Abstract
Hemophilia A (HA) and B are inherited bleeding disorders caused by a deficiency of factor VIII or factor IX, respectively. The current standard of care is the administration of recombinant or purified factor. However, this treatment strategy still results in a high economic and personal burden to patients, which is further exacerbated by the development of inhibitors—alloantibodies to factor. The treatment landscape is changing, with nonfactor therapeutics playing an increasing role in what we consider to be the standard of care. Emicizumab, a bispecific antibody that mimics the function of factor VIIIa, is the first such nonfactor therapy to gain US Food and Drug Administration approval and is rapidly changing the paradigm for HA treatment. Other therapies on the horizon seek to target anticoagulant proteins in the coagulation cascade, thus “rebalancing” a hemorrhagic tendency by introducing a thrombotic tendency. This intricate hemostatic balancing act promises great things for patients in need of more treatment options, but are these other therapies going to replace factor therapy? In light of the many challenges facing these therapies, should they be viewed as a replacement of our current standard of care? This review discusses the background, rationale, and potential of nonfactor therapies as well as the anticipated pitfalls and limitations. This is done in the context of a review of our current understanding of the many aspects of the coagulation system.
Learning Objectives
Describe the basis of emicizumab action
Describe potential targets used in rebalancing therapies for hemophilia treatment
Introduction
Congenital hemophilia A (HA) and B (HB) are inherited bleeding disorders caused by a deficiency of factor VIII (FVIII) and factor IX (FIX), respectively. Many barriers have been overcome leading up to our current standard of care in the form of modern factor therapies.1
The administration of factor concentrates (derived from human plasma) to replace the missing factor is the most straightforward treatment approach and started in the 1970s. However, many hemophilia patients fell victim to the HIV pandemic, when contaminated factor products infected many patients. With the sequencing and cloning of the FVIII and FIX genes in the 1980s and technological advances to inactivate and purify concentrates, modern recombinant and plasma-derived factor products ushered in a new era of treatment.1,2 Recombinant and plasma- derived factor products have ascended as the major hemophilia treatment and have remained the standard of care in hemophilia in subsequent years.3 However, even with infectious concerns all but eradicated in new patients, treatment with factor products poses a risk of developing inhibitors, which are neutralizing alloantibodies to exogenous FVIII or FIX proteins recognized as foreign by the body's immune system.
Inhibitor development remains a significant complication in the treatment of patients with HA and HB and leads to bleeding despite factor therapy. The phenomenon is more common in severe HA than in nonsevere HA, with an incidence of 25% to 30%.4 In HB, inhibitor incidence is 3% to 5% in general but is higher in populations enriched for null mutations.5 Even with attempted immune tolerance induction and immunosuppression, inhibitors recur in up to 30% of HA and 20% of HB patients.5-7 First-line bleed treatment in inhibitor patients is generally with bypassing agents (BPA) such as recombinant activated factor VII (rFVIIa) or activated prothrombin complex concentrates (aPCC), which have similar efficacy and side effect profiles in retrospective analyses. Although generally effective, BPA cannot be monitored by standard lab assays and have a reported failure rate ranging from 7% to 11.6% and thrombosis rates between 4% and 6.5%.8 As such, nonfactor alternatives to circumvent these complications have been of interest. These therapies are part of 2 broad categories: factor mimetics and rebalancing therapies. This review discusses the individual drugs either currently in use, in trial, or in preclinical investigation. A discussion of gene therapy, desmopressin, and antifibrinolytics is omitted in favor of a focus on the basic science, rationale, and considerations of these emerging nonfactor therapies.
CLINICAL CASES
Case 1: A 10-year-old boy with severe HB developed an inhibitor at age 4, successfully completing immune tolerance induction at that time. He experienced bleeding while on prophylactic recombinant FIX infusions years later and was found to have a recurrence of his inhibitor. He had a central line inserted for BPA use while restarting immunosuppression. He still has evidence of an inhibitor, and his parents are asking about alternative therapies, especially those that could allow him to live without central venous access and such frequent infusion center visits.
Case 2: A 26-year-old man has severe HA and has not been on factor prophylaxis since age 12. He is afraid of bleeding and avoids various activities out of caution but still experiences spontaneous bleeding, predominantly in his ankles. He never learned to infuse factor and states that he is afraid of needles. Additionally, he has no insurance coverage. He is asking if there are now any options other than infusing intravenous factor to treat his hemophilia.
Hemostatic targets of nonfactor therapies
A review of the clotting cascade and its role in the hemophilia phenotype is necessary to a discussion of mimetic and rebalancing therapies.
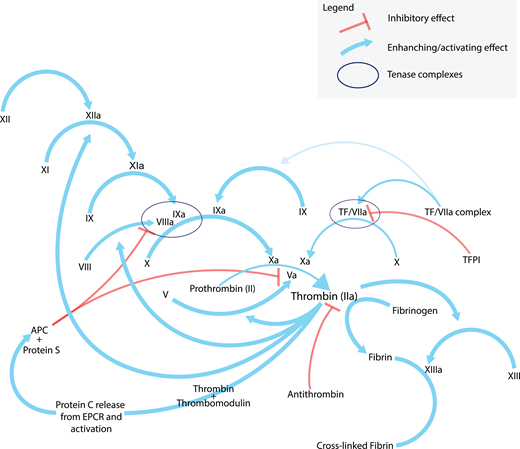
Hemostasis is initiated by the extrinsic pathway of the coagulation cascade and then amplified by the contact, or intrinsic, pathway. These interactions generate thrombin, which then cleaves to fibrinogen to form a stable fibrin clot (Figure 1).
Coagulation cascade. A simplified representation of the “coagulation cascade”. Note the role that the tenase complexes play in thrombin generation. The intrinsic tenase complex consists of factors VIIIa, IXa, and X on a phospholipid surface with phosphatidylserine exposure, usually a platelet, and facilitates the activation of factor X. The extrinsic tenase complex consists of factor VIIa, tissue factor, and factor X, likewise leading to the activation of factor X. Note the many feedback mechanisms of activation that thrombin performs. Although the generation and exposure of TF at the site of vascular endothelial is the primary initiator of coagulation via the extrinsic pathway, the intrinsic tenase pathway is important because active TF has only limited availability in vivo and TFPI’s constitutive activity inhibits the extrinsic tenase complex from generating adequate thrombin for a stable clot (see reference 19 for a more detailed treatment of this topic). In a PTT test, a test on which clot-based factor assays are built, phospholipid and calcium are added to a sample anticoagulated with sodium citrate (a calcium chelator that inhibits the Ca++ dependent steps as noted in the figure). Thrombin is added to the assay and further generated by the thrombin burst (see text). The activation of factor VIII or IX is the rate-limiting step in the assay. Factors are labeled by their traditional roman numeral. TF, tissue factor; TFPI, tissue factor pathway inhibitor; EPCR, endothelial protein C receptor; APC, activated protein C.
Coagulation cascade. A simplified representation of the “coagulation cascade”. Note the role that the tenase complexes play in thrombin generation. The intrinsic tenase complex consists of factors VIIIa, IXa, and X on a phospholipid surface with phosphatidylserine exposure, usually a platelet, and facilitates the activation of factor X. The extrinsic tenase complex consists of factor VIIa, tissue factor, and factor X, likewise leading to the activation of factor X. Note the many feedback mechanisms of activation that thrombin performs. Although the generation and exposure of TF at the site of vascular endothelial is the primary initiator of coagulation via the extrinsic pathway, the intrinsic tenase pathway is important because active TF has only limited availability in vivo and TFPI’s constitutive activity inhibits the extrinsic tenase complex from generating adequate thrombin for a stable clot (see reference 19 for a more detailed treatment of this topic). In a PTT test, a test on which clot-based factor assays are built, phospholipid and calcium are added to a sample anticoagulated with sodium citrate (a calcium chelator that inhibits the Ca++ dependent steps as noted in the figure). Thrombin is added to the assay and further generated by the thrombin burst (see text). The activation of factor VIII or IX is the rate-limiting step in the assay. Factors are labeled by their traditional roman numeral. TF, tissue factor; TFPI, tissue factor pathway inhibitor; EPCR, endothelial protein C receptor; APC, activated protein C.
Given the primacy of the extrinsic, or initiation, pathway in physiologic hemostasis, why should a deficiency of FVIII or FIX lead to such severe bleeding? Because once fibrin deposition begins and thrombin is formed, thrombin also acts “upstream” to activate additional FVIII, FXI, and FV, leading to more thrombin generation via the intrinsic pathway (also called the amplification pathway) in what is referred to as the thrombin burst. Without FVIII or FIX, this phenomenon does not occur, and thrombin generation remains too meager to form a stable fibrin clot. The objective of either a factor mimetic or a rebalancing therapy is to restore thrombin generation in patients with hemophilia, thus achieving clinical hemostasis without thrombotic complications.
Factor mimetic therapy
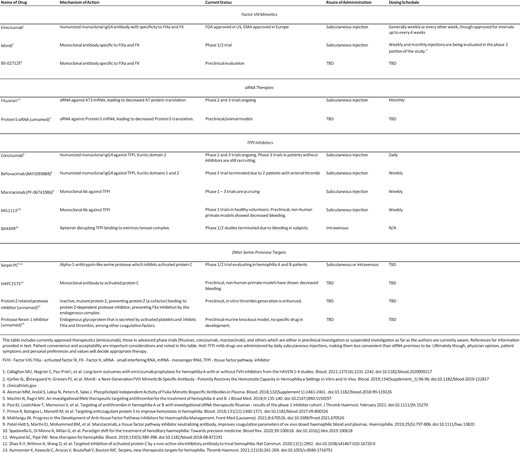
Emicizumab (Genentech, San Francisco, CA) is a humanized, bispecific, monoclonal immunoglobulin G4 antibody (mAb) that binds to activated FIX (FIXa) and FX, thereby performing the function of FVIII by bringing FIXa and FX into close enough proximity to facilitate FX activation.
First approved for bleed prophylaxis in HA with inhibitors in the US by the US Food and Drug Administration in 2018, emicizumab has been appropriately recognized as signaling a new era of HA treatment. In all phase 3 trials, emicizumab prophylaxis has led to drastic reductions in annualized bleeding rates (ABRs), with median ABRs of 2.6 for all dosing regimens in patients through the HAVEN 1 through 4 trials and with over 80% of participants experiencing no bleeds after week 24 of therapy.9,10
Considering data showing diminishing FVIII expression months to years after adeno-associated virus gene therapy,11 emicizumab or a similar mimetic therapy may well be the dominant paradigm in HA treatment for some time. In clinical practice it is being successfully used in infants and other previously untreated patients, who now grow up without bleeding or factor exposure. Given the low incidence of antidrug antibodies, the ease of administration with minimal instruction, and the extremely long half-life, patients may experience a de facto cure with regular administration.
However, much remains unanswered in emicizumab use, and optimal management requires an experienced hematologist. Standard coagulation tests and thus one-stage, clot-based FVIII activity assays (FVIII OSCA) are rendered unreliable due to the independence of emicizumab from thrombin generation in initiating its activity.
Thromboses and thrombotic microangiopathy were observed in early trials, and all were associated with the concomitant use of aPCC.10 The mechanism of this potentially devastating adverse effect is still unknown, though elucidating this may yield insights helpful for clinicians treating with emicizumab in general.
Other antibody factor VIIIa mimetics with the same mechanism of action, binding factors IXa and X in close proximity, leading to thrombin-independent FXa activation, are in development. Mim8 (Novo Nordisk, Bagsvaerd, Denmark) is a next- generation bispecific antibody to FIXa and X that has been shown in vivo and in vitro to potently promote thrombin generation in the absence of FVIII.12 It is currently being investigated as a next-generation FVIII mimetic in a phase 1/2 trial (clinicaltrials.gov). Another next-generation bispecific FVIII mimetic in development is BS-027125 (Bioverativ, Waltham, MA), currently in preclinical evaluation.13,14
FVIII is a cofactor rather than a serine protease, making it amenable to therapy replacing it with a nonfactor compound, leaving HB treatment wanting a similar paradigm change. Fortunately, ingenious manipulation of the clotting cascade promises a similar new era for HB. Approaches with roots in observational science are driving emerging hemophilia treatments known as rebalancing therapies. These therapies seek to “rebalance” coagulation to a more normal state by altering the inherent physiologic modulation of coagulation (Table 1).
Rebalancing therapies as a treatment for hemophilia
siRNA therapeutics
With the advent of genome sequencing, scientists identified patients with severe hemophilia who coinherited various prothrombotic gene mutations and displayed a milder phenotype.15 A preponderance of evidence exists for coinheritance of FVIII and prothrombin mutations, so it is perhaps fitting that targeting the function of thrombin would be an early contender for a rebalancing therapy.
Antithrombin (AT) is an endogenous protein that naturally regulates the function of active thrombin. Fitusiran (Alnylam, Cambridge, MA) is a small-molecule RNA interference therapeutic that acts by binding and degrading the mRNA encoding AT, leading to increased total thrombin generated with a hemostatic challenge.16,17
In early-phase clinical trials to date, doses of fitusiran were targeted to lower AT levels to 20%, which normalizes thrombin generation and reduces bleeding. However, trials were briefly put on hold in 2017 after a patient died after developing a dural sinus thrombosis following high-dose rFVIII administration to treat a bleed during the open-label extension of the trial.17 The hold was lifted after protocol amendments were made. The drug had no thrombotic complications in a phase 1 cohort of patients with inhibitors,18 and results of the phase 2 trial have not been published. Subsequently, following further nonfatal thrombotic events, clinical trials were again held, and doses were reduced in October 2020. Revised dosing and AT targets have been adopted for ongoing phase 3 participants (clinicaltrials.gov).
In trials, fitusiran is now administered as a subcutaneous injection every other month. Since the decrease in AT affects the common pathway, this can treat either HA or HB, potentially with or without inhibitors.
Another preclinical siRNA therapeutic shares fitusiran's mechanism but silences protein S expression rather than AT. It is in preclinical investigation for eventual use in hemophilia.19
Tissue factor pathway inhibitors
Another way to exploit inherent mechanisms to promote hemostasis is by blocking tissue factor pathway inhibitor (TFPI), an endogenous serine protease inhibitor that prevents the activation of FX by the TF-FVIIa complex, thus limiting the degree of thrombin generation via the contact pathway. By disrupting TFPI binding to this extrinsic tenase complex, Xa and thrombin generation are increased. This approach has been successful via monoclonal antibodies to various domains of the TFPI molecule.20
Concizumab (Novo Nordisk) is a humanized immunoglobulin G4 anti-TFPI antibody to the second Kunitz (K2) domain of TFPI. Building on observational data in humans, animal models demonstrated restored thrombin generation and decreased bleeding despite deficiencies in FVIII or FIX.21 Phase 1 and 2 trials in hemophilia patients without inhibitors demonstrated reduced ABRs and no thromboembolic events. Phase 3 trials in HA and HB were temporarily suspended due to nonfatal thrombotic events in early 2020 but have since resumed. In addition, antidrug antibodies have been observed in trials for concizumab.22,23
Befovacimab (BAY1093884, Bayer) is specific to both K2 and K1 domains, blocking TFPI binding to both FVIIa and FX in the extrinsic tenase complex and enhancing thrombin generation despite deficiencies in the contact pathway. In an early study, it decreased bleeding episodes, but the trial was terminated when 2 patients developed cerebral arterial thrombi, and one developed a cerebral venous thrombus.22,23
PF-06741086 (Marstacimab, Pfizer) has been shown to normalize coagulation in hemophilia patient plasmas ex vivo and is being evaluated in HA and HB patients (clinicaltrials.gov). There have been no thrombotic complications to date, with reduction of ABRs to zero in most participants.22,24 MG1113 (Green Cross Corporation) is another TFPI mAb likewise being tested in healthy volunteers (clinicaltrials.gov), with nonhuman primate models having demonstrated in vivo reduction of blood loss.22
An aptamer derived from recombinant human TFPI (BAX499, Takeda) was also developed and found to efficiently inhibit TFPI in vitro and in vivo, with dose-dependent increases in thrombin generation and decreased bleeding in animal models and in early-phase human trials.15,25 Development is on hold due to bleeding in subjects.15
Other serine protease targets
SerpinPC is an alpha-1-antitrypsin-like serine protease modified to inhibit activated protein C (APC).26 By preventing the action of APC, which inhibits FV activation, thrombin generation is likewise restored despite factor deficiencies in the contact system via enhanced common pathway activity (Figure 1).27 This is currently in a phase 1/2 trial, with approximately 50 participants having both HA and HB (clinicaltrials.gov). Recently, a monoclonal antibody to activated protein C (HAPC1573) has been shown in nonhuman primates to prevent bleeding.28
Protein Z (PZ)-related protease inhibitor is an inactive mutant PZ, cofactor to PZ-dependent protease inhibitor. Endogenously, the PZ-dependent protease inhibitor complex inhibits FXa. By preventing this complex formation through various methods, preclinical data have shown increased thrombin generation in vitro.29
Finally, protease nexin-1 has been proposed as a target. Endogenously, this glycoprotein is expressed by activated platelets and inhibits FXIa, thrombin, and other factors. Hemophilic mice showed decreased bleeding in protease nexin-1 knockout models, making this another promising target.29
The economics of nonfactor therapy
Although potentially paradigm changing for patients, nonfactor therapies may come at a paradigm-changing price. Cost analyses of new monoclonal antibodies for various indications are common in the literature. Although analyses suggest cost-effectiveness for some mAb according to many metrics for rare diseases, equity in access to treatment with these expensive medications must be ensured for these benefits to be realized.30
Some authors have found that mAb costs for malignant and nonmalignant hematologic disorders skew higher than those marketed for use in other disorders.31 However, emicizumab has actually been shown to be highly cost-effective in several real-world analyses, owing to its superb efficacy and the already high cost of hemophilia care with factor infusions and inhibitor development.32,33 Current data describe an existing high burden in treating those patients who fail factor therapy or cannot secure access to effective factor therapy.34,35 In this context, costs of new therapies may be offset by a reduced burden of the complications of standard therapy. However, concerns remain about the long-term effects of these therapies, which may carry hidden economic costs and disadvantages.
Concerns and pitfalls
For factor mimetic and rebalancing therapies that have reached a sufficiently advanced stage in clinical trials, a reduction in ABRs has indeed been noted. Note that this efficacy has been shown thus far in prophylaxis only, including in emicizumab. This is to be expected considering the mechanism of action of these therapeutics but is important in evaluating the limitations and potential application of these new and emerging drugs.
As noted in the discussion of these agents, every nonfactor therapy that has reached clinical trial has had thrombotic side effects with the exception of marstacizumab.23,29,36 With emicizumab, all thromboses and thrombotic microangiopathy episodes were associated with aPCC use, and safety otherwise is becoming well established.10 But safety in the setting of surgeries and trauma remains an unresolved problem. Although the current aim of nonfactor therapy is prophylaxis, the concomitant management of perioperative and trauma-related bleeding is of growing interest,35-37 with consensus that factor concentrate use is still necessary in these cases. Though standardized, optimized approaches do not yet exist, current consensus guidelines provide detailed guidance based on real-world use thus far.35,38,39 Additionally, experience in competitive athletes is lacking, though expert opinion generally considers monotherapy insufficient if breakthrough bleeding is observed.23,37
Because mimetic and rebalancing therapies alter hemostasis either independently or by directly modifying regulatory mechanisms of coagulation, patient selection will also need to take into account thrombotic risk factors such as obesity, existing cardiovascular disease, diabetes, and other conditions.
Aside from efficacy and safety in general, meaningful trial end points are essential for emerging therapies. For example, some extended half-life factor products, despite showing adequate trough levels in clinical trial, have been found in real-world use to have inferior bleeding response.38 The approval and wide clinical adoption of emicizumab reflect its proven efficacy in reducing ABRs in patients with and without inhibitors.39 As monitoring will not be as straightforward as with factor replacement, ABR reduction must remain the end point of choice in nonfactor therapy trials.15,23,40
The monitoring of nonfactor therapies is complicated and impossible with standard screening tests and assays, a prominent subject of concern.40 This is because these drugs exert their effect independently of activation by thrombin itself, the reaction noted above being the rate-limiting step in such clot-based assays,39 or by directly modifying thrombin generation. Though OSCA and chromogenic factor assays are widely available, validated monitoring of nonfactor agents is forthcoming.
Patients started on emicizumab while still having active inhibitors are another population who will benefit from further research to optimize inhibitor eradication, with approaches likely to differ between pediatric and adult patients.41,42 Alloantibodies to the drugs themselves and inhibitor recurrence while on mimetic therapy are emerging issues as well.29,43,44
Other concerns with the advent of factor-mimetic therapies, especially in long-term use, are that they do not perform some important functions of the factor molecules in maintaining vascular integrity and interacting with the cellular components of blood.39 Additionally, TFPI knockout mice develop premature atherosclerosis, suggesting that modulating the hemostatic system could have unintended consequences outside of the acute hemostatic effect.45 Indeed, the act of rebalancing a system that exists in such a complex balance is bound to be a difficult proposition, and long-term follow-up data will be critical in all emerging therapies.
Factor mimetic and rebalancing therapies will continue to coevolve with factor therapy. These therapies and the accompanying complexity involved in their use will continue to require comprehensive, expert care. The myriad offerings will match the complex needs of our patients and eventually lead to superior care overall. While expert opinion still regards factor replacement as the current standard of care for hemophilia,3,37 this “standard” is continuing to change. Future therapy, protean as it may be, has a common goal: a standard of result that our patients do not bleed.
Final thoughts
There will be a need for factor replacement therapy in the treatment of hemophilia for the foreseeable future, but nonfactor therapies fulfill a great need and represent a new phase of hemophilia care. Some of those needs not currently amenable to nonfactor therapies include the management of surgeries and trauma.
Are nonfactor therapies the end of factor therapy? Perhaps the more important question is whether they should be the end of factor therapy. Preliminary data seem to suggest that altering the hemostatic balance is a fraught proposition, and ultimately, the best option is that which yields the best result for a given patient. Some patients will not want new options to replace current treatments. Maybe they will be reassured by the number of choices. Clinicians should find reassurance in this as well.
Returning to our introductory cases, are they candidates for nonfactor therapies? Certainly, our young man in Case 1 would clearly benefit from therapy to free him from frequent BPA use in a central line and the risks of ongoing immunosuppression. Our gentleman from Case 2 may have more to think about. Someone who has never had factor therapy optimized lacks a compelling reason to consider newer therapy, though the decision would ultimately rest on his values and perception of his quality of life. As we have seen with emicizumab, even patients without inhibitors and other difficulties have benefited from nonfactor treatment. Also, the simplicity of subcutaneous dosing in a patient who has not learned self-infusion is likely to improve bleed prophylaxis.
Even if these nonfactor tools are not the end of factor therapy, they do seem poised to inaugurate a new chapter in the history of hemophilia—a chapter of precision treatment, in which many roads lead to a life unencumbered by the threat of bleeding.
Conflict-of-interest disclosure
Patrick Ellsworth: Is an NHF-Takeda Clinical Research Fellowship award recipient.
Alice Ma: research funding and honoraria: Takeda.
Off-label drug use
Patrick Ellsworth: no off-label drug use has been discussed.
Alice Ma: no off-label drug use has been discussed.