Abstract
Inhibitor formation is among the most severe complications of hemophilia treatment. With a cumulative incidence of ∼30% in those with severe hemophilia A and ∼3% in those with severe hemophilia B, inhibitors are caused by a T-cell response directed against infused coagulation factor; these inhibitors neutralize factor VIII or IX activity and disrupt normal hemostasis. Inhibitor patients become unresponsive to standard factor treatment and, as an alternative, use bypass treatment (eg, recombinant factor VIIa or factor VIII inhibitor bypass activity). However, response to bypass agents is poorer and the burden of disease is higher, with greater morbidity, hospitalization, cost, and mortality, than in noninhibitor patients. Furthermore, inhibitor formation interferes with prophylaxis to prevent bleeding episodes and is a contraindication to gene therapy. Thus, more effective therapies for inhibitor patients are greatly needed. In the last several years, there has been an explosion of novel alternative hemostatic agents for hemophilia patients with and without inhibitors. These agents take advantage of technologic manipulation of coagulation factors and natural anticoagulants to promote hemostasis. The approaches include the following: (1) mutants or mimics of coagulation factors, rendering them resistant to natural anticoagulants; or (2) knock-down or disruption of natural anticoagulants, preventing degradation of coagulation factors. The purpose of this article was to review these novel alternative hemostatic agents and their mechanisms of action, as well as the preliminary pharmacokinetic, safety, and efficacy data available from early-phase clinical trials.
Learning Objectives
Describe novel alternative hemostatic agents in development
Describe coagulation proteins they target and their mechanisms of action
Review pharmacokinetic variables and safety
Describe efficacy in bleeding episodes, surgery, and prophylaxis
Discuss patients who might benefit from these agents
Introduction
Despite the gains made in improving the safety and half-life of coagulation factor concentrates for the treatment of individuals with hemophilia, problems remain. This scenario is particularly true for the ∼30% who develop inhibitors to factor VIII (FVIII), as inhibitors neutralize FVIII activity and disrupt normal hemostasis, requiring bypass therapy (eg, recombinant factor VIIa [rFVIIa] or factor VIII inhibitor bypass activity [FEIBA]). However, response to rFVIIa and FEIBA is less effective than standard factor in noninhibitor patients, resulting in 2-fold the hospitalizations, 10-fold the cost, and 3.5-fold the mortality of noninhibitor patients.1,2 Furthermore, inhibitors interfere with prophylaxis to prevent bleeding episodes and are a recognized contraindication to gene therapy. Thus, simpler, more effective treatment of hemophilia inhibitor patients is needed.
In the last several years, there has been an explosion of novel alternative hemostatic agents for patients with hemophilia A and B, with and without inhibitors. These hemostatic agents, many of which are in clinical trials, show potential to simplify treatment by reducing treatment frequency, invasiveness by subcutaneous administration, and immunogenicity by avoiding factor use. Furthermore, these agents promote hemostasis in inhibitor patients comparable to that in noninhibitor patients. The mechanisms by which these hemostatic agents promote hemostasis include the following: (1) mutants or mimics of coagulation proteins (eg, FVIII, factor V, FX) to render them resistant to natural anticoagulants; or (2) knock-down or disruption of natural anticoagulants (eg, AT, tissue factor pathway inhibitor [TFPI], activated protein C [APC]) to allow unopposed hemostasis. Some of these agents take advantage of the modulating prothrombotic effects observed in patients with coagulation factor mutations (eg, factor V Leiden) or in patients with deficiencies of natural anticoagulants (eg, antithrombin deficiency). These novel agents use unique technologies to promote hemostasis by interfering with physiological regulation of coagulation, including RNA interference, bispecific monoclonal antibodies, paired basic amino acid cleaving enzyme (PACE)/furin cleavage, super-active factor mutants, zymogen-like factor variants, and serpin mutants. For each novel agent, the present article reviews the mechanism of coagulation inhibition, pharmacokinetic variables, and, where available, safety and efficacy data from early clinical trials.
Mutants and mimics of coagulation factors
Bispecific antibody mimicking FVIII
FVIII is the target of the novel bypass agent known as ACE910 (Tables 1 and 2; Figure 1). Also known as emicizumab, ACE910 is a bispecific antibody that binds and bridges factor IXa and factor X, thereby mimicking their interaction with FVIII.3-6 In a phase 1 study in patients with hemophilia A, emicizumab, when given subcutaneously once weekly at doses ranging from 0.3 to 3 mg/kg per week, shortened the activated partial thromboplastin time and improved thrombin generation in a dose-dependent manner.7 Peak plasma emicizumab levels occurred at 12 weeks, with a half-life of 4 to 5 weeks. With improved thrombin generation, a dose-dependent reduction in the annualized bleed rate (ABR) was observed, with a 90% to 100% reduction in ABR in subjects receiving the highest emicizumab dose (3 mg/kg per week). Improvement in ABR occurred in both noninhibitor and inhibitor subjects, including those with high-titer inhibitors, up to 77 Bethesda units.6,7 Mild skin erythema at the injection site was observed in some subjects. In a phase 1, 2 trial of emicizumab in 18 subjects with hemophilia A aged 12 to 59 years, with and without inhibitors, once-weekly subcutaneous dosing resulted in sustained improvement in thrombin generation and a dose-dependent reduction in ABR, up to 100% in those subjects in the weekly high-dose arm (3 mg/kg). In subjects with hemophilia A receiving emicizumab prophylaxis, ABR was significantly lower than with FVIII; similarly, in subjects with inhibitors (hemophilia A-I) receiving emicizumab prophylaxis, ABR was significantly lower than with rFVIIa or FEIBA. In 1 subject, an appendectomy was performed without FVIII requirement. In addition to local injection site reactions, 5 subjects experienced thrombotic complications, 3 of whom developed thrombotic microangiopathy (1 died) and 2 of whom developed thromboembolism (1 with superficial thrombophlebitis and 1 with cavernous sinus thrombosis).7 All 5 subjects had received FEIBA concomitantly with emicizumab. A phase 3 trial of emicizumab prophylaxis in hemophilia A-I subjects is ongoing.
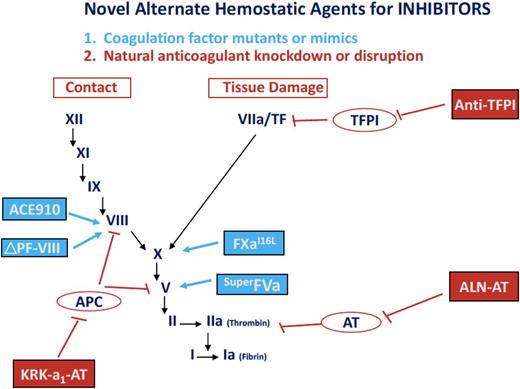
Novel alternative hemostatic agents for hemophilia. These hemostatic agents in early-phase trials in subjects with hemophilia A and B, with and without inhibitors, include the following: (1) coagulation factor mutations or mimics (eg, the bispecific monoclonal antibody mimicking FVIII, ACE910; a site-specific mutation of factor V, superFVa; an amino acid substituted factor Xa, factor XaI16→L; and the PACE/furin cleaved FVIII, FVIII ΔP/F); and (2) natural anticoagulant knock-down or disruption (eg, RNA targeting antithrombin, ALN-AT; an albumin-fusion inhibitor of TFPI, an anti-TFPI fusion peptide; and an APC-specific serpin, KRK α1AT).
Novel alternative hemostatic agents for hemophilia. These hemostatic agents in early-phase trials in subjects with hemophilia A and B, with and without inhibitors, include the following: (1) coagulation factor mutations or mimics (eg, the bispecific monoclonal antibody mimicking FVIII, ACE910; a site-specific mutation of factor V, superFVa; an amino acid substituted factor Xa, factor XaI16→L; and the PACE/furin cleaved FVIII, FVIII ΔP/F); and (2) natural anticoagulant knock-down or disruption (eg, RNA targeting antithrombin, ALN-AT; an albumin-fusion inhibitor of TFPI, an anti-TFPI fusion peptide; and an APC-specific serpin, KRK α1AT).
Super factor Va
Factor V is the target of the novel bypass agent known as superFVa. Factor V is physiologically regulated by APC, and another approach to promote hemostasis in inhibitor patients is to therefore reduce inhibitory regulation of factor V by APC. The superFVa protein was developed by the introduction of 4 unique mutations in factor V, including in 3 APC cleavage sites (R306, R506, and R679) and in the interchain disulfide bond H609C-E1691C, between the A2 and A3 domains of factor V.8,9 The resulting protein was shown to be resistant to APC cleavage. In hemophilia A mice, superFVa was comparable to rFVIIa in thrombin generation and clot lysis, and in blood loss correction after tail clip.10 SuperFVa was also synergistic with rFVIIa in reducing blood loss after tail clip: hemophilia A mice receiving 10 to 40 U/kg of superFVa exhibited dose-dependent reductions in blood loss, which further decreased with the addition of rFVIIa 1 to 3 mg/kg.11,12 Furthermore, when superFVa was given before rFVIIa, the rFVIIa dose could be reduced 10-fold to achieve similar thrombin generation. In vitro studies of plasmas from 2 hemophilia A inhibitor patients with titers of 32 to 64 Bethesda units exhibited improvement in thrombin generation and clot lysis after superFVa treatment of joint and muscle bleeding episodes, comparable to findings after treatment with rFVIIa.11 SuperFVa seemed to be safe, nonimmunogenic, and nonthrombogenic in animal models, and, in human plasma, the half-life was 4.9 hours.12 Based on these findings, a phase 1 clinical study is planned.
Zymogen-like factor X variant
Factor X, the primary substrate for coagulation by the extrinsic (TF-based) and intrinsic (Xase) pathways, is the target of a novel bypass agent, factor XaI16L. This FXa variant involves a single amino acid change at position no. 16 at the N terminus from isoleucine (I) to leucine (L), which decreases FXa sensitivity to AT degradation.13,14 Thus, factor XaI16L promotes hemostasis, allowing coagulation to bypass FVIII whether missing or neutralized by an inhibitor. In preclinical studies in hemophilia B mice, factor XaI16L increased thrombin generation, and, when given intravenously in escalating doses up to 450 µg/kg, factor XaI16L reduced tail clip blood loss by 69%.15,16 The FXa variant seemed to be safe, with only transient thrombin antithrombin complexes detected at the highest dose but no change in D-dimer, fibrinogen, or platelet levels.16 Subsequent in vitro studies in hemophilia subjects with and without inhibitors showed greater shortening in clotting time with XaI16L than with rFVIIa or FXa alone.17 There was a dose-dependent improvement in thromboelastography, which, in subjects with inhibitors, was further boosted by rFVIIa. These studies suggest the potential utility of factor XaI16L in the treatment of hemophilia inhibitor patients. A phase 1 study is ongoing.
PACE/furin cleaved FVIII
FVIII is targeted by another novel approach to promote hemostasis in inhibitor patients, the FVIII variant known as PACE/furin cleaved FVIII or FVIII-∆P/F. In this variant, there is a deletion of 4 amino acid residues (1645–1648) in the PACE/furin FVIII cleavage site, which renders FVIII resistant to degradation.18,19 In preclinical studies in hemophilia A mice, when the FVIII-∆P/F variant was delivered by AAV gene transfer, there was a fourfold improvement in FVIII expression and correction of the whole blood clotting time.20 The AAV–FVIII-∆P/F was safe, with no evidence of inhibitor formation. In preclinical studies in hemophilia A mice, when ∆P/F-FVIII variants 3 and 4 were given subcutaneously, blood loss was reduced after tail clip. FVIII antigen increased, as did FVIII pro-coagulant activity, the latter by twofold over that observed with standard B-domain deleted FVIII used in clinical practice.21,22 Despite the FVIII-∆P/F mutation, there was no increased immunogenicity in preclinical animal studies, but whether this outcome will be true in humans must await confirmation in clinical trials. The data suggest the potential for PACE/furin cleaved FVIII in the treatment of hemophilia A. Preclinical studies are ongoing.
Knock-down or disruption of natural anticoagulants
RNA targeting AT
A small interfering RNA, ALN-AT (fitusiran), targets and binds to AT mRNA in the liver. There it interferes with AT translation and blocks AT synthesis.23,24 Because AT is a regulatory natural anticoagulant targeting thrombin (factor IIa), ALN-AT blocks AT synthesis and interferes with thrombin breakdown, thereby promoting hemostasis. In a phase 1 study, weekly dosing in hemophilia A and B subjects of subcutaneous ALN-AT over a range of doses (15-45 µg/kg) led to a reduction in AT levels to 80%, which persisted for the period of treatment but was reversible after stopping treatment.25 When given monthly at 225 to 1800 µg/kg per month in subjects with hemophilia, with or without inhibitors, ALN-AT also led to improved thrombin generation and a dose-dependent reduction in bleeding, as measured by using ABR.26-28 Thrombin generation and bleed reduction were comparable between hemophilia subjects with and without inhibitors, and adverse effects were mild (primarily local erythema at the injection site).25-28 Phase 3 studies of ALN-AT for prophylaxis and patients with and without inhibitors are planned.
TFPI
Tissue factor (TF) activates the initial phase of coagulation after tissue damage via the extrinsic coagulation pathway. The major inhibitor of TF-initiated coagulation is endogenous TFPI. Thus, a novel strategy to promote hemostasis in patients with hemophilia is to inhibit TFPI by the novel monoclonal antibody, anti-TFPI, a single-chain polypeptide that inhibits TF-VIIa–initiated coagulation.29 TFPI is a Kunitz-type protease inhibitor consisting of 3 Kunitz domains (KD): KD-1, which binds TF-VIIa; KD-2, which binds factor FXa; and KD-3, which binds protein S.29,30 Several novel anti-TFPI monoclonal proteins have been developed that target 1 or more of these domains. These include a human anti-TFPI monoclonal antibody, BAY1093884, targeting TFPI KD1 and KD2; an anti-TFPI monoclonal antibody, NNC172-2021 (concizumab), which targets KD2; and an anti-TFPI monoclonal antibody, PF06746086, which targets KD1 and KD2.29-34 In preclinical studies, anti-TFPI, when given subcutaneously in doses ranging from 0.5 to 8.0 mg/kg, increased thrombin generation in hemophilia A patient plasma and reduced tail clip bleeding in the hemophilia A mouse.31 In phase 1 studies in hemophilia A and B patients given doses of concizumab from 250 to 9000 µg/kg intravenously or 1000 to 3000 µg/kg subcutaneously, plasma levels were detected for up to 43 days, with a half-life of 4 hours and suppression of TFPI levels for ≥14 days.31 The drug was well tolerated, with local injection site reactions and 1 episode of superficial thrombophlebitis that resolved spontaneously. An anti-TFPI targeting KD2 has shown improved clotting times in hemophilia A and B patient plasma samples and better rFVIIa generation by rotational thromboelastography (EXTEM clotting time) which mirror PT shortening.34 Phase 2, 3 clinical trials are planned in patients with hemophilia A and B, and in those with inhibitors.
APC-specific serpin
Another approach to bypass the Xase complex through inhibition of endogenous natural anticoagulants is the APC-specific serpin. APC, which plays a critical role in regulating factors Va and VIIIa through their proteolytic inactivation after physiological clot formation, is regulated by protein C inhibitor and α1AT. This target was selected because of the reduced bleeding severity observed in patients with hemophilia A who also have the factor V Leiden mutation and associated APC resistance. APC inhibitors, however, are inefficient and lack specificity. Thus, a set of mutations was generated in and around the P1-P1′ bond in protein C inhibitor to determine if more specific inhibitors could be developed. A novel variant, KRK α1AT (a Pittsburgh variant of α1AT), was found to have the best anti-APC serpin inhibitory profile, inhibiting APC and preventing degradation of factor FVa and factor FVIIIa.35 In preclinical studies, KRK α1AT, given intravenously at 7.5 to 15 mg/kg, promoted normal thrombin generation in vitro and restored in vivo hemostasis after intravital laser injury in hemophilia B mice. Based on its similarity to α1AT, KRK α1AT is anticipated to have a prolonged half-life, potential for subcutaneous administration, and low immunogenic potential. A phase 1 study is in development.
Conclusions
Novel alternative hemostatic agents are now in early-phase clinical trials. Developed to promote hemostasis in individuals with hemophilia A and B, with and without inhibitors, these agents seem to improve thrombin generation and reduce bleeding episodes in hemophilia patients with or without inhibitors. The technologic approaches by which these novel agents promote hemostasis include the following: (1) mutants or mimics of coagulation factors that render them resistant to degradation; and (2) knock-down or disruption of natural anticoagulants to block coagulation factor degradation. By these mechanisms, these hemostatic agents interfere with normal regulation of coagulation to promote hemostasis. Preliminary data suggest these alternative hemostatic agents have the potential to increase thrombin generation into the therapeutic range and to reduce frequency of bleeding episodes in both inhibitor and noninhibitor patients. Generally, adverse events have been mild local reactions, with no inhibitor antibodies and no antidrug antibodies, but the occurrence of thrombosis in a small number of subjects receiving concomitant FEIBA and emicizumab is concerning. These findings suggest the need for caution when using bypass agents with alternative hemostatic agents and, importantly, the need for studies to understand the mechanism. Requiring less invasive and less frequent dosing than standard factor, these novel agents have the potential to simplify treatment and improve outcomes for individuals with hemophilia with and without inhibitors.
Many questions remain. What is the long-term safety and efficacy of these novel alternative hemostatic agents in the treatment of acute hemorrhages? Should supplemental bypass with rFVIIa or FEIBA be avoided, given the potential for thrombotic complications? Will these hemostatic agents reduce immunogenicity and inhibitor formation by avoiding the need for standard coagulation factor treatment? Will these agents be safe in children, in those with acquired anti-VIII inhibitors or rare bleeding disorders, and will they be effective in surgery and in trauma? Finally, will monitoring be required, and, if so, by what assays? Clinical trials, ongoing and in development, should hopefully help to answer many of these questions.
Correspondence
Margaret V. Ragni, Department of Medicine, Division Hematology/Oncology, University of Pittsburgh, Hemophilia Center of Western Pennsylvania, 3636 Boulevard of the Allies, Pittsburgh, PA 15213-4306; e-mail: ragni@pitt.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from Sangamo, Shire, SPARK, Bayer, Bioverativ, Biomarin, Genentech/Roche, and Alnylam; and has consulted for and received honoraria from Shire, Bayer, Bioverativ, Biomarin, MOGAM, Alnylam, and Novo Nordisk.
Author notes
Off-label drug use: None disclosed.