, such as active bleeding or high bleeding risk.34,35 COVID-19–specific guidelines from the major scientific societies suggest thromboprophylaxis with low- or intermediate-dose LMWH after a careful assessment of the bleeding risk (Table 2).
Low (prophylactic), intermediate (half-therapeutic), and full (therapeutic) doses of anticoagulants (mainly LMWH or UFH) have been investigated as primary thromboprophylaxis regimens in acutely ill and critically ill patients (Antithrombotic Therapy to Ameliorate Complications of COVID-19; A Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia; Accelerating COVID-19 Therapeutic Interventions and Vaccines 4 ACUTE, Inspiration trials). New data showing the superiority of therapeutic doses in the acutely ill setting have recently emerged.41 On the contrary, in critically ill patients, therapeutic and intermediate-intensity anticoagulation have not shown advantages compared with low-dose thromboprophylaxis, while increasing significantly the risk of bleeding and thrombocytopenia42 (R. Zarychanski, unpublished data). The guidelines are constantly evolving and updating as the results of ongoing trials become available.
MPN plus COVID-19
Given the high thrombotic risk of both MPN and COVID-19, it is quite natural to wonder how patients with MPN deal with the SARS-CoV-2 infection. The recent retrospective MPN-COVID trial, promoted by the European LeukemiaNet, found a cumulative incidence of 8.6% thrombosis in hospitalized patients with MPN and COVID-19.7 Thrombotic events were mainly venous (VTE = 7.4% vs arterial thromboembolism = 1.9%), suggesting a closer link to infection rather than MPN (in which arterial events are more common), and were significantly more frequent in ET than in PV or MF. Data on thromboprophylaxis with various regimens of LMWH were very heterogeneous.
In March 2020 (as well as now), there was no specific evidence on which dose of LMWH had a most favorable risk/efficacy profile in MPN/COVID-19. Therefore, in practice, the dose could only be chosen on an empirical basis, evaluating the presence of generic vascular risk factors.
CLINICAL CASE (continued)
For VTE prophylaxis, we adopted the ACCP and ASH guidelines' recommendations for acutely ill medical patients,34,35 and LMWH enoxaparin 4000 IU subcutaneously daily was started. Treatment with aspirin was also continued due to the patient's history of transient ischemic attack. Ruxolitinib was reduced to 5 mg twice a day, given the possible drug-drug interaction with lopinavir/ritonavir, as it is known that the antiviral can interfere with JAK inhibitor metabolism, slowing down its excretion and enhancing toxicity.
The patient's clinical condition improved with treatment. However, 3 days after admission, he developed epistaxis. Both aspirin and anticoagulation were withdrawn, and bleeding stopped in 1 day. Only prophylactic LMWH was resumed.
Three days later, he suddenly became significantly more breathless—his oxygen saturation was now 75% on air. Computed tomography pulmonary angiography showed widespread bilateral ground-glass opacities consistent with extensive COVID-19 pneumonitis plus bilateral segmental pulmonary emboli. No DVT of legs was detected by ultrasound.
Blood tests showed the typical COVID-19 profile with mild lymphopenia, mildly raised alanine aminotransferase and lactate dehydrogenase, modestly raised C-reactive protein, raised ferritin, and markedly raised D-dimer.
He was put on high-flow oxygen, and therapeutic-dose LMWH enoxaparin 1 mg/kg twice daily was started. He did not require mechanical ventilation or intensive care unit management.
Thrombosis treatment in MPN and in COVID-19
Treatment of VTE, including DVT and PE, is divided in 3 phases: acute (first 5-10 days), long term (from end of acute treatment to 3-6 months), and extended (beyond 3-6 months). Anticoagulation with therapeutic-dose LMWH or fondaparinux is recommended over UFH intravenously or subcutaneously for initial treatment, before switching to a long-term anticoagulation regimen.44-46 This schema is not different for patients with MPN10 or COVID-19.
Before starting anticoagulant therapy, a careful assessment of the bleeding risk of both patients with MPN9 and COVID-1927 is required. In addition, an increased risk of heparin-induced thrombocytopenia in MPN calls for special care in monitoring the patient platelet count during the heparin course.47
CLINICAL CASE (continued)
In the initial phase, we opted for therapeutic doses of LMWH as long as the patient was hospitalized.
After 10 days, the patient's condition improved, and antiviral drug was stopped. However, the patient's blood counts had worsened from admission, with mild thrombocytopenia (110 × 109/L), hemoglobin of 110 g/L, and white blood cell count of 5 × 109/L. Ruxolitinib suspension was evaluated, also considering the need to keep the patient on anticoagulation.
How to manage specific MPN therapy under COVID-19 infection
The management of MPN-directed cytoreductive therapy after the onset of SARS-CoV-2 infection has been questioned since the beginning of the pandemic, and expert consensus platforms appointed by ASH have been issued (https://www.hematology.org/COVID-19/COVID-19-and-myeloproliferativeneoplasms). The results from an observational study promoted by the European LeukemiaNet group, focusing on clinical/laboratory presentation and risk factors for overall survival in patients with MPN during the acute phase of SARS-CoV-2 infection, show a high mortality risk among patients with MPN (28.6%), especially in MF, and demonstrate that withdrawal of ruxolitinib was a negative prognostic factor.48
Abrupt suspension of ruxolitinib appears associated with a “ruxolitinib discontinuation syndrome,”49 with debilitation, progressive splenomegaly, or, rarely, cytokine storm. Moreover, reports from observational studies suggest that JAK inhibitors may be beneficial for critically ill patients with COVID-19,50 although these results have not been confirmed in a large randomized commercial study (Study to Assess the Efficacy and Safety of Ruxolitinib in Patients With COVID-19 Associated Cytokine Storm, NCT04362137; results on clinicaltrials.gov). Adjustments of other cytoreductive drugs (ie, HU, interferon, or anagrelide) are not recommended in patients with MPN and COVID-19 (https://www.hematology.org/COVID-19/COVID-19-and-myeloproliferativeneoplasms).
CLINICAL CASE (continued)
We opted to keep the patient on reduced-dose ruxolitinib, strictly monitored the laboratory and clinical parameters, and contemplated that the infection state and the concomitant medications could in part be responsible for the mild pancytopenia observed. Indeed, blood values did not further decrease, and a slight improvement in platelet count was observed apart from antiviral suspension.
The patient recovered sufficiently to be discharged home, and long-term anticoagulant and PV-specific treatments were planned.
Long-term/extended duration and type of anticoagulation in MPN and in COVID-19
The aim of long-term anticoagulation (3-6 months) is to prevent thrombosis recurrence. DVT of legs or PE in patients with MPN should be treated the same as DVT or PE occurring in patients without MPN. Therefore, continuation of anticoagulant therapy is based on evaluation of the underlying risk factors for VTE recurrence.44,46 This risk in MPN, particularly in patients with PV, is significantly higher than in the general population.51 Long-term and, possibly, extended (after 6 months) anticoagulation is recommended in MPN due to the presence of a permanent risk factor, such as a chronic malignant disease. Other strong predictors for recurrence in MPN are age older than 60 years and history of thrombosis.
The risk of VTE recurrence in COVID-19 is not well defined. Recent data show that postdischarge thromboembolic outcomes and mortality may be frequent after COVID-19 hospitalization, and anticoagulation may reduce this risk by 46%,52 but these results are controversial.53,54 Currently, postdischarge thromboprophylaxis is not recommended.
Concerning the type of long-term anticoagulant regimen, we can choose among the following oral agents: vitamin K antagonists (VKAs) aiming for targeting an international normalized ratio of 2.5 (range, 2.0-3.0) or direct oral anticoagulants (DOACs).45,46
So far, after acute treatment with LMWH, early initiation of VKAs is the most used strategy in patients with MPN.
At present, there are no evidence-based data on the efficacy and safety of DOACs in patients with MPN with VTE. Data from a randomized controlled trial conducted in noncancer and cancer populations have shown that these drugs are as effective as VKAs, with a lower risk of bleeding. In addition, DOACs do not need laboratory monitoring and have less dietary interference, and therefore, they have become widely used in clinical practice. Small series of patients with MPN treated with DOACs have been published.1 Of interest, recently, the results of a large retrospective study of 442 patients with MPN receiving DOACs (factor Xa inhibitors) or VKAs either for VTE or atrial fibrillation confirm a similar risk/benefit profile of both regimens for treatment of VTE in MPN.55
CLINICAL CASE (continued)
For long-term home anticoagulant treatment, we chose to stop LMWH and shift to therapy with a DOAC, based on our personal experience with these drugs and because of their easy handling, particularly with the concurrent difficulties due to the pandemic and hospital crash. I discussed the 2 options with the patient, and he shared my decision and preferred DOAC. He started oral rivaroxaban 20 mg daily.
After 2 weeks from discharge, the patient was visited as an outpatient. Blood counts had recovered, so we decided to resume ruxolitinib at the regular dose of 10 mg twice daily.
After a 6-month follow-up, he was doing well, with no recurrence of VTE.
Summary and conclusions
In conclusion, MPN and COVID-19 are both high-risk conditions for thrombosis. Thus, it is likely that patients with MPN, particularly patients with PV and ET, are more prone to develop thrombotic complications during SARS-CoV-2 infection. In this work, the journey is illustrated by a 71-year-old patient with PV who faced an acute infectious illness, COVID-19, during his chronic disease and related treatment. Not only did he have to deal with hospitalization and COVID-19 treatments, but despite thromboprophylaxis, he also experienced a PE. As a consequence, all tasks related to the choice and duration of anticoagulation in the acute phase and during the long-term postdischarge, as well as the issues related to the PV-specific treatments, had to be handled.
There is a clear need to protect patients with MPN from SARS- CoV-2 infection and its complications. Nowadays, vaccination represents our best weapon against COVID-19. Although an endogenous production of anti-PF4/heparin antibodies, also involved in the pathogenesis of vaccine-induced thrombotic thrombocytopenia,56 has been described in patients with MPN,57 no cases of vaccine-induced thrombotic thrombocytopenia syndrome associated with MPN have been published so far.58,59 A recent small cohort study on chronic myeloid neoplasms showed an impaired seroconversion after vaccination, especially in patients receiving ruxolitinib,60 but larger studies with a longer follow-up are required to confirm these findings. Moreover, there are reassuring data concerning the efficacy of the COVID-19 mRNA vaccine in the general MPN population.61,62 Therefore, at this time, patients with MPN should be encouraged to receive vaccination against COVID-19.
Acknowledgments
The author thanks doctors Francesca Schieppati and Marina Marchetti for invaluable assistance and critical review of the manuscript.
Conflict-of-interest disclosure
Anna Falanga discloses no relevant conflicts of interest.
Off-label drug
Anna Falanga: nothing to disclose.