Abstract
The post-thrombotic syndrome (PTS) is an important chronic complication of deep vein thrombosis (DVT). The present review focuses on risk determinants of PTS after DVT and available means to prevent and treat PTS. More than one-third of patients with DVT will develop PTS, and 5% to 10% of patients develop severe PTS, which can manifest as venous ulcers. PTS has an adverse impact on quality of life as well as significant socioeconomic consequences. The main risk factors for PTS are persistent leg symptoms 1 month after acute DVT, anatomically extensive DVT, recurrent ipsilateral DVT, obesity, and older age. Subtherapeutic dosing of initial oral anticoagulation therapy for DVT treatment may also be linked to subsequent PTS. By preventing the initial DVT and DVT recurrence, primary and secondary prophylaxis of DVT will prevent cases of PTS. Daily use of elastic compression stockings for 2 years after proximal DVT appears to reduce the risk of PTS; however, uncertainty remains regarding optimal duration of use, optimal compression strength, and usefulness after distal DVT. The cornerstone of managing PTS is compression therapy, primarily using elastic compression stockings. Venoactive medications such as aescin and rutosides may provide short-term relief of PTS symptoms. Further studies to elucidate the pathophysiology of PTS, to identify clinical and biological risk factors, and to test new preventive and therapeutic approaches to PTS are needed.
Introduction
The post-thrombotic syndrome (PTS) is an important, underappreciated, chronic consequence of deep vein thrombosis (DVT). PTS develops in one-third to one-half of patients with DVT,1,2 even when appropriate anticoagulant therapy is used. Based on its high incidence and prevalence, PTS is the most frequent complication of DVT. PTS is a burdensome and potentially debilitating condition for which patients frequently seek medical advice. Manifestations of PTS vary from mild clinical symptoms or signs to more severe manifestations such as chronic leg pain that limits activity and ability to work, intractable edema, and leg ulcers.3 PTS has adverse effects on quality of life and productivity,4 and is costly as measured by health resource utilization and direct and indirect costs.5 The present review focuses on the risk determinants of PTS after DVT and available means to treat and prevent PTS. In addition, gaps in our understanding of PTS that merit further research are highlighted. This paper focuses on PTS in adults; readers with an interest in PTS in children are referred to a recent review of this topic.6
How Is Post-Thrombotic Syndrome Diagnosed?
PTS is called a “syndrome” because it manifests as symptoms and clinical signs that can vary from patient to patient. Patients with PTS experience aching pain, heaviness, swelling, cramps, itching, or tingling in the affected limb. Symptoms can be present in various combinations and may be intermittent or persistent, and tend to be aggravated by standing or walking and improved by resting and leg elevation. Signs on physical examination of the limb include edema, perimalleolar or more extensive telangiectasiae, brown pigmentation, and venous eczema. Secondary varicose veins may occur, and thickening of the subcutaneous tissues of the medial lower limb known as lipodermatosclerosis may develop. Leg ulcers can also occur, which may be precipitated by minor trauma. These are characteristically chronic, painful, and slow to heal; require close medical attention; and often recur.3
PTS is diagnosed on clinical grounds based on the above-noted characteristic symptoms and signs in patients with prior DVT. In some patients, it may take a few months for the initial pain and swelling associated with acute DVT to resolve, so a diagnosis of PTS should be deferred until after the acute phase has passed.
A number of clinical tools or scales have been used in clinical investigations to measure PTS.3 Recently, in a step toward standardizing the measurement of PTS, the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis recommended that the Villalta Scale be adopted in clinical studies to diagnose and grade the severity of PTS.7 The Villalta Scale is a clinical scoring system that is based on severity ratings of PTS symptoms and signs.8 The scale can be used as a binary (e.g., yes/no), categorical (e.g., none, mild, moderate, severe), or continuous (e.g., score range: 0–33) measure. A recent review of the scale's measurement properties indicates that it is a reliable and valid measure of PTS.9
In the absence of the above-noted characteristic clinical features of PTS, the demonstration of venous abnormalities (e.g., venous reflux, venous obstruction, venous hypertension) on invasive (e.g., contrast venography) or noninvasive (e.g., ultrasonography, plethysmography) imaging or testing should not lead to assigning a diagnosis of PTS to a patient with previous DVT. This is because while many patients with symptomatic PTS have detectable venous abnormalities, many DVT patients who do not have symptoms of PTS can also be shown to have such abnormalities. Therefore, if a patient with previously documented DVT develops clinical features compatible with PTS, there is generally no need for further diagnostic investigation. However, objective confirmation of previous DVT can be useful in patients who have a characteristic presentation of PTS but who do not have a history of previous DVT. In one study, the combination of a standardized clinical evaluation, compression ultrasonography, and continuous-wave Doppler analysis was able to reliably diagnose or exclude previous proximal vein thrombosis in almost 90% of patients.10
Determinants of Risk of PTS after DVT
Why Does PTS Develop after DVT, and What Is Its Overall Incidence?
PTS is thought to occur as a consequence of venous hypertension, which leads to impaired venous return, reduced calf muscle perfusion, abnormal function of the microvasculature with increased tissue permeability, and consequently the characteristic clinical manifestations of PTS.3,11 DVT can lead to chronic venous hypertension via persistent (residual) venous obstruction and valvular reflux. Standard anticoagulant treatment of DVT prevents thrombus extension and embolization to the pulmonary arteries, but does not directly lyse the acute thrombus, and in many cases only partial clearance of the thrombus occurs.12,13 Valvular reflux occurs frequently after DVT, likely via thrombus-induced activation of inflammation, fibrous scarring by acute and resolving thrombosis, or by venous dilation distal to the obstructed vein segment.14–16 Of the two mechanisms, persistent venous obstruction appears to be more important.17,18
Based on data from contemporary prospective studies with 12 months or longer follow-up, one-third to one-half of DVT patients can expect to develop PTS and 5% to 10% of patients will develop severe PTS, which may include venous ulcers.1,2,19–23 In most cases, PTS develops within the first year or two after DVT.
Individual Patient Risk Factors for PTS
New information has been gained in the last few years about clinical and biological factors that influence the risk of developing PTS. In a recent prospective study of 387 patients with acute symptomatic DVT, we found that incomplete resolution of leg symptoms and signs by 1 month after DVT strongly predicted the development of PTS during the subsequent 2 years.2 As summarized in a recent review,24 we and others have also shown that venous thrombosis of the common femoral or iliac vein (as compared with DVT of the distal or popliteal vein), previous ipsilateral venous thrombosis, higher body mass index, and older age are associated with increased risk of PTS. One study reported that DVT patients with a subtherapeutic INR (international normalized ratio) more than 50% of the time during the initial 3 months of treatment with vitamin K antagonists had a 3-fold higher risk of developing PTS,22 reinforcing the importance of close monitoring to ensure an adequate intensity of oral anticoagulant therapy. PTS has been inconsistently associated with female gender, and is not associated with having the factor V Leiden or prothrombin mutation or by circumstances of the initial DVT (i.e., spontaneous, due to reversible risk factors, or cancer-related).24
Biomarkers may prove to be useful in predicting the risk of PTS. Recent studies have reported that elevated levels of markers of inflammation (e.g., ICAM-1 [intercellular adhesion molecule 1], interleukin-6, and C-reactive protein)25,26 and D-dimer21,27 early after diagnosis or within a few months of DVT were associated with the development of PTS. Further work in this area is under way.28
Can PTS Be Prevented?
Preventing First and Recurrent DVT
Improving the systematic use of thromboprophylaxis to prevent DVT in high-risk hospitalized patients as recommended in evidence-based consensus guidelines will prevent some cases of PTS.29 Because recurrent ipsilateral DVT is an important risk factor for PTS, reducing the risk of recurrent DVT by providing anticoagulation of appropriate intensity and duration to treat the initial DVT is an important clinical goal.30
Use of Elastic Compression Stockings
A meta-analysis of the three trials that have evaluated the effectiveness of long-term use of graduated elastic compression stockings (ECS) to prevent PTS after symptomatic proximal DVT concluded that use of ECS after DVT substantially reduces the risk of any PTS (odds ratio [OR] 0.3; 95% confidence interval [CI] 0.20–0.48) and of severe PTS (OR 0.39; 95% CI 0.20–0.76).31 Based on these data, the American College of Chest Physicians guidelines recommend that patients with acute symptomatic proximal DVT wear ECS with an ankle pressure gradient of 30 to 40 mmHg for a minimum of 2 years or even longer if patients have symptoms of PTS, providing that it is feasible for patients or their caregivers to apply and remove the stockings.30 However, due to various limitations of the above-noted trials, important questions remain regarding the true magnitude of effectiveness and general feasibility of using ECS to prevent PTS in patients with DVT.24 It is not known if ECS should be used in patients with symptomatic distal DVT. It is unclear if wearing ECS prevents PTS or merely palliates it, in which case it may be as effective and more convenient for patients to start using ECS at the time of PTS onset. It is not known if easier-to-apply, lighter-compression (20–30 mmHg) ECS are as effective as 30 to 40 mmHg ECS. Finally, it is not known how long ECS need to be worn: while the trials to date evaluated the use of ECS for 2 years or longer, a recent trial reported that beyond an initial 6-month period of use, there was no incremental benefit in prolonging compression therapy for an additional 18 months,32 suggesting that the use of ECS for 6 months might be adequate and would be easier for patients than the currently recommended 2 years.
Although ECS are unlikely to cause harm, they are difficult to apply, uncomfortable, expensive, and require replacement every few months. Based on the current state of evidence on the use of ECS to prevent PTS and pending the results of ongoing trials in this area,28 a reasonable clinical approach is to prescribe below-knee, 30 to 40 mmHg compression stockings to patients who have residual leg pain or swelling after proximal or distal DVT, and to continue them for as long as the patient derives symptomatic benefit or is able to tolerate them.
Thrombolysis of Acute DVT to Prevent PTS
Some studies of systematic thrombolytic therapy and, more recently, catheter-directed thrombolysis techniques support the tenet that early thrombolysis of acute DVT reduces the occurrence of PTS, presumably by rapidly restoring venous patency and preserving or limiting damage to venous valves.33 However, studies to date have been small and have had various methodological limitations; therefore, uncertainty remains regarding the effectiveness of thrombolysis in reducing PTS and the associated risks of these procedures. The safety, effectiveness, and cost-effectiveness of newer endovascular strategies such as catheter-directed thrombolysis, including pharmacomechanical catheter-directed thrombolysis (i.e., with inclusion of thrombus fragmentation and/or aspiration), are currently undergoing evaluation in well-designed, randomized controlled trials in which PTS is the primary study outcome.11,34
Based on the current state of evidence, the routine use of early thrombolytic therapy for the prevention of long-term sequelae of DVT is not recommended. In selected cases, however, thrombolytic therapy may be considered. In the current American College of Chest Physicians guidelines, it is suggested that in selected patients with extensive acute proximal DVT (e.g., those with iliofemoral DVT, symptoms < 14 d, good functional status, life expectancy of more than 1 year) in whom the risk of bleeding is low, catheter-directed or pharmacomechanical thrombolysis or, less ideally, systemic thrombolytic therapy followed by standard anticoagulant therapy, may be used to reduce acute DVT symptoms and PTS, providing that the appropriate expertise and resources are available.30
Treating PTS
Compression Therapy
Compression therapy and frequent leg elevation are the cornerstones of managing established PTS. ECS are helpful to reduce leg swelling, heaviness, and aching,35 but require compliance on the part of the patient to maximize effectiveness. Patients should be advised to apply their stockings in the morning and to remove them at bedtime or in the early evening. The principal contraindication to using ECS is symptomatic peripheral arterial disease, as claudication can worsen when stockings are worn. To start, a knee-length, 30 to 40 mmHg ECS should be prescribed; however, if patients find these constricting or difficult to apply, a 20 to 30 mmHg stocking can be tried. Some patients will require higher compression strengths (e.g., 40–50 mmHg) to adequately control edema. Knee-length stockings are easier to apply and are more comfortable than thigh-length stockings, with similar physiologic effects in decreasing venous stasis of the lower limb.36
Other types of compression therapies such as intermittent pneumatic compression units can be used to treat severe PTS.37 Recently, a randomized crossover trial in 32 patients with severe PTS tested the effectiveness of a portable, battery-operated lower-limb venous return assist device (Venowave™, Saringer Life Science Technologies, Inc., Stouffville, Ontario, Canada) that provides compression of the calf and increases upward volumetric displacement of venous blood. Use of the device alone or in combination with ECS was associated with improvement in quality of life and reduced severity of PTS.38
Medications
There is limited evidence that “venoactive” or “phlebotonic” remedies such as aescin (a derivative of the horse chestnut seed) or rutosides reduce symptoms of PTS. In a clinical trial of 120 patients conducted in Italy, the use of oral hydroxyethylrutosides for 1 year improved symptoms of PTS (improvement defined as reduction in Villalta PTS score to <5, or decrease in score by at least 30% in comparison to the baseline score, at any time during the follow-up) to a similar extent as below-knee ECS, with no added benefit of combining the two therapies.39 A 12-week trial of ECS vs. twice-daily horse chestnut seed extract in 240 patients with chronic venous insufficiency showed that both therapies were well tolerated and had similar effects in reducing edema.40 A Cochrane review of 17 trials comparing horse chestnut seed extract against placebo, ECS, or other medications concluded that horse chestnut seed extract is effective for short-term treatment of chronic venous insufficiency symptoms such as leg pain and edema, and that adverse events were mild and infrequent; however, larger, more rigorous trials are needed to assess its long-term effectiveness and safety.41 A short-term (i.e., up to 3 weeks) trial of twice-daily horse chestnut seed extract (available at natural product stores) may be suggested to patients whose PTS symptoms are not adequately controlled by ECS.
There is no evidence that short- or long-term use of diuretics is effective for the treatment of PTS-related edema, or that nonsteroidal anti-inflammatory drugs improve symptoms of PTS beyond their analgesic effects.
Venous Ulcer Management
Post-thrombotic venous ulcers are usually treated conservatively with compression bandages or stockings, leg elevation, topical dressings, and various pharmacologic compounds, but can be refractory to therapy. Patients with ulcers should be managed by a specialist with expertise in managing post-thrombotic ulcers. Surgery may be advocated in select patients whose ulcers do not respond to conservative treatment. Detailed discussion of venous ulcer management is beyond the scope of this paper. The interested reader is referred to recent reviews of this topic.3,42
Endovascular or Surgical Treatment of PTS
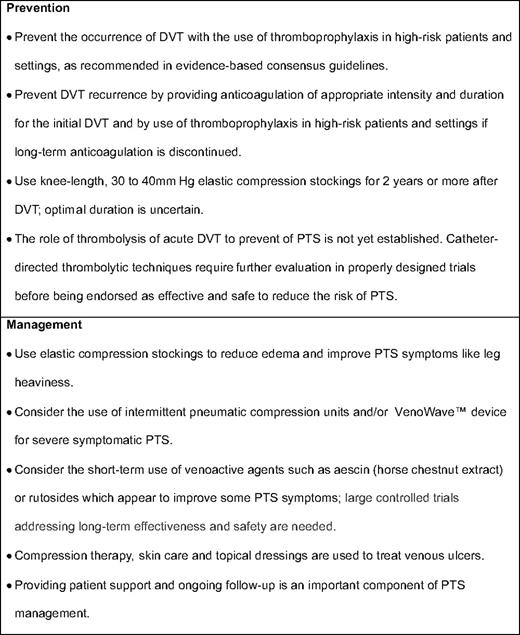
A few case reports and small case series suggest that procedures such as endovascular stent recanalization for patients with iliac vein or inferior vena cava obstruction or surgical valvuloplasty for chronic femoro-popliteal obstruction and deep venous valvular reflux may be beneficial for selected patients with moderate or severe PTS.11 To date, however, there have been no methodologically rigorous, comparative trials that have addressed whether these therapies improve the clinical manifestations of PTS. Table 1 summarizes available strategies to prevent and treat PTS.
Upper-Extremity PTS
PTS of the upper extremity has been less well studied than lower-extremity PTS, but is estimated to occur in up to 15% to 25% of patients after treated upper-extremity DVT.43 Upper-extremity PTS is a potentially disabling condition, particularly if the dominant arm is involved.44 Symptoms include arm swelling, heaviness, and fatigue with exertion.43,44 Dilation of the superficial veins of the upper arm and chest wall and dependent dusky cyanosis of the arm may be noted. To date, no controlled studies have evaluated the effectiveness of elastic bandages, compression sleeves, or venoactive drugs to prevent or treat PTS after upper-extremity DVT. However, anecdotal evidence suggests that patients with persistent arm swelling and pain after upper-extremity DVT may derive symptomatic relief from elastic bandages or compression sleeves. Some reviews have advocated staged, multidisciplinary approaches to the management of primary upper-extremity DVT that involve thrombolysis and angioplasty or stent placement, followed by early or late surgical decompression of the thoracic outlet.45,46 Results of a case series suggest that combined endovascular and surgical treatment of idiopathic axillo-subclavian vein thrombosis may lead to favorable long-term symptomatic and functional improvement.47 However, randomized controlled trials evaluating such approaches are presently lacking.
Research Needs
PTS-related research needs are substantial. Important subjects for future research include better characterization of the pathophysiology of PTS; identification of clinical, anatomic, physiologic and genetic risk factors for PTS; and derivation and validation of PTS risk-prediction models that integrate clinical and biomarker information. Further investigation of the link between inflammation and PTS could lead to identifying new therapeutic targets for preventing or treating PTS. Finally, careful evaluation of the long-term effectiveness and safety of catheter-directed thrombolytic techniques to prevent PTS and of endovascular or surgical procedures to treat moderate-to-severe PTS are also needed.
Acknowledgments
Dr. Kahn is a recipient of a National Research Scientist Award from the Fonds de la Recherche en Santé du Québec.
Disclosures
Conflict-of-interest disclosure: The author is a member of advisory boards of Boehringer Ingelheim and Sanofi-Aventis. She also receives research funding from Sanofi-Aventis and Sigvaris. Off-label drug use: None disclosed.
Correspondence
Susan R. Kahn MD, MSc, Professor of Medicine, McGill University, Division of Internal Medicine, and Centre for Clinical Epidemiology and Community Studies, Jewish General Hospital, 3755 Cote Ste. Catherine, Rm. A-114, Montréal, Québec, Canada H3T 1E2; Phone: 514-340-7587; Fax: 514-340-7564; e-mail: susan.kahn@mcgill.ca