Abstract
Iron deficiency is the commonest cause of anemia during pregnancy; however, its prevalence is highly determined by nutritional and socioeconomic status. Oral iron is the frontline therapy, but is often poorly tolerated. Awareness of the available intravenous formulations is essential for management. Before delivery, risk factors such as multiparity and heavy uterine bleeding increase the prevalence of iron deficiency and should be motivation for early diagnosis and treatment. Neonates born with iron deficiency have a statistically significant increment in both cognitive and behavioral abnormalities that persist after repletion, highlighting the need for heightened awareness of the diagnosis. A smartphone application providing information on nutrition and treatment is provided. New formulations of intravenous iron with carbohydrate cores, which bind elemental iron more tightly, minimize the release of labile free iron to allow complete replacement doses of intravenous iron in 15 to 60 minutes, facilitating and simplifying care.
Learning Objectives
To understand the prevalence of iron deficiency in pregnancy and the importance of recognizing the presence of iron deficiency without anemia
To understand the different laboratory tests currently available to secure a firm diagnosis of iron deficiency
To learn the methods of administration of the currently available intravenous iron formulations that can be safely administered to women in the second and third trimesters of pregnancy and to those with heavy uterine bleeding and iron deficiency
Introduction
Iron deficiency is known to be among the commonest nutritional deficiency states worldwide, with physical sequelae and symptoms depending on duration and severity. Although countries with chronic malnutrition have a high prevalence of iron-deficiency anemia (50%-80%), iron-deficiency states without anemia are frequently found in countries with normal nutrition (prevalence up to 20%).1 Successful iron therapy depends, on one hand, on correct diagnosis and, on the other, on the choice of effective iron formulations and treatment of the cause. Women naturally have a much higher prevalence of iron deficiency than do men. Women with regular periods have a prevalence of iron deficiency that is about 10 times higher than do men of the same age. Additionally, it can be shown that among blood donors it is nearly always women who have absent iron stores. The reason for this is regular blood or iron loss during menstruation with concomitant insufficient intake.2,3
Gynecologic causes of iron deficiency include recurrent hypermenorrhea, menorrhagia, or metrorrhagia. They all come under the heading of heavy uterine or menstrual bleeding. It has long been known that the incidence of anemia significantly increases with increasing menstrual blood loss. If menstrual blood loss is 61 to 80 mL per cycle, the frequency of anemia is 10.3%, and it increases up to 50% with menstrual blood loss between 151 and 240 mL. Published evidence reports that with one definition of iron deficiency (hemoglobin <12 g/dL and a ferritin level <16 ng/mL), the prevalence of iron-deficiency anemia is 0% with blood loss of <20 mL; if blood loss is 60 to 80 mL, the prevalence is 17%, and with blood loss >100 mL the prevalence is 26%.
Gynecologic bleeding caused by diseases such as adenomyosis, uterine fibroids, or endometrial hyperplasia frequently results in considerable iron-deficiency anemia. In registration studies for a hormonal treatment of heavy uterine bleeding, in the baseline groups of 231 patients, a mean blood loss of 640 mL was demonstrated by using the very specific alkaline-hematin method in a 90-day reference period (WHO). The amount of blood loss correlated well with low hemoglobin and ferritin levels.2-4
Pregnancy and puerperium
Anemia in pregnancy and the puerperium is associated with increased maternal and fetal morbidity and mortality, depending on severity and comorbidity. It is among the commonest risk factors in obstetric and perinatal medicine (Table 1). According to the WHO, the estimated worldwide prevalence of anemia in pregnancy is 30% to 50%, depending on geographic area. The incidence of iron deficiency without anemia is not known. Especially in developing countries, postpartum anemia continues to be among the commonest causes of death in women who have just given birth. In Europe, approximately 10% of women in the puerperium have moderate-to-severe anemia, and adequate treatment of which without recourse to allogeneic blood is a current problem.5-7
Symptoms of iron deficiency
Symptoms of iron deficiency cause women considerable suffering and are the main reason for the use of iron therapy. Symptoms of iron deficiency, including fatigue, headache, hair loss, poor concentration, pagophagia or other forms of pica, restless legs syndrome, and reduced physical performance, are in general indications for intervention. More severe symptoms can include decreased physical and work capacity, increased cardiovascular stress (tachycardia, hypotension), reduced thermoregulation, and increased susceptibility to infection. Among other things, a consequence of the iron deficiency in various enzyme systems, such as oxidoreductases, mono-oxidases, dioxygenases, and especially decreased mitochondrial activity in the body cells,1,2,8-10 may lead to symptoms independent of anemia. Although traditionally this has been referred to as nonexchangeable iron, placebo-controlled studies have shown a positive influence of iron administration on symptoms independent of anemia. In this context, the effect of iron does not correlate directly with the amount of iron administered or the ferritin level.11,12 It is important to note that certain symptoms, such as fatigue, may only suggest but not prove iron deficiency. People without iron deficiency may have the same degree of fatigue as people with iron deficiency. The specificity of the symptom “chronic fatigue” for iron deficiency (ferritin < 15 µg/L) is only 20%. Thus, if iron deficiency is suspected as the cause it always needs laboratory conformation.13
Consequences of iron deficiency in pregnancy
In gravidas, tolerance to peripartum blood loss is greatly reduced.2,5,14,15 Mortality increases with worsening anemia. Depending on the severity of iron-deficiency anemia, mortality is increased. Reasons for this include an increased rate of cardiovascular insufficiency, a higher risk of hemorrhagic shock, higher infection rates in the puerperium, and poorer wound healing. Maternal morbidity is linked to factors such as socio-economic status, availability of medical care, and nutritional state. A problem interpreting available published evidence is that maternal and fetal outcomes are related to the severity of the anemia but not to the duration and time of first appearance of anemia or the duration and time of onset of iron deficiency. Taking this limitation into account, some authors postulate a correlation between maternal mortality and degree of anemia, but there are no supporting prospective studies, and it is unclear what the critical hemoglobin level is in relation to maternal mortality (Table 2).
There are currently no studies on the relationship between iron-deficiency anemia before pregnancy and subsequent outcomes, and there are no prospective studies in large cohorts that show the effect of early intervention and treatment of anemia on maternal, fetal, and neonatal outcome5,16-18 (Table 3).
Diagnostic principles
The diagnosis and consequent management of anemia in pregnancy are based on the differentiation between the relative or physiologic anemia of pregnancy due to increased plasma volume and “true anemia” with its different pathophysiological causes. When defining the cutoff value for anemia in pregnancy, the degree of changes in plasma volume at varying gestational age must be taken into account. Accordingly, hemoglobin concentrations <11.0 g/dL in the first and third trimester and <10.5 g/dL in the second indicate possible anemia, which requires further investigation.19 The pathogenesis of anemia in pregnancy is multifactorial. It is not enough simply to diagnose anemia based on the hemoglobin concentration; rather, the causes should always be investigated.
Various etiological factors, which are accompanied by reduced hemoglobin synthesis, increased hemoglobin breakdown, or hemoglobin loss, need to be considered in the differential diagnosis. A not uncommon example is the combination of decreased hemoglobin synthesis and increased cell death seen in thalassemia syndromes, a result of which may complicate diagnosis and treatment. The most important differential diagnoses include iron-deficiency anemia and its precursors (to which often too little attention is paid), hemoglobinopathies (thalassemias, sickle cell anemia), anemia of infection, and anemia of chronic kidney disease.7,20
A thorough history and clinical examination of the pregnant woman often lay the foundation for correct diagnosis. The current gold standard for detecting iron deficiency is still the serum ferritin level. However, because of its acute phase reactivity, it may be spuriously elevated, missing the diagnosis. If the serum ferritin is not below the lower limit of normal, the percentage of transferrin saturation remains the most reliable indicator of iron need. The reticulocyte hemoglobin content, when routinely available, promises to facilitate the diagnosis of iron deficiency even further.15,21,22
Work-up beyond the complete blood count
Measurement of the serum ferritin level has the highest sensitivity and specificity for detecting iron deficiency. Ferritin levels of <20 ng/mL are diagnostic of iron deficiency, regardless of the hemoglobin concentration. Ferritin levels between 20 and 50 ng/mL are regarded as a gray area. If ferritin levels are within the normal range (>50 ng/mL), iron deficiency anemia can be virtually ruled out, unless a concomitant active infection or other inflammatory process is present.
In this situation and in inflammatory reactions even postoperatively, the ferritin levels may yield a false-normal result, because apoferritin is, like C-reactive protein (CRP), an acute-phase protein. The serum ferritin level correctly represents the iron stores ∼6 weeks after surgery or childbirth. If the concomitant presence of iron deficiency and anemia is suspected, the presence of infection or inflammation must always be excluded by using the sedimentation rate or CRP. In special cases as outlined below, iron investigations may be supplemented by various parameters, such as serum transferrin receptors, ferritin index, zinc protoporphyrin, and percentage of hypochromic red cells.9,22
Serum iron, transferrin, transferrin saturation
In most cases, assay of serum iron and transferrin levels (usually represented by the total iron binding capacity) does not provide any additional benefit to the investigation of iron deficiency, even in pregnancy, since the serum iron levels are subject to many influences such as diurnal, intra-individual and inter-individual variations. Conclusions for iron need are possible only in combination with transferrin levels by determining the percentage of transferrin saturation.
If the ferritin levels are within the normal range, but the transferrin saturation is less than 15%, this is indicative of latent iron deficiency since iron is being released in higher quantities from circulating transferrin to maintain erythropoiesis. However, it needs to be remembered that fluctuations in serum iron levels also affect calculation of transferrin saturation and thus may lead to incorrect interpretations.23 Therefore it is recommended that the sample be drawn after an overnight fast as dietary iron may influence the percent transferrin saturation.
Transferrin receptors
Various studies have shown that the soluble transferrin receptor (sTfR) is a sensitive and specific indicator of change in iron kinetics. It increases in iron deficiency or if there is an increased cellular iron requirement. Transferrin receptors are probably not affected by infections and thus represent a useful addition to ferritin assays. The first studies in pregnancy have already been conducted, and low sTfR levels in early pregnancy appear to be associated with inhibited erythropoiesis in the first trimester. The increase in sTfR during pregnancy is attributed to an increasing stimulation of erythropoiesis and an increasing iron requirement by iron-dependent cell proliferation. It is not known whether inhibited erythropoiesis at the start of pregnancy negatively influences detection of concomitant iron deficiency through measurement of sTfR. There is nothing to suggest that the sTfR concentration is influenced by inflammatory reactions. Thus, this parameter would also be useful for the investigation of unclear situations in pregnancy (normal ferritin in the presence of an elevated CRP) and in the early puerperal phase. In our own studies, we showed that after labor sTfR concentrations are not influenced by the inflammatory reaction at birth, in contrast to ferritin levels20 (Table 4).
Prevention and treatment of iron deficiency in nonpregnant women
Ideally, a woman can compensate for iron losses through adequate dietary intake. Iron intake in the diet depends on the iron content of food, amount ingested, and absorption of iron in the intestine and subsequent bioavailability. Foods high in iron, such as meat, contain up to 2 mg/100 mg, but absorption in the intestine ranges from 1% to 20%, depending on whether the food is of animal or vegetable origin. A daily requirement of 2 mg iron per day is covered by 300 g meat/fish. For vegetable sources, lacking heme iron, requirements are higher (1000 g soya beans or 5000 g spinach). Therefore, compensating for higher iron losses through diet is unrealistic with existing eating habits and quantities.8,12,14 The fact that the population’s knowledge about iron content of food is usually very low, with most unaware of which foods are actually high in iron and how much they should consume, motivated us to develop a smartphone application (MyIronfriend), which aims to help women with this issue and to provide guidance for daily shopping (www.myironfriend.com).
Both oral iron preparations (tablets or drops/syrup) and intravenous iron formulations can be used for treatment. Oral iron preparations are available as Fe II salts or Fe III complexes, with the absorption being between 1% and 8% depending on composition. An 80-mg tablet/d corresponds to just under 8 mg iron absorption/d. It has been shown in intervention studies (oral iron vs placebo) that even daily doses of 20 mg Fe II salts result in a significant improvement in symptoms. As the dosage increases, the gastrointestinal side effects of oral iron increase because of the toxic oxidative effect of iron in cells.24 This usually occurs at daily doses >100 mg/d, which results in a reduction in adherence. Irrespective of the preparation, almost 20% of women stop oral iron therapy. In general, iron (III) complexes show better gastrointestinal tolerability but are absorbed to a lesser extent.25 Supporting this conclusion is a meta-analysis of randomized trials of oral iron in which 70% of those to whom it was prescribed reported significant gastrointestinal perturbation.26
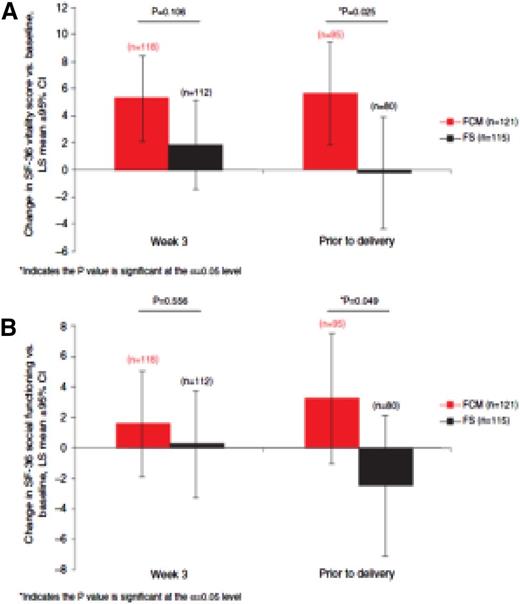
Iron deficiency states that do not respond to oral iron should be treated with intravenous iron (iron sucrose, ferric carboxymaltose [FCM], iron dextran, iron gluconate). In gynecology, most experience has been with iron sucrose. However, there exists a growing body of evidence with the use of FCM and low-molecular-weight iron dextran (LMW ID). A recently published international, open-label, randomized controlled trial compared FCM with oral iron and randomly assigned 252 women with ID at 16-33 weeks gestation to FCM or oral iron. The primary objective was efficacy, with safety and quality of life secondary. Significantly more in the intravenous iron group achieved anemia correction (hemoglobin > 11.0 g/dL; 84% vs 70%; odds ratio, 2.06; 95% confidence interval, 1.07, 3.97; P = .031) and within a shorter time frame (median, 3.4 vs 4.3 weeks). Compared with oral iron, FCM improved vitality (P = .025) and social functioning (P = .049) before delivery (Figure 1).
Women taking oral iron reported higher rates of gastrointestinal disorders (16 vs 3). No newborn morbidity was noted. The authors concluded that during late-stage pregnancy, intravenous, and not oral, iron is the appropriate first-line option for rapid and effective anemia correction, with additional benefits for vitality and social functioning.27
LMW ID has been compared with FCM with equivalent safety and efficacy.28 Two recent publications support the safety and efficacy of 1000 mg LMW ID in 1 hour.29 Based on all credible published evidence, there is no difference in safety or efficacy among the available listed formulations.
However, as a result of much less tight binding of the elemental iron to the carbohydrate carrier by iron sucrose and ferric gluconate, multiple visits are required to accomplish what a single infusion of LMW ID or FCM can do in 15-60 minutes. Consequently, we prefer the latter for convenience and cost to patients, physicians, and ancillary personnel.
The rate of adverse reactions is approximately 1% to 5%, which include dizziness, flushing, pressure in the chest or back, limb pain, and flu-like symptoms. Severe allergic reactions with any of the formulations are extremely rare, occurring in <1:250 000 administrations. Extravasation must be avoided during parenteral iron administration because intravenous iron causes persistent skin discoloration.30-32
Sustained iron therapy
Iron therapy is useful only if the cause of the iron deficiency or iron loss is simultaneously treated. Thus, a woman may once again have absent iron stores a few months after intravenous iron therapy if the sources of loss are not corrected at the same time. Vegetarians who menstruate and consume little iron in their diet are not able to replenish or maintain replenished stores. Women with periodically high iron losses (menstruation, blood donors) or consumption (competitive sportswomen because of increased loss in extreme exercise, pregnant women) are similarly unable to maintain stores.
Prevention and treatment of iron-deficiency anemia in pregnancy
The treatment of anemia is directed at its cause and severity. Maternal and fetal risk factors that may be complicated by anemia need to be considered. The time period available for treatment of anemia before childbirth influences the choice of intervention.
Later complications, such as preterm labor and peripartum bleeding, can be prevented by proactive intervention. Such intervention is especially important given the known delay in growth and development and statistically significant increments in cognitive and behavioral abnormalities, which persist after treatment, in infants born iron deficient.33 The administration of allogeneic blood should be a last resort in pregnant women and those in the puerperium. The availability of safe, well-tolerated parenteral iron formulations able to be administered as a complete replacement dose in 15-60 minutes facilitates treatment. Rarely, recombinant erythropoietin may be necessary in extreme cases.7,34
Prevention
There are 3 ways to improve the body’s iron status: reduce losses, limit consumption, and increase intake. Depending on preexisting stores immediately after pregnancy, sufficient intervals before further pregnancies may be used for replenishment. In the puerperium, worthwhile strategies to reduce or avoid major blood loss make a contribution at the time of birth. In pregnancy, however, the only option is pharmacologic supplementation.
Pharmacological supplementation
The benefit of preventive iron administration, regular administration of iron preparations without actual knowledge of iron stores, continues to be a subject of debate. This approach is recommended and practiced in industrialized countries. Critical arguments against it include the lack of evidence that worldwide prevalence of iron deficiency and the consequences of anemia are reduced and possible harmful effects of nonselective iron administration on the mother. According to the last update to the Cochrane Database, there is no scientific or medical justification for prophylactic iron administration in pregnancy in countries with adequate nutritional resources because epidemiological data do not show any positive effect on the course of pregnancy and/or maternal and fetal outcome.35 In terms of hematological data (ferritin levels, hemoglobin), randomized, placebo-controlled trials do show a positive effect. Women with low iron stores at the start of pregnancy develop anemia less often if they receive iron supplementation. In contrast, it has been shown that iron supplementation in countries with a high prevalence of iron-deficiency states is indeed justified.10,36,37 It is unclear whether studies on iron supplementation in developing countries can be extrapolated to industrialized countries. The correct dosage for pure prophylactic administration is also unclear. Current guidelines on prophylactic iron supplementation are 60-120 mg elemental iron/d.36 Lower dosages appear to be less effective. At dosages of ≥120 mg/d, adverse reactions increase and adherence is poor.23,38 Because women with low iron stores benefit most from prophylactic iron administration, selective iron administration in pregnancy would be the optimum solution. This presupposes investigating iron stores because the hemoglobin concentration usually measured in isolation shows a poor correlation with iron stores. The ferritin level must be used for this.
Treatment of iron-deficiency anemia in pregnancy
The preceding sections clearly support treatment of iron-deficient states. Change or worsening over time cannot be predicted even for mild forms of anemia. In deciding on the method of treatment, various factors such as time until birth, severity of anemia, additional risks, especially maternal comorbidities, and patients’ wishes should be taken into account.
A Jehovah’s Witness with severe anemia requires a different approach compared with a woman who has moderate anemia in the second trimester without additional risk factors. The current options for the treatment of pregnancy-related iron-deficiency anemia are oral iron, parenteral iron, rarely in severe cases stimulation of hematopoiesis by using human recombinant erythropoietin, and administration of allogeneic blood.
Oral iron
Although oral iron remains frontline therapy, 70% of those to whom it is prescribed report significant gastrointestinal side effects.26 Worsening constipation is an especially difficult problem given the high incidence of constipation due to high progesterone levels, which slow bowel transit worsened by the enlarging gravid uterus pressing posteriorly on the rectum. Additional adverse events, such as metallic taste and gastric cramping, further decrease adherence. Nonetheless, when tolerated, oral iron is effective, easy to obtain, and inexpensive. It is unclear whether weekly or intermittent administration of oral iron is equivalent to daily administration. A recent study reported increased hepcidin levels after iron intake with subsequent incremental decrements in absorption with increasing doses.39 Hepcidin, the hepatic-synthesized iron regulatory protein, decreases iron absorption by inactivating ferroportin, the only known export protein from intestinal epithelial cells. Further confirmatory studies on this issue are currently underway. The ideal dosage for intermittent or weekly administration is unknown because the proportional absorption is inversely proportional to the administered dose. Dosages between 100 and 200 mg daily are a compromise in relation to the hemoglobin increase and tolerability of iron. The recommended dosage is 80-160 mg elemental iron/d.
If there is a good response to oral iron, reticulocytosis occurs within 3 to 5 days and increases until 8 to 10 days after treatment. The hemoglobin increase follows after a delay and is, at best, approximately 0.2 g/dL per day or approximately 2.0 g/dL within 3 weeks. Once the hemoglobin levels have returned to normal, oral iron should be continued for at least another 4 to 6 months until a target ferritin level of approximately 50 ng/mL and a transferrin saturation of at least 30% have been reached.34,35,40
Gastrointestinal side effects, such as constipation, heartburn, metallic taste, and nausea, which occur in up to 70% of patients, limit the dose and are a key disadvantage of oral iron preparations (Table 5). In this case, the dose must be either reduced or switched to a different formulation. Unfortunately, no formulation has shown superiority over another in prospective studies. As a result, adherence remains a problem. It has been shown that only 36% of pregnant women regularly take oral iron despite being specifically instructed about the problem of iron deficiency. Studies from countries such as Tanzania and Indonesia also show adherence rates of only 36% to 42%1 (Table 6).
Parenteral iron formulations
Parenteral iron is the most important alternative to oral iron because it circumvents the natural mechanism of iron absorption via the intestine and facilitates transferrin saturation. Free iron is toxic because it promotes the formation of hydroxide and oxygen radicals, which in turn result in cell and tissue damage via peroxidation. Newer formulations, which bind elemental iron more tightly to the carbohydrate core, limit the amount of labile free iron released, minimizing reactions. This conclusion is supported by the absence of serious adverse events in published studies extant.41
In a meta-analysis comprising more than 10 000 patients who received intravenous iron, although minor infusion reactions were observed, serious adverse events were not increased compared with any comparator, including placebo.42 Gastrointestinal toxicity was rare. No difference in safety or efficacy among the available formulations was reported. Independently from the type of iron complex, all present studies that compare intravenous iron formulations with oral iron in pregnancy show advantages for parenteral iron regarding hemoglobin and ferritin increase during pregnancy and before and after birth. The currently available formulations are shown in Table 7.
Most studies in pregnancy and postpartum were performed with intravenous iron sucrose complex (Venofer; Vifor Int., Switzerland). It was shown that iron sucrose complex is safe and effective in pregnancy and postpartum, and the side effect profile is better than it is for oral iron. In 6 studies, the hemoglobin increase in 28 days ranged from 1.3 to 2.5 g/dL compared with 0.6 to 1.3 g/dL in the oral iron groups.7,43-45 However, because the sucrose moiety binds elemental iron less tightly, doses between 200 and 300 mg are proscribed.46
Recently, in Switzerland, iron sucrose complex has been substituted by FCM complex (Ferinject; Vifor Int., Switzerland or InjectoFer; American Regent/Luitpold). The carbohydrate moiety binds the elemental iron more tightly, allowing high dosages up to 1000 mg/administration over a short period (15 minutes). It has been shown that FCM does not cross the placenta in a placenta-perfusion model. In a retrospective study on 103 patients, comparing data from pregnant anemic women who were treated with either iron sucrose or FCM, FCM was reported to have fewer side effects than iron sucrose, despite a considerably higher single dosage.47
Several studies have shown safety and effectivity of FCM in pregnancy and postpartum, and the largest randomized multicenter study (Ferrric Carboxymaltose Assessment of Anemia Therapy and Safety in Pregnancy [FER-ASAP]) comparing ferric carboxymaltose with oral iron in the treatment of iron deficiency anemia was recently published.27,48,49 Other parenteral iron preparations, such as iron polymaltose (Ferosig; Ferrum Hausmann), iron gluconates (Ferrlicit), and LMW ID (INFeD or CosmoFer) have been studied in various gynecologic settings. Although the numbers of treated patients are low, no severe or anaphylactic reactions were reported. A recent study with iron polymaltose in postpartum women showed a significant positive effect of intravenous iron on quality of life indices in the treated women and an ongoing positive effect on iron stores over a longer period after birth.50,51 In a recent publication, 189 oral iron–intolerant gravidas in the second and third trimesters who received 1000 mg LMW ID in 1 hour reported similar efficacy and safety with no serious adverse events observed.29
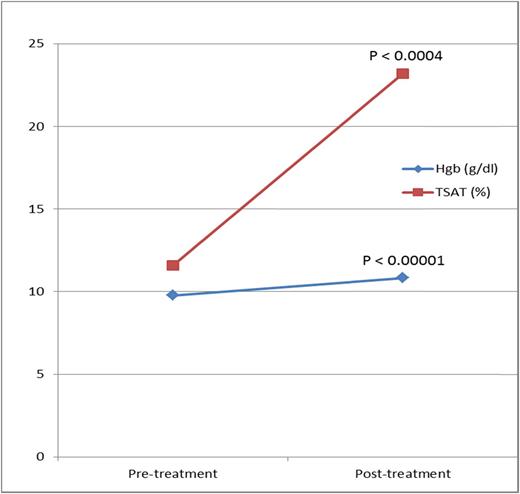
Supporting these data, the first prospective study with intravenous iron in gravidas ever performed in the United States, also with LMWID, in 74 oral iron–intolerant iron-deficient gravidas reported nearly identical efficacy and safety (Figure 2).52
Change in hemoglobin and TSAT. Reprinted from Auerbach et al52 with permission.
Change in hemoglobin and TSAT. Reprinted from Auerbach et al52 with permission.
Conclusion
Iron deficiency in nonpregnant and pregnant women requires treatment. Oral iron remains the frontline therapy but is rife with gastrointestinal side effects. Published evidence supports early intervention with intravenous iron when oral iron is poorly tolerated or ineffective. Because there are no first-trimester safety data, it should not be given until the second trimester. The preponderance of published evidence suggests intravenous iron is effective and safe in nonpregnant women and in pregnancy with no serious adverse events reported in numerous published studies, supporting moving intravenous iron closer to the front line.
Correspondence
Christian Breymann, Obstetric Research–Feto Maternal Hematology Unit, University Hospital Zurich, 8091 Zurich, Switzerland; e-mail: breymann@ggs-zh.ch.
References
Competing Interests
Conflict-of-interest disclosure: C.B. has consulted for and received honoraria from Vifor International Company. M.A. has received research funding from AMAG Pharma, Pharmacosmos, and Luitpold and has consulted for AMAG Pharma and Pharmacosmos.
Author notes
Off-label drug use: The total dose infusion of intravenous iron, which is off label in the United States but not in Europe.