Abstract
Up to two-thirds of menstruating women experience abnormal uterine bleeding (AUB) when treated with oral anticoagulants. However, the true prevalence of AUB for specific agents remains uncertain, as many of these episodes, while interfering significantly with quality of life and overall health, are not captured by definitions of major bleeding (MB) or clinically relevant nonmajor bleeding (CRNMB) used in clinical trials. A 2017 systematic review determined that women taking rivaroxaban, but not edoxaban or apixaban, had a twofold higher risk of AUB than women taking warfarin. Since then, new data have become available from extension trials, cancer-associated venous thromboembolism trials, pediatric trials, and a few observational studies specifically examining AUB as an outcome. Reported rates of uterine CRNMB were low (around 1%) and similar for rivaroxaban and apixaban in all these studies, and no episodes of uterine bleeding meeting MB criteria were reported. Rates of AUB not meeting MB or CRNMB criteria were much higher, affecting up to 50% of women on rivaroxaban. Only 1 such study included women on apixaban, and no AUB was reported. In pediatric trials, 19% of girls experienced menorrhagia when treated with rivaroxaban. In conclusion, rates of uterine MB and CRNMB were low in all studies, but rates of other types of AUB not meeting these criteria ranged from 15.8% to 50%. We conclude that AUB is underreported due to the limitations of MB/CRNMB criteria despite its substantial impact on quality of life. We urge future investigators to include broader definitions of AUB to better capture the impact of this outcome in menstruating women treated with oral anticoagulants.
Learning Objectives
Quantify the risk of abnormal uterine bleeding with rivaroxaban and apixaban
Manage a patient with a history of heavy menstrual bleeding and venous thromboembolism
Clinical case
A 22-year-old woman is found to have a pulmonary embolism 3 months after starting a combined oral contraceptive for the management of heavy menstrual bleeding severe enough to require a blood transfusion. She is concerned about a worsening of her menstrual bleeding with starting an anticoagulant. Which is the most appropriate oral anticoagulant to offer?
Discussion
“Abnormal uterine bleeding” (AUB) is defined as uterine bleeding that is excessive and/or occurs outside of the normal menstrual cycle and encompasses heavy menstrual bleeding/menorrhagia, intermenstrual bleeding, prolonged menstrual bleeding, or postmenopausal bleeding. Up to two-thirds of menstruating women experience AUB when treated with oral anticoagulants, which can, in turn, lead to premature discontinuation and increased risk of venous thromboembolism (VTE) recurrence.1 The majority of studies of anticoagulants define uterine bleeding as that meeting International Society on Thrombosis and Haemostasis definitions of major bleeding (MB) and clinically relevant nonmajor bleeding (CRNMB),2 which do not capture many forms of AUB. A 2017 review by Godin et al3 summarized existing data on AUB in women receiving direct oral anticoagulants (DOACs) for VTE and included uterine bleeding events from registry trials of apixaban, rivaroxaban, and edoxaban, including those reported only in product monographs. The authors concluded that women receiving rivaroxaban have a twofold increased risk of AUB (relative risk [RR], 2.10; 95% confidence interval, 1.64-2.69; P < .0001) as compared with those receiving vitamin K antagonists (VKAs), an increase not seen with apixaban or edoxaban (RR, 1.18; P = .37; RR, 1.26; P = .044, respectively). One observational study included in this review found that, although rates of AUB were similar in users of rivaroxaban and VKAs, women receiving rivaroxaban had significantly increased incidence of prolonged menstrual bleeding and intermenstrual bleeding. Women receiving rivaroxaban also had more medical or surgical interventions as a result of AUB and had more modifications of anticoagulant therapy than women receiving VKAs.3 Since the publication of this review, new data have become available from extension trials, cancer-associated VTE trials, and pediatric trials. The present review provides an update on the incidence of AUB in women treated with full and reduced doses of rivaroxaban and apixaban, the most commonly prescribed DOACs for women of childbearing age.
We conducted a literature review of the MEDLINE, PubMed, and Cochrane databases from the earliest available date until 18 May 2020 to retrieve any study in English reporting AUB as an outcome or adverse effect in women receiving rivaroxaban or apixaban for VTE. Newly published randomized controlled trials (RCTs) of rivaroxaban or apixaban for VTE were also included when MB and CRNMB events were reported by site, allowing the identification of uterine bleeds. Each abstract was screened by 2 reviewers.
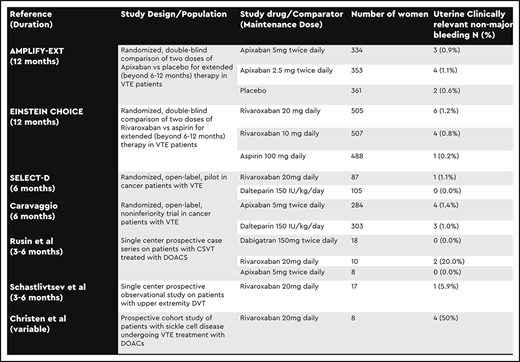
Our literature search identified a total of 11 new publications specifically addressing AUB in anticoagulated menstruating women. Seven did not include new data, and 4 did not include AUB outcomes specific to drug; thus, all were excluded. We identified 2 additional studies of apixaban and rivaroxaban for extended prophylaxis after VTE; 3 on cancer-associated thrombosis; and 5 prospective studies in unique VTE populations, including pediatric patients. Three publications did not report bleeding events by site, but the authors of one were able to provide us a detailed breakdown via personal communication. The remaining studies were excluded. Outcomes were defined differently between studies but included AUB, menorrhagia, uterine MB, and uterine CRNMB (Table 1).
Pivotal extension and cancer-associated VTE trials
The randomized, double-blind extension trials compared standard and reduced doses of apixaban (AMPLIFY-EXT)4 and rivaroxaban (EINSTEIN CHOICE)5 with placebo (apixaban) or aspirin (rivaroxaban) for the treatment of VTE beyond the initial 6 to 12 months. Uterine bleeds were reported as uterine MB (0%) or CRNMB (apixaban, 0.9% to 1.1%; rivaroxaban, 0.8% to 1.2%) (Table 2).4,5 Other types of AUB were not included as outcomes. In the 2 randomized, open-label cancer-associated thrombosis trials, standard dose rivaroxaban (SELECT-D)6 or apixaban (Caravaggio)7 was compared with low-molecular-weight heparin (LMWH). Outcomes included uterine MB (0%) and uterine CRNMB (apixaban, 1.4%; rivaroxaban, 1.1%).6,7
Observational studies
Three observational studies reported AUB in adult women receiving rivaroxaban or apixaban (Table 2). In a study of rivaroxaban in patients with upper extremity deep vein thrombosis, 1 (5.9%) of 17 women reported uterine bleeding classified as CRNMB, and none reported uterine MB events.8 In a study of patients treated with DOACs for cerebral venous sinus thrombosis, menorrhagia was reported in 2 (20%) of 10 women treated with rivaroxaban, whereas no AUB was reported in women treated with apixaban or dabigatran.9 In a study that included patients with sickle cell disease treated with rivaroxaban for VTE, 4 (50%) of 8 women reported menorrhagia.10 Neither of the latter 2 studies classified uterine bleeds as MB or CRNMB.
Pediatric trials
In the EINSTEIN-Jr phase 2 single-arm study of rivaroxaban in pediatric patients previously treated with LMWH, VKAs, or fondaparinux, 15.8% of girls in the 6- to 17-year-old age group reported menorrhagia.11 In the randomized, open-label, phase III study comparing rivaroxaban with heparin, LMWH, or VKAs, 19% of girls (ages 6-17 years) in the rivaroxaban group reported menorrhagia compared with 7.1% of girls in the control group (Table 3).12 No uterine bleeding events meeting criteria for MB or CRNMB were reported.
Conclusions
No uterine MB events were reported in any of the 10 studies included in this updated review. Reported rates of uterine CRNMB were overall very low (0% to 5.9%). Rates of menorrhagia or other types of AUB not meeting MB or CRNMB criteria, however, ranged between 15.8% and 50% in females treated with rivaroxaban and were higher than rates of bleeding in females treated with apixaban (0%) or LMWH/VKA (7.1%) when these groups were directly compared. This finding is congruent with the conclusion of Godin et al’s review.3 Though AUB in women receiving anticoagulants has significant effects on quality of life and can lead to early discontinuation of anticoagulation, we suspect rates are underreported because many such bleeds do not meet criteria for MB or CRNMB.
Rates of uterine MB or CRNMB in women in the extension RCTs who were receiving both full and reduced doses of rivaroxaban and apixaban were virtually identical (0.9% vs 1.1% for apixaban; 1.2% vs 0.8% for rivaroxaban). Once again, the overall low rates of uterine MB or CRNMB events, compared with the broader scope of AUB outcomes reported in observational studies, suggest that cases of AUB went unreported, and therefore a true difference may have been missed. In addition, because subjects in the extension RCTs had already completed 6 months of full-dose anticoagulation and were deemed eligible for longer-term anticoagulation, it is likely that any who experienced intolerable AUB in the early months of treatment were either successfully treated for it or were excluded from these studies.
This dramatic increase in rates of AUB when including broader definitions, such as menorrhagia, in addition to uterine MB or CRNMB events, is also a likely explanation for the substantially higher rates of AUB reported in the pediatric studies than in adult studies. An overall higher prevalence of AUB in adolescents due to anovulatory cycles likely also played a role.
Limitations of our review include, as discussed, inconsistent definitions used between studies (uterine MB and CRNMB only vs inclusion of menorrhagia). We were also unable to clearly define denominators of the population at risk (menstruating women), and therefore rates of AUB are, in general, underestimates. Our findings are consistent with those of Godin et al,3 suggesting increased rates of AUB with rivaroxaban vs apixaban. An RCT specifically assessing menstrual blood loss in patients receiving apixaban vs rivaroxaban (RAMBLE; NCT02761044) is currently enrolling patients, and 2 additional cohort studies of AUB in women receiving DOACs (NCT4477837, NCT03772366) will be enrolling patients soon. We hope that the results of these trials will provide more conclusive data. We strongly urge investigators of future RCTs of anticoagulants to evaluate broader definitions of AUB in addition to standard MB and CRNMB criteria to avoid an ongoing underestimation of the significance of this problem.
Graded recommendations
In menstruating women with an indication for oral anticoagulant therapy, we recommend taking a careful menstrual history both before and after initiation of anticoagulation (strong recommendation, very low certainty in the evidence of effects).
In menstruating women who are experiencing ongoing consequences of AUB, we recommend apixaban over rivaroxaban as first-line oral anticoagulant therapy for VTE (strong recommendation, moderate certainty of the evidence about effects).
In menstruating women without a history of AUB or with a successful, ongoing management strategy for AUB, we suggest apixaban over rivaroxaban as first-line oral anticoagulant therapy for VTE (conditional recommendation, moderate certainty of the evidence about effects).
Correspondence
Bethany T. Samuelson Bannow, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, OC14HO, Portland, OR 97239; e-mail: samuelsb@ohsu.edu.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.