Abstract
Somatic mutations are an unavoidable consequence of aging tissues. Even though most mutations are functionally silent, some may affect genes critical to proper tissue self-renewal and differentiation, resulting in the outgrowth of affected cells, also known as clonal expansion. In hematopoietic tissue such clonal dominance is known as clonal hematopoiesis (CH). Sporadic CH is frequent in aging and affects over 10% of individuals beyond the fifth decade of life. It has been associated with an increased risk of hematologic malignancies and cardiovascular disease. In addition to aging, CH has been observed in other hematologic conditions and confers an adaptation of hematopoietic stem cells (HSCs) to various environmental stressors and cell-intrinsic defects. In the presence of extrinsic stressors such as genotoxic therapies, T-cell-mediated immune attack, or inflammation, somatic mutations may result in augmentation of HSC fitness. Such attuned HSCs can evade the environmental insults and outcompete their unadapted counterparts. Similarly, in inherited bone marrow failures, somatic mutations in HSCs frequently lead to the reversion of inherited defects. This may occur via the direct correction of germline mutations or indirect compensatory mechanisms. Occasionally, such adaptation may involve oncogenes or tumor suppressors, resulting in malignant transformation. In this brief article, we focus on the mechanisms of clonal dominance in various clinical and biological contexts.
Learning Objectives
Review the etiology and nomenclature of CH
Discuss the mechanism of clonal expansion and stem cell fitness in various clinical and biological contexts
CLINICAL CASE
The patient is a 69-year-old man who is a computer programmer and has a medical history of hypertension, hyperlipidemia, coronary artery disease, and rheumatoid arthritis. He was recently found to have mild normocytic normochromic anemia during his annual physical exam. His initial workup is shown in the table below (abnormal values are in bold).
A bone marrow biopsy revealed normocellular marrow for age and trilineage hematopoiesis with no dysplasia and no increased blasts. Normal iron stores and no ringed sideroblasts were noted on iron stain. Cytogenetics studies showed a normal male karyotype. A next-generation sequencing (NGS) targeted myeloid panel revealed a DNMT3A R882C mutation at a variant allele frequency (VAF) of 2.5%.
Introduction
The term “clonal hematopoiesis” (CH) describes the dominance of a hematopoietic clone arising from a single hematopoietic stem cell (HSC) relative to the rest of the hematopoietic cells. Since the introduction of NGS technology in hematopoiesis research, an association between CH and various hematologic and nonhematologic conditions has been reported. Thus, a number of new terms and acronyms comprising CH have been proposed.
For the purpose of this review, CH of indeterminate potential (CHIP) and age-related clonal hematopoiesis (ARCH) will be considered synonymous and will denote CH characterized by the presence of 1 or more somatic mutations in genes associated with hematologic malignancies in otherwise healthy individuals without obvious hematologic conditions. Moreover, the mutations must be present at a VAF ≥2%, although consensus on the biologically and clinically meaningful VAF cutoff is unknown and has been a subject of ongoing research.1-3
Clonal cytopenia of undetermined significance (CCUS) describes the presence of CH in individuals with persistent uni- or multilineage cytopenias that cannot be explained by other conditions.3 Since cytopenia in CCUS is thought to be due to the underlying clonal process, this condition often represents an early myeloid neoplasm.2 A detailed description and the clinical consequences of CCUS are provided in an accompanying review by Dr. Osman.
Idiopathic cytopenia of undetermined significance (ICUS) denotes persistent uni- or multilineage cytopenia unexplained by other conditions and having no evidence of CH.4
We propose that the term CH be used for any clonal process that does not fulfill the criteria for the above-mentioned conditions (Table 1).
The etiology of clonal dominance
Aging
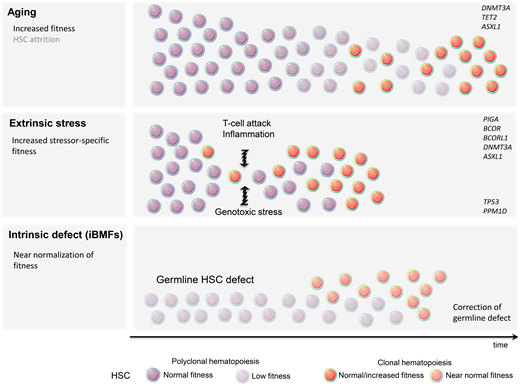
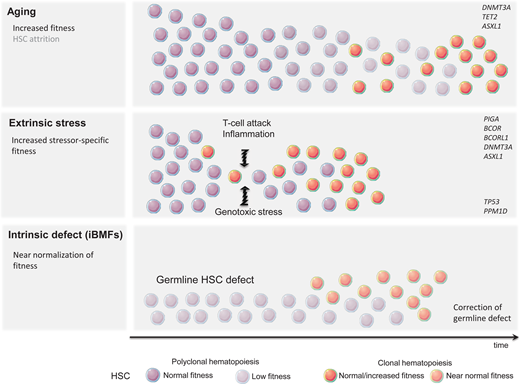
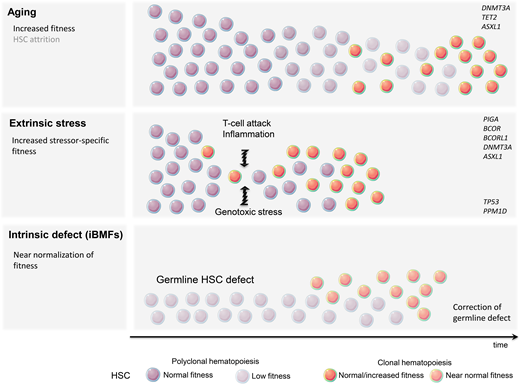
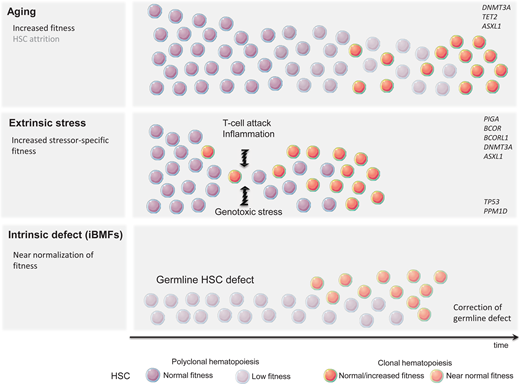
Despite the human body's very effective DNA repair machinery, somatic mutations are inevitable, and their number increases with time. In fact, every tissue accumulates somatic mutations during development and aging, resulting in the generation of cells with different genotypes, a phenomenon known as somatic mosaicism.5,6 This is particularly prevalent in highly proliferative tissues such as the hematopoietic system, and somatic mutations are present in the blood of nearly all older adults.7,8 Fortunately, because of their predominantly stochastic nature, most mutations affect noncoding regions and thus remain functionally silent. Assuming that approximately 1000 HSCs are active and contribute equally to human hematopoiesis, somatic mosaicism would be expected at a level of 0.001 in the absence of clonal advantage of an individual HSC. In fact, analysis of the peripheral blood of patients in the fifth decade of life using ultradeep sequencing showed the presence of somatic mosaicism with a median VAF of 0.002 in 95% of individuals. Interestingly, most clones remained stable over time.8 Thus, somatic mosaicism is frequent with aging but rarely results in clonal advantage and subsequent CH. The relative expansion of a single clone can arise either due to the increased fitness and growth advantage of an HSC (large numerator) or as a result of the HSC's pool contraction (small denominator). Pathogenic mutations, often in cancer-associated genes, frequently result in the expansion of HSCs and clonal dominance. Nonfunctional variants, on the other hand, serving only as “clonal markers,” may become detectable in the case of HSC pool attrition and when ultradeep or wide breadth of coverage NGS is applied. Consistent with this notion, CH was found to be significantly more prevalent when whole-genome sequencing methods were applied.7 What then is the underlying mechanism of hematopoietic clonal expansion in aging hematopoiesis? Undoubtedly, the HSC pool decreases with age; however, stochastic HSC attrition does not appear to be the major means behind clinically consequential CH (Figure 1) CH seems to be predominantly driven by a cell autonomous increase in HSC self-renewal and positive selection rather than genetic drift.9 This is mostly due to qualitative and/or quantitative changes in proteins encoded by so-called driver genes. DNMT3A and TET2 are among the most commonly mutated genes in ARCH and belong to the class of epigenetic modifiers. Since both are essential in self-renewal and differentiation, they impose their effects in a cell autonomous manner.
The DNMT3A gene encodes a methyltransferase responsible for de novo DNA methylation that is critical for transition from one cell state to another and thus is required for proper tissue development and stem cell maintenance. Animal studies have shown that the loss of DNMT3A augments HSC self-renewal with only a modest impact on differentiation, leading to the gradual expansion of mutated HSC and clonal dominance.10
TET2 is the second most frequently mutated gene in CHIP. The product of this gene belongs to the family of dioxygenases. TET2 mediates DNA demethylation by catalyzing the oxidation of 5-methylcytosine to 5-hydroxymethylcytosine that remains unmethylated during subsequent cell division.11 Similarly to DNMT3A loss, animal models of TET2 knockout exhibit increased HSC self-renewal and skewed hematopoietic differentiation favoring myelomonocytic lineage.12 Similar gain of self-renewal has been observed with ASXL1 mutations.13 The impact of less common CH mutations on clonal growth advantage, particularly in genes involved in mRNA splicing, is not well understood.
Cell-extrinsic factors
A very modest increase in the fitness of an individual HSC often requires years or decades to gain dominance over unmutated counterparts. This mostly indolent process may be further accelerated by a plethora of extracellular stressors, such as increased proliferative pressure, inflammation, and genotoxic agents. The most profound stress that can be imposed on the hematopoietic system is related to hematopoietic reconstitution after allogeneic bone marrow transplantation. Recent studies have demonstrated that CH clones present in allogeneic stem cell donors undergo accelerated expansion in the recipients while remaining relatively dormant in the donors.14-16 Inflammation is another presumptive modifier of CH and has been frequently associated with tissue aging. Mutated HSC may gain a selective growth advantage when exposed to proinflammatory signals such as IL-6 or IFN-gamma.17,18
Immune attack
The immune destruction of hematopoietic cells is a hallmark of acquired aplastic anemia (AA). CH is present in up to 50% of AA patients.19 Perhaps the best example of clonal immune escape is the clonal advantage of the paroxysmal nocturnal hemoglobinuria (PNH) clone arising from phosphatidylinositol glycan class A-deficient HSC. The PNH clone can be found at low levels in most healthy individuals, but the cells lack a competitive advantage under homeostatic conditions. Under immune pressure, glycosyl-phosphatidylinositol-deficient cells are less susceptible to T-cell-mediated killing and outcompete their unmutated counterparts.20 This observation raises the possibility that the glycosyl-phosphatidylinositol-anchored protein might be a critical target recognized by T cells. Inactivating mutations or loss-of-heterozygosity of HLA class 1 alleles, allowing for resistance against autoreactive T cells, is another example of HSC adaptation to immune-mediated stress.21 The role of other mutations in increased HSC fitness is less clear. DNMT3A and ASXL1 are seen in up to 15% of patients with AA.19 Also, mutations in BCOR and BCORL1 (transcriptional corepressors) are frequently associated with AA. Unlike clones with DNMT3A and ASXL1 mutations that may expand over time and lead to malignant transformation, clones carrying BCOR and BCORL1 mutations remain stable and seldom result in progression to acute myeloid leukemia/myelodysplastic syndrome.19 The exact mechanism of the aforementioned mutations to augment HSC fitness in the presence of T-cell-mediated attack is unclear.
Genotoxic therapies
Unlike already primed HSCs with mutations resulting in improved fitness, some hematopoietic clones lack the clonal advantage under homeostatic conditions but are capable of evading extracellular insults or intracellular defects (Figure 1). Patients treated with genotoxic chemotherapy or radiotherapy are at risk for a therapy-related myeloid neoplasm. It is now well recognized that miniscule hematopoietic clones carrying TP53 or PPM1D mutations frequently not detectable by standard NGS techniques are present long before treatment with genotoxic agents.22 It is likely that functionally inconsequential somatic mutations in TP53 and PPM1D, under homeostatic conditions, are able to resist genotoxic stress. Given that these mutations likely confer an inadequate response to DNA damage, it is not surprising that while exposed to genotoxic therapy they may circumvent apoptosis and acquire additional DNA defects. The common example is the loss of the wild-type TP53 allele resulting in a complete loss of function and subsequent accumulation of additional genetic defects and leukemic transformation.23,24
Cell-intrinsic defects
In addition to sporadic CH associated with aging or shaped by extracellular stressors, CH can also represent an adaptation to intrinsic HSC defects underlying inherited bone marrow failure syndromes (iBMFs). iBMFs occur as a result of germline HSC mutations and include short telomere syndromes (STS), impaired ribosome biogenesis (Shwachman-Diamond syndrome; SDS), an increase in DNA damage (Fanconi anemia; FA), and SAMD9/ SAMD9L mutations (MIRAGE syndrome). Several compensatory mechanisms may lead to an increase in HSC self-renewal in iBMFs. Randomly occurring mutations in HSCs may result in somatic reversion of the genetic defect, allowing the corrected clone to “rescue” hematopoietic output. This may be achieved via the direct correction of germline mutation or an indirect compensatory mechanism (Table 1).25 Somatic reversion of DKC1, a gene frequently mutated in STS, or gain-of-function mutation in the TERT promoter leading to increased mRNA expression have been observed in STS. Direct correction of damaging mutations in FA patients and the full or partial function restoration of several genes in the FANC complex have also been observed. Since bone marrow failure in FA is mainly mediated by p53-induced apoptosis, the emergence of CH with TP53 loss-of-function may provide better survival and improvement in hematopoietic output. The latter is an example of the indirect correction of a germline defect. Similarly, TP53 mutations may rescue bone marrow failure in SDS patients in whom abnormal ribosome biogenesis triggers p53-mediated apoptosis. The cleavage of EIF6 by SBDS protein is essential for proper ribosome assembly. Thus, either mutations in EIF6 or chromosome 20q deletion resulting in EIF6 haploinsufficiency confers the indirect correction of SBDS mutations.26 Finally, the duplication of the hypomorphic SBDS allele, through the emergence of CH with isochromosome 7q, may partially reverse the disease phenotype.27 In contrast, in SAMD9/SAMD9L-mediated bone marrow failure, somatic reversion may be accomplished by the loss of the mutant allele located on chromosome 7q (Table 2).28 The role of CH in iBMFs is discussed in detailed by Dr. Jung in an accompanying article.
CH is common in BMFs and frequently represents an adaptation of HSCs to extracellular or cell-intrinsic stressors, improving stem cell fitness and peripheral blood count. However, such correction may result in a higher incidence of hematologic malignancies, especially when involving putative oncogenes or tumor suppressors.
CLINICAL CASE (continued)
The patient had a single hot spot mutation in DNMT3A with a VAF of 2.5%. This likely represents sporadic CH associated with aging. The patient's normocytic normochromic and hypoproliferative anemia is likely related to chronic inflammation given the patient's history of rheumatoid arthritis and the elevation of inflammatory markers (ferritin). The hemogram and iron studies are highly suggestive of anemia of inflammation (AI), particularly in the absence of nutritional deficiencies or other apparent abnormalities, including an unremarkable bone marrow examination. It appears that the anemia is not caused solely by an underlying CH. Additionally, the presence of a minor clone accounting for only 5% of the total hematopoietic output (heterozygous mutation with a VAF of 2.5%) would not be sufficient to have a significant impact on the hematopoietic output from 95% nonclonal counterparts in a cell-autonomous manner. However, the exact VAF cutoff associated with cytopenias is not yet established, and the values are likely mutation-specific. Of note, DNMT3A mutations seem to be more frequent in older patients with AI, but the presence of small clones (<5% VAF) has no impact on clinical outcome.29 Even though the patient may be at risk for progression to myelodysplastic syndrome or acute myeloid leukemia, the probability appears to be small given the lack of high-risk CH features such as multiple mutations, spliceosome, IDH1/2 or TP53 mutations, and high VAF.30-32 Since the presence of CHIP is an important risk factor for a major cardiovascular event, the patient should be counseled on lifestyle modification to address modifiable cardiovascular risk factors.
Conflict-of-interest disclosure
Lukasz P. Gondek: no competing financial interests to declare.
Off-label drug use
Lukasz P. Gondek: nothing to disclose.