Abstract
The development of the oral tyrosine kinase inhibitors (TKIs) to treat chronic myeloid leukemia (CML) is one of the great triumphs of cancer research. Although the efficacy of TKIs has dramatically improved the disease-specific overall survival rate, the prevalence of CML is increasing worldwide. Currently, CML patients receive prolonged (even lifelong) treatment, and over the last decade, clinical decision making has become challenging. Therefore, consideration of the effects of TKI therapies on patients’ quality of life (QoL) and symptom burden (ie, patient-reported outcomes [PROs]) is now critical to more robustly inform patient care and improve health care quality. Over the last 5 years, a number of studies have generated valuable PRO data, for example, on long-term QoL effects of imatinib therapy or symptom burden of patients switching from imatinib to second-generation TKIs. PRO findings are important, as they provide a unique patient perspective on the burden of the disease and treatments effects. We will review main evidence-based data on the use of PROs in clinical research and highlight the importance of methodological rigor of PRO assessment. Also, we will describe the potential value of using PRO assessment in routine clinical practice, for example, to facilitate timely management of side effects. Areas for future research will also be discussed.
Learning Objectives
List main evidence-based data on the impact of tyrosine kinase inhibitors on patients’ quality of life and symptom burden
Describe the value of quality of life assessment in CML clinical research
Illustrate the importance of integrating quality of life assessment into routine practice and the relationship between quality of life assessment and adherence to therapy
Introduction
After the introduction of imatinib and other tyrosine kinase inhibitors (TKIs), the prognosis of chronic myeloid leukemia (CML) has improved dramatically. The pivotal phase 3 International Randomized Study of Interferon versus STI571 (IRIS) trial has landmarked the treatment of this disease and documented that patients diagnosed with chronic-phase (CP) CML and treated with imatinib have an 8-year overall survival (OS) of 85% and freedom from progression to the accelerated phase (AP) or blast crisis (BC) of 92%.1
Since imatinib was approved in 2001, the therapeutic armamentarium for CML has been enriched by other TKIs such as nilotinib, dasatinib, bosutinib, and ponatinb. While some of the adverse events (AEs) associated with these TKIs are common to all, such as nausea and fatigue, others are more specific to a given drug. In any case, there is evidence indicating that the occurrence of AEs tends to decrease over time and that severe AEs are not common in all TKIs.
The external validity of results from randomized controlled trials (RCTs) introducing TKIs in CML treatment has been confirmed by long-term survival improvement documented by large, single-institution experiences and population-based studies.2 Long-term survival of CP-CML patients who were in stable disease remission (complete cytogenetic response [CCyR]) and in treatment with imatinib was shown to be not statistically different from that of the general population.3
Presently, published evidence-based guidelines for the management of CML recommend initial treatment with any frontline-approved TKI (ie, imatinib, nilotinib, or dasatinib) and lifelong TKI treatment in cases of optimal response in routine practice. However, the results of the registration studies for frontline approval of nilotinib and dasatinib (ENESTnd [Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients] and DASISION [Dasatinib Versus Imatinb Study in Treatment-Naive Chronic Myeloid Leukemia Patients], respectively) have shown that second-generation TKIs promote faster and deeper responses (major molecular response and CCyR) than imatinib, but this did not translate into statistically significant improved long-term OS.4,5 This scenario broadly illustrates why selection of TKI treatment has become a challenge and should involve the consideration of several factors, including comorbidity, toleration of therapy, and disease characteristics (ie, prognostic factors).6
The importance of patients’ self-reported data in oncology: QoL and other patient-reported outcomes
Health status information obtained through patient self-reporting provides clinically relevant information that cannot be captured by standard laboratory or clinical measures typically used in oncology. For example, there is convincing evidence indicating the independent prognostic value of such information for clinical outcomes (ie, survival) across several cancer populations with solid tumors.7
Integrating the patient’s view in cancer care is typically achieved using standardized questionnaires that are to meet several psychometric characteristics, including validity, reliability, and responsiveness.8 These questionnaires may range from single-item tools assessing a narrow health domain to broader multidimensional constructs such as quality of life (QoL). Examples of widely used questionnaires to assess QoL in oncology are the EORTC QLQ-C30 (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30)9 and the FACT-G (Functional Assessment of Cancer Therapy – General).10 In general, instruments devised to assess QoL include a mix of items and scales that evaluate both symptoms and more general aspects of the patient’s life such as physical, social, and emotional functioning. However, there are also other questionnaires that are meant not to assess such broad aspects of the patient’s life but to focus on narrower health concerns, such “fatigue” and “symptom burden.”
The US Food and Drug Administration (FDA) has recently introduced the term “patient-reported outcomes” (PROs), which can be considered an umbrella term encompassing several different constructs. According to the FDA, PRO is defined as a measurement of any aspect of a patient’s health status that comes directly from the patient, without the interpretation of the patient’s responses by a clinician or anyone else.8 Therefore, regardless of the construct being measured, the setting of application, or the specific questionnaires used, the most important aspect is that this information has to be collected from patients themselves. In this article, we generally refer to PROs, as we discuss not only studies dealing with QoL but also other types of patient self-reported health status information.
Why do we need PRO assessment in CML clinical research?
We have thus far accumulated a wealth of clinical and laboratory data for patients with CML treated with TKI, and the choice of the therapy traditionally has been based on efficacy and safety (physician-reported) criteria.
However, in the current CML arena, dominated by targeted drugs that are to be administered lifelong, it becomes essential to obtain information on patients’ QoL to more comprehensively assess treatment effectiveness.11 This information, however, cannot be obtained or inferred by any other type of standard measure traditionally collected in clinical research, such as the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) reporting system. The CTCAE system has been the gold standard for comparing toxicities of drugs in RCTs, and it has been critical for informing on the potential harm of various drugs on a patient’s life. Many of the CTCAE-coded toxicities are biochemical or laboratory abnormalities (eg, QT prolongation, anemia, and neutropenia) that can be recorded objectively, as they broadly rely on laboratory examinations. However, there is also another category of AEs (albeit a minority of the full list of CTCAE) that can be considered as symptomatic toxicities (eg, sensory neuropathy, nausea, fatigue, pain, and diarrhea) that are experienced by patients but whose grading as well as reporting in medical files is performed by clinicians.
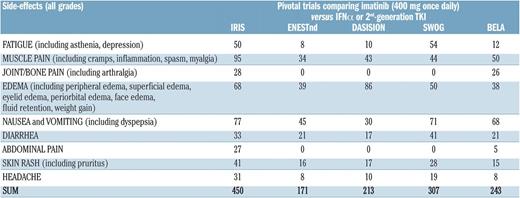
Over the years, a substantial body of evidence has accumulated regarding the limitations of the current practice of documenting this latter category of AEs across various cancer populations.12 For example, there are important discrepancies between clinicians and patients in the way symptoms are reported. Also, there is evidence indicating that clinicians may miss a large proportion of patients’ symptomatic AEs in clinical trials.12 Specifically in CML research, we note that even in settings in which one would expect that side effects be collected in the most rigorous way (ie, registrative RCTs), extensive variation exists in their reporting, raising some concerns regarding the extent to which they reflect the actual patient burden.13 For example, by comparing data from pivotal RCTs in newly diagnosed CP-CML patients treated with 400 mg/d imatinib,14-18 we note that the reported proportion of patients with a given side effect (any grade) is not consistent across studies (Figure 1).
Percentage of newly diagnosed CP-CML patients who reported the listed side effects of imatinib. The data are from 5 prospective, company-sponsored, Good Clinical Practice, contract research organization–monitored studies testing imatinib vs interferon-α plus low-dose arabinosyl cytosine (IRIS)15 vs nilotinib (ENESTnd)16 vs dasatinib (DASISION and SWOG)14,17 and vs bosutinib (BELA).18 In the original reports, the figure represented the proportion or percentage of patients reporting each side effect. In all studies, the sum of the figures was >100%, because many patients reported >1 side effect. The differences among each side effect underscore the variability in collecting and reporting side effects, although all patients were treated frontline with the same dose of imatinib (400 mg/d). The differences among studies are quite impressive. The difference is also impressive for grade 3/4 side effects: from a total of 18.1% in IRIS to a total of 3.6% in ENESTnd (data not shown). Adapted from Baccarani et al.13 Image and legend obtained from the Haematologica Journal website (www.haematologica.org) and reproduced with permission of the rights holder (Ferrata Storti Foundation, Pavia, Italy).
Percentage of newly diagnosed CP-CML patients who reported the listed side effects of imatinib. The data are from 5 prospective, company-sponsored, Good Clinical Practice, contract research organization–monitored studies testing imatinib vs interferon-α plus low-dose arabinosyl cytosine (IRIS)15 vs nilotinib (ENESTnd)16 vs dasatinib (DASISION and SWOG)14,17 and vs bosutinib (BELA).18 In the original reports, the figure represented the proportion or percentage of patients reporting each side effect. In all studies, the sum of the figures was >100%, because many patients reported >1 side effect. The differences among each side effect underscore the variability in collecting and reporting side effects, although all patients were treated frontline with the same dose of imatinib (400 mg/d). The differences among studies are quite impressive. The difference is also impressive for grade 3/4 side effects: from a total of 18.1% in IRIS to a total of 3.6% in ENESTnd (data not shown). Adapted from Baccarani et al.13 Image and legend obtained from the Haematologica Journal website (www.haematologica.org) and reproduced with permission of the rights holder (Ferrata Storti Foundation, Pavia, Italy).
It is noteworthy that the US National Cancer Institute, as early as in 2008, started a large project to formally incorporate direct patient reporting in the documentation of AEs. This effort resulted in the development, and recent validation, of the PRO-CTCAE measurement system.19 This major paradigm shift in outcome reporting pointed out that the potential harm of therapy should also be collected through patients’ self-reports and that the collection of PROs is critical in drug development. There are excellent examples in other hematologic malignancies such as myelofibrosis, where the inclusion of PROs in clinical trials has been very informative and even contributed to the approval of new drugs (ie, ruxolitinib).20 Notably, also in CML, PRO data from the pivotal IRIS study21 were used by the FDA in support of imatinib approval.
Assessing PROs in CML clinical research (eg, RCTs or population-based registries) is important to generate data that are unique to the patient’s perspective on the disease and treatment burden and therefore helpful in making more informed clinical decisions.
State-of-the-art evidence-based data on the effects of TKIs on QoL in CML
In order to synthesize the most robust evidence in this area, we performed a systematic literature search in PubMed (from January 2000 to March 2016) to identify and summarize original studies that have analyzed PROs in CML patients. Studies only linking PROs with adherence to therapy were not included. Case reports and abstracts were also not included given the lack of reported details on full PRO assessment methodology used, and only English full-length published manuscripts were considered. Full details on our search strategy and a list of studies not fully discussed here are available from the authors. Out of 632 records screened, 22 eligible articles were identified. These studies were heterogeneous with regard to purpose, design, and PRO methodology. Nonetheless, some noteworthy findings can be gleaned from them, and selected main results are provided in Table 1. Six studies dealt with CP-CML patients treated with imatinib therapy; 14 studies included patients also receiving second-generation TKIs or in the advanced phase of the disease (ie, AP or BC); and 2 studies specifically investigated QoL and symptoms of patients switching or discontinuing TKIs. Interestingly, all studies (except one) were published within the last 5 years, indicating the rising interest of the CML community in generating PRO data to keep improving the management of patients.
QoL in CP-CML patients receiving first-line therapy with imatinib
The main historical evidence, clearly demonstrating the superiority of QoL outcomes for patients treated with imatinib vs those treated with standard interferon therapy, was provided by the pivotal phase 3 RCT IRIS study.15,21 This well-conducted analysis can be considered an important milestone in CML science, and, interestingly, the difference found in QoL results between treatment arms was one of the largest ever noted in RCTs conducted in patients with other cancer malignancies. In this study, however, patients were followed up for 18 months to assess QoL.
The first evidence-based data regarding the long-term QoL impact (mean time from diagnosis of 5 years) of TKIs was published in 2011.22 This study compared the QoL profile of 422 CP-CML responding patients (ie, at least in CCyR) in first-line treatment with imatinib with that of their peers in the general population and found that patients aged at least 60 years had a QoL profile similar to that of their peers in the general population. However, patients aged between 18 and 59 years reported significantly worse QoL outcomes than their peers in the general population. Another important result, in line with findings from the IRIS study,21 was that the QoL profile of female patients was generally worse than that of male patients. This study showed for the first time that although responding to therapy, younger patients are those most affected by therapy and therefore should receive special consideration. Patient-reported symptom assessment in this study showed that fatigue was the most prevalent symptom, with 82% of patients reporting it with any level of concern. In addition, edema, musculoskeletal pain, muscle cramps, and fatigue were reported as severe in at least one-fourth of the overall sample.
Additional multivariate analysis from the same population23 showed that of all the key sociodemographic and disease-related variables examined, only fatigue showed a consistent association across all QoL dimensions. Even small differences in fatigue severity corresponded to clinically meaningful impairments in all physical and mental QoL domains, indicating the importance of targeting fatigue to possibly improve more general aspects of QoL. Another finding from this study was that fatigue does not occur by itself but clusters with other symptoms, especially musculoskeletal pain and muscular cramps. These data suggest that symptom management is also critical in patients who are successfully in treatment with the same drug for several years.
QoL in patients receiving second-generation TKIs or in the advanced phase of the disease
In our systematic review, we did not find any study primarily designed to assess the effects of nilotinib or dasatinib on patients’ QoL and symptom burden. Therefore, knowledge of the effects of these TKIs is still mainly confined to efficacy and (physician-reported) safety data.
In a large sample of patients enrolled in 2 trials (phase 2 and phase 3), Trask et al24 analyzed baseline QoL profiles of CP-CML patients about to receive first-, second-, and third-line therapy, as well as the QoL profiles of patients with CP-CML vs patients with AP-CML or BC-CML. Interestingly, only in the latter comparison (by phase of disease) were clinically meaningful differences found favoring patients with CP-CML over patients with AP-CML or BC-CML. Although this report was a secondary analysis, the findings suggested that QoL can be influenced more by the phase of the disease than by the number of lines of therapy.
Two prospective studies have been conducted in patients treated with bosutinib.25,26 Trask et al25 prospectively examined QoL in 2 cohorts of CP-CML patients who were either imatinib-resistant (n = 167) or imatinib-intolerant (n = 80) and treated with bosutinib. The authors showed that during the 96 weeks of bosutinib therapy, patients experienced statistically significant and/or clinically meaningful improvements in several QoL domains. However, QoL benefits over time were more prominent in the cohort of imatinib-intolerant patients. Until publication of this study, very little was known regarding QoL of an imatinib-resistant or imatinib-intolerant patient. Recently, another study reported a QoL prospective investigation of bosutinib therapy in 2 cohorts of AP- and BC-CML patients,26 extending findings from the Trask et al study.25 Witheley et al26 analyzed separately AP-CML (n = 76) and BC-CML (n = 64) patients who were resistant or intolerant to imatinib with or without prior exposure to other TKIs. Over the course of 96 weeks of observation, improvements in QoL occurred from baseline assessment at multiple time points. Of interest, the magnitude of improvements from the baseline in some QoL scales was generally greater for the BC-CML cohort than for the AP-CML cohort. Taken together, these studies provide novel insights on the potential value of bosutinib therapy that complement already known data on efficacy and safety of this drug.
QoL in patients after TKI switch or discontinuation
Cortes et al27 recently showed that switching to nilotinib was an effective strategy to improve chronic low-grade (nonhematologic) treatment-related AEs in patients experiencing reduced imatinib tolerance. Importantly, after switching to nilotinib, many patients not only resolved imatinib AEs but also reported improved symptom relief and better QoL. This is an important finding, suggesting that QoL can be improved by timely switching of TKI therapy. This prospective study further confirmed the detrimental effects that low-grade AEs have on patients’ well-being and daily functioning.
A Korean study recently reported some novel data on the QoL of patients who discontinued imatinib therapy.28 Six months after discontinuation of therapy, most symptoms (including nausea, indigestion, peripheral edema, skin whitening, and fragility) had resolved. However, unexpectedly, musculoskeletal pain, pruritus, and fatigue were newly developed or worsened in some patients. The proportion of patients reporting improvements in physical and mental health outcomes was modest, with a substantial proportion of patients reporting no changes since therapy discontinuation. These findings challenge the common-sense idea that stopping therapy necessarily yields only QoL benefits for patients. However, further confirmation of these data is needed from ongoing larger studies on the discontinuation of therapy.
The importance of rigorous PRO methodology in clinical research
An important lesson we can learn from decades of PRO measurement in other cancers is that a naive approach to PRO implementation in research protocols, as well in data management, analysis, and data interpretation, is unlikely to inform patient care. If PROs are to fulfill their potential of allowing patients and physicians to facilitate treatment decisions, then methodological rigor is critical and several methodological issues should be carefully considered. Regulatory stakeholders have often cited methodological shortcomings in the PRO design as a reason for the lack of impact of PRO findings in regulatory decisions. Evaluating PROs in clinical trials requires making a number of decisions and international consensus-based guidelines, including the recently published CONSORT PRO recommendations,29 available to guide investigators. The success of a PRO study can be jeopardized by a number of factors, including, for example, a large volume of missing PRO data over time, poorly performed statistical analyses, and failure to comprehensively report study findings. Therefore, clinicians should carefully consider the robustness of methodology when interpreting study reports from PRO findings. On the basis of previous literature on the major drawbacks on PRO assessment in clinical trials, we have summarized some of the most critical issues to consider in future CML studies (Table 2).
The inclusion of PROs in future comparative effectiveness research or population-based registry studies will be important to generate data to help better inform treatment decisions.
How should we measure PROs in CML clinical research?
There is no universally correct answer to the question of which PRO instrument is best to use in your research. There might be equally valid options, and this really depends on the specific research question being asked in a particular setting. The decision should be guided by a number of factors. Various PRO instruments have been successfully used in CML research. For example, although not exclusively validated on CML patients receiving TKIs, previous PRO questionnaires, such as the SF-36 (36-Item Short Form Health Survey) and the FACT-Leu (Functional Assessment of Cancer Therapy – Leukemia),22,24,25 have provided relevant information to help better understand treatment impact on patients’ QoL. However, increasing the sensitivity of PRO measurements by using questionnaires devised for the specific population under study can be crucial to informative results.
Recently, 2 CML-specific questionnaires, the EORTC QLQ-CML24 (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Chronic Myeloid Leukemia 24)30 and the MDASI-CML (MD Anderson Symptom Inventory Chronic Myeloid Leukemia Module),31 have been published. The EORTC QLQ-CML24 has been tested in 655 CML patients in treatment with various TKIs from 10 different countries (Europe, the United States, and Asia) and has shown satisfactory validity and applicability across multiple languages and cultures. Additional prospective validation data will be collected soon for this questionnaire. The MDASI CML has been tested on 187 patients in treatment with different TKIs in the United States, and it was devised to specifically assess symptom burden (rather than QoL). The strength of this tool was the longitudinal analysis performed in the development process, which further supported validity data. Basic characteristics of both measures are reported in Table 3.
What is the added value of assessing PROs in routine practice, and what are the implications for adherence to TKI therapy?
With 3 TKIs that can be used for frontline treatment (ie, imatinib, dasatinib, and nilotinib), and the same drugs plus bosutinib that can be used as second-line or greater therapy,32 treatment decisions in daily practice are often challenging. For example, appropriate management of intolerance and AEs is crucial in clinical practice, and several strategies can be used to help patients reduce symptom burden33 and improve QoL, including timely switching to another TKI.27
Considering the chronic and possibly lifelong nature of TKI administration, even low-grade AEs might significantly interfere with patients’ daily functioning and negatively affect QoL.23,31,34 Although the literature shows that AEs of current TKIs are typically of low-to-mild intensity, these symptoms can be particularly insidious, as they are most likely to go unrecognized by clinicians in daily practice. For example, a recent study on patients treated with long-term imatinib therapy has shown that clinicians most typically underestimate symptom severity, such as fatigue, muscular cramps, and musculoskeletal pain, and overestimate the health status of their patients.35 Notably, in this study, more than two-thirds of patients were in treatment for at least 3 years with the respective physician who made the evaluation, suggesting that even physicians who have known their patients for a long time may not be able to fully and accurately capture the symptom burden experienced by their patients.35
This mismatch between the physicians’ perception and the actual burden of therapy experienced by patients might have major implications in disease management, for example, when considering the relationship between adherence to therapy and treatment outcomes.
Noens et al36 showed that nonadherence is a major challenge and that only 14% of CML patients can be considered as fully adherent to therapy. Marin et al37 found a correlation between low adherence rate (≤90%) and the 6-year probability of achieving a major molecular response and a complete molecular response. It is now recognized that full adherence to therapy is a critical factor to obtain and maintain an optimal response to therapy36,37 and thus to increase drug effectiveness.
Several factors might influence adherence to therapy, including financial aspects and social as well as patient- and disease-related factors. However, 2 broad non–mutually exclusive reasons for nonadherence have been reported in the literature: nonintentional (eg, forgetfulness) and intentional (eg, avoiding side effects).38 Intentional nonadherence was found to be associated with a greater patient-reported symptom burden,39 and, in a systematic review by Noens et al,40 AEs were also found to be the most frequent cause of nonadherence to therapy. The direct link between patient self-reported QoL and poor adherence to therapy has also been recently reported.41
In the modern era of TKIs, one of the key goals of therapy is to keep patients’ QoL within satisfactory levels for as long as possible. The main risk of not being aware of how satisfied our patients are with their own QoL, and the real burden of the symptoms that they can tolerate, is not promptly identifying those patients who might be at heightened risk of poor adherence behavior.
Interestingly, Kakele et al conducted a study to compare adherence reported by patients with that perceived by their physicians42 and found that physicians were too optimistic in assessing their patients’ adherence.
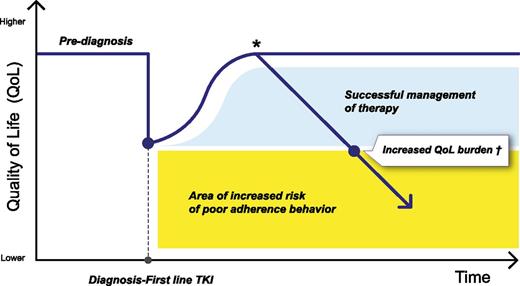
Therefore, introducing standardized PRO tools in our routine practice has the great potential to (1) more accurately monitor treatment burden and (2) promptly adopt strategies to lessen side effects or to switch to another TKI27 before treatment becomes too burdensome. Indeed, it should also be acknowledged that each patient has a different threshold for considering a given TKI therapy as too burdensome. Also, susceptibility to a specific type of AE (eg, muscle cramps rather than headache) may vary from patient to patient, therefore requiring individualized treatment strategies. Being able to early identify this patient-specific threshold has major implications for reducing nonadherence (at least intentional nonadherence), thus possibly maximizing long-term treatment outcomes (Figure 2). Prospective studies aimed at investigating whether interventions to improve QoL outcomes are possibly associated with increased adherence and, eventually, better long-term treatment outcomes are warranted.
The importance of monitoring patient-reported QoL and symptom burden over time. *For a number of reasons (including side effects), it is possible that QoL might decline at any point along the disease/treatment trajectory. Also, it is likely that the QoL decline might not be as steady as shown in this figure (the trend of the QoL curve reported here is for descriptive purposes only). †One goal of first-line TKI therapy should be to ensure optimal QoL for as long as possible. Any effort should be made to maintain an “acceptable” QoL level for each patient (ie, before reaching the critical threshold below which the risk of poor adherence behavior is heightened).
The importance of monitoring patient-reported QoL and symptom burden over time. *For a number of reasons (including side effects), it is possible that QoL might decline at any point along the disease/treatment trajectory. Also, it is likely that the QoL decline might not be as steady as shown in this figure (the trend of the QoL curve reported here is for descriptive purposes only). †One goal of first-line TKI therapy should be to ensure optimal QoL for as long as possible. Any effort should be made to maintain an “acceptable” QoL level for each patient (ie, before reaching the critical threshold below which the risk of poor adherence behavior is heightened).
Previous experience of implementation of PRO instruments in clinical practice in other cancer malignancies has shown that an “integrated” patient-centered approach is feasible and is associated with a number of benefits, such as improved symptom control, enhanced patient-physician communication, and patient satisfaction and well-being.43,44
Assessment of patients’ self-reported symptoms in clinical practice has been shown to improve not only QoL but also traditional clinical outcomes (eg, reductions in emergency room visits, hospitalizations, and quality-adjusted survival) also in advanced metastatic cancer populations.45
This evidence now challenges the CML community to rapidly move toward personalized patient care by formally integrating patient’s self-reported health status data into the mainstream of other standard clinical and laboratory measures. Routine monitoring can be implemented in several ways; for example, a number of electronic PRO systems are already available and used in various oncology clinics.46 These systems are typically Web-based and generally provide real-time, easily interpretable, graphical feedback of questionnaire scorings that are used by clinicians to specifically focus on symptoms (or other health concerns) in need of special attention.
Future work is needed in this area to generate evidence-based policies that can inform how to best incorporate standard PRO assessment into CML patients’ treatment.
Areas for future research and conclusions
The number of CML studies including a PRO assessment has been increasing in recent years, reflecting the scientific CML community’s interest in obtaining additional valuable data to make more informed decisions. Although the PRO evidence accumulated thus far is likely to contribute to improving patient management, major research efforts are still needed in this area. For example, there is paucity of information regarding the QoL impact of second-generation TKIs (ie, nilotinib and dasatinib), used either as first- or second-line therapy, and their effects over the long-term period is also not known. Prospective studies are also warranted to evaluate whether interventions aimed at better monitoring and improving QoL outcomes might significantly improve adherence and, possibly, maximize drug effectiveness. Another important area of research is the documentation of QoL trajectories in patients switching TKIs at any step or even discontinuing TKI therapy. It is recommended that all future clinical trials incorporate a QoL assessment in the study protocol (at least as a key secondary outcome) in addition to other clinical efficacy measures typically assessed in CML. However, as the methodological rigor of PROs evaluation in clinical research is essential to robustly inform patient care, investigators should pay special attention to several PRO design, analysis, and outcome reporting issues.
The inclusion of PRO instruments in routine practice also holds the promise of helping clinicians with patient management. Standard PRO assessment is necessary to provide a comprehensive evaluation of the treatment burden experienced by patients, and it can facilitate early identification of patients at heightened risk of poor adherence behavior. The development of shared strategies on how to best integrate patient’s self-reported health status information into routine practice is also needed to facilitate a transition to a more patient-centered approach.
Although continued efforts toward the cure of CML are necessary, high-quality PRO information will likely play a major role in improving health care quality and patient treatment in the near future.
Correspondence
Fabio Efficace, Health Outcomes Research Unit, Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA), GIMEMA Data Center, Via Benevento 6, 00161 Rome, Italy; e-mail: f.efficace@gimema.it.
References
Competing Interests
Conflict-of-interest disclosure: F.E. has received research funding from Lundbeck and TEVA and has consulted for Seattle Genetics, TEVA, and Bristol-Myers Squibb. L.C. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.