Abstract
Despite an improved understanding of the risk factors underlying venous thromboembolism (VTE), extensive clinical investigation, and detailed clinical guidelines, the decision to extend anticoagulation indefinitely for an individual patient with VTE is often problematic. Patients with VTE in association with major surgery, trauma, immobilization, or pregnancy are at relatively low risk of recurrence and generally do not require more than 3 to 6 months of anticoagulant therapy. For patients with a first unprovoked, or idiopathic, episode of VTE, an individualized approach should be taken in deciding on the duration of anticoagulation based on the patient's recurrence and bleeding risk, as well as their personal preference. Although the presence of genetic thrombophilic disorders (factor V Leiden and prothrombin G20210A gene mutations; deficiencies of antithrombin, protein C, and protein S) predispose patients to a first episode of VTE, there is inconsistent data on whether testing for these defects changes patient outcomes or should alter their management. In patients with a single unprovoked VTE, measurement of D-dimer several weeks following the completion of anticoagulant therapy appears useful in stratifying patients with a first unprovoked episode of VTE with regard to recurrence risk. Through a series of clinical vignettes, the utility of the laboratory in risk-stratifying patients with respect to recurrence risk will be discussed, along with decision making regarding the duration of anticoagulation. The potential impact of having a nonremovable inferior vena caval filter will also be addressed.
The American College of Chest Physicians (ACCP) and other groups have published evidence-based guidelines regarding the duration of anticoagulant therapy following venous thromboembolism (VTE). A grade “1” recommendation denotes a high level of certainty that the benefit of an intervention does or does not outweigh the risk. Less certainty of the magnitude of the benefits and risks, burden, and costs leads to a grade “2” recommendation. Assignment of an “A” rating to the grade indicates that the evidence supporting the recommendation is strong and comes from methodologically sound randomized clinical trials or high-quality observational studies with large effects. Recommendations derived from moderate or low-quality evidence are given a “B” or “C” rating, respectively.
The 8th ACCP Evidence-Based Clinical Practice Guidelines, which were published in 2008, recommend that warfarin be continued indefinitely after at least 3 months of initial anticoagulation for patients with a first episode of idiopathic proximal deep venous thrombosis (DVT) or pulmonary emboli (PE).1 This is provided that good anticoagulant monitoring is achievable, and the patient has a low bleeding risk (grade 1A). This recommendation includes a caveat stating that it places a relatively a high value on the prevention of recurrent VTE and a lower value on the bleeding risk and burden of regular International Normalized Ratio (INR) monitoring. Interestingly, using essentially the same data, the 7th ACCP Guidelines published in 2004,2 recommended at least 6 to 12 months of anticoagulation after a first unprovoked VTE (grade 1A); indefinite anticoagulation was a grade 2A recommendation, denoting uncertainty that the benefit exceeds the risk. Only 3 months of anticoagulation is recommended for patients with VTE secondary to a transient risk factor (grade 1A) or a distal DVT of the leg (grade 2B).
Therapy for patients with a first episode of symptomatic VTE typically includes initiation of a parenteral anticoagulant (either unfractionated heparin, low molecular weight heparin [LMWH], or fondaparinux), along with the vitamin K antagonist warfarin. The parenteral anticoagulant is discontinued after a minimum of 5 days of overlap with warfarin provided that an INR of 2 to 3 has been achieved for at least 24 hours. Warfarin is then continued for a period of 3 to 6 months. Through a series of clinical vignettes, this paper will discuss the use of the laboratory, as well as clinical factors in risk-stratifying a patient's recurrence risk and determining the need for a more extended duration of anticoagulant therapy.
Prognosis of VTE
After the cessation of warfarin therapy, unprovoked VTE is associated with an annual recurrence rate of approximately 10% in the first 2 years. In subsequent years, the annual recurrence rate drops to 3%, such that the cumulative recurrence rate at 4 years is approximately 25%. The cohort study conducted by Prandoni and colleagues,3 along with a number of randomized trials comparing different durations of anticoagulant therapy4–9 have led to the recognition that unprovoked VTE is a chronic disease state. Although the continuation of warfarin therapy is highly effective in preventing recurrences (> 90% relative risk reduction), prolonging initial therapy beyond 3 months does not reduce the recurrence risk after warfarin is discontinued; a longer initial course of anticoagulation (eg, 12 months instead of 3 months) does not change the risk of recurrence after oral anticoagulation is discontinued.7,8 Differences in types of VTE and patient characteristics have made application of the current guideline recommending indefinite anticoagulation for a first unprovoked event problematic in clinical practice. Many consultants with expertise in thrombosis management favor an individualized approach to determining the duration of anticoagulant treatment following a first episode. This is based on multiple considerations:
A mortality benefit has not been clearly demonstrated by continuing oral anticoagulation indefinitely; the reduction in fatal PE is offset by fatalities from major bleeding.10 In patients with a first unprovoked VTE event, the recurrence rate in the first year off-therapy is approximately 8%, with a case fatality rate of 0.3% to 1%, and the major bleed rate is 2% to 6%, with a case fatality rate of 0.2% to 0.6%.
Although DVT and PE result from the same pathophysiologic disease process, it has been observed that patients presenting with symptomatic PE are more likely recur with PE than DVT7 ; similarly, patients with a first DVT are more likely to recur with a DVT.8
The use of warfarin requires INR monitoring at approximately monthly intervals with the potential for food, medication, and alcohol interactions; many patients require dose adjustments if the INR is outside the therapeutic range of 2 to 3 and then must be brought back within 1 to 2 weeks for a follow-up INR measurement. Many patients, particularly if they are younger (ie, under age 50) and in otherwise good health, do not want to be committed to long-term warfarin therapy. In young women who are considering pregnancy, warfarin is potentially teratogenic; this risk is highest at 6 to 12 weeks gestation.
After understanding the benefits and risks of long-term anticoagulation, some patients express a clear preference to either continue or discontinue anticoagulation. However, for many physicians and patients, this decision presents a conundrum, and input from consultants—usually hematologists, pulmonologists, or vascular surgeons—is frequently obtained (Figure 1).
Considerations in extending anticoagulant therapy beyond 3 to 6 months in patients with a first unprovoked VTE.
Considerations in extending anticoagulant therapy beyond 3 to 6 months in patients with a first unprovoked VTE.
Evaluation of the Patient With Unprovoked VTE
Among patients with unprovoked VTE, a substantial percentage will prove to have an acquired and/or a hereditary risk factor. The literature suggests that up to 10% of older patients (i.. over age 50) harbor an occult malignancy that will be diagnosed within 1 to 2 years. However, about two-thirds of these patients will have a diagnosis of cancer when they initially present with VTE.11 Although extensive screening at baseline using computed tomography of the abdomen and pelvis can significantly increase the number of cancers detected in patients with unprovoked VTE, there is insufficient evidence to recommend aggressive investigation unless there are symptoms (ie, weight loss, cough, change in bowel habits) or findings (i.e., lymphadenopathy, guiac-positive stools) that suggest the presence of an underlying malignancy. The best strategy remains a thorough history and physical examination at clinical presentation, routine blood work, and age-appropriate cancer screening, along with ongoing clinical surveillance.
In patients with a first unprovoked venous thrombotic event, determination as to whether the patient meets criteria for the antiphospholipid antibody syndrome (APLAS) is arguably the single most important laboratory diagnosis to ascertain. APLAS has been empirically defined as the occurrence of venous or arterial thrombosis or recurrent fetal loss in association with a positive lupus anticoagulant or elevated cardiolipin or β2-glycoprotein I antibody levels. These tests must remain persistently positive for a minimum of several months following the thrombotic event to warrant assignment of a diagnosis of APLAS. An unexplained prolongation of the activated partial thromboplastin time (aPTT) is found in approximately two-thirds of patients; the presence of a lupus anticoagulant is identified by failure of the patient's prolonged aPTT to normalize following a 1:1 dilution with normal plasma with relative stability during 2 hours of incubation. The presence of a lupus anticoagulant is confirmed using one of several more specialized phospholipid-dependent coagulation tests (eg, neutralization of the lupus anticoagulant effect in an aPTT-based assay by the addition of hexagonal phase phosphoplipid, dilute Russell Viper Venom time). APLAS is frequently identified in patients with systemic lupus erythematosus, but is associated with the use of certain medications (ie, thorazine, hydralazine), or in association with infections or an underlying malignancy. It can occur in the absence of any underlying disease state (ie, idiopathic). Patients with an unprovoked episode of VTE who meet criteria for APLAS appear to be at higher risk for recurrent events than those without this diagnosis.
Evaluation for Hereditary Thrombophilia
Clinical Vignette: A 61-year-old male in excellent health sustains a symptomatic DVT involving the left popliteal and femoral veins following a 4-hour car trip. There is no family history of VTE. He is treated with anticoagulation for 6 months, with complete resolution of symptoms. He had a normal colonoscopy 1 year ago, and his PSA (prostate-specific antigen) is normal. A follow-up ultrasound shows no residual thrombus. A complete workup for an underlying hypercoagulable state 3 weeks after discontinuing warfarin showed that the patient has protein C deficiency (protein C activity 34%; protein C antigen 37%; reference range 70–130%).
Although the literature suggests that hereditary deficiencies of protein C and protein S confer a higher risk for initial, as well as recurrent, VTE than the more common factor V Leiden, this may be attributable to selection bias.12 In this patient with a first DVT relatively late in life and no family history of VTE, I would argue that heterozygosity for protein C deficiency does not substantially increase his risk for recurrent VTE over that of a patient without an identifiable thrombophilia, and it does not mandate indefinite anticoagulation. Studies suggesting that deficiencies of antithrombin, protein C, and protein S confer an increased risk of recurrent VTE included individuals from thrombosis-prone families.13,14
With the discovery of the factor V Leiden and prothrombin G20210A mutations as frequent genetic risk factors for VTE in Caucasians in the 1990s, it was anticipated that testing would help identify those patients most likely to develop a recurrence. This, however, has not turned out to be the case because most individual studies have not found an increased risk of recurrent VTE among heterozygotes with the factor V Leiden or prothrombin G20210A mutations. A pooled analysis of the literature found the risk associated with factor V Leiden mutation to be significantly increased, but the magnitude was so modest that this by itself does not merit committing a patient to long-term anticoagulation (odds ratios of approximately 1.5 each for factor V Leiden and prothrombin G20210A).15,16
Hereditary deficiencies of antithrombin, protein C, or protein S are much less frequently encountered in clinical practice, and one of these disorders will be identified in fewer than 5% of patients presenting with a first unprovoked episode of VTE; this is increased up to about 15% if the patient is under age 50 and has a positive family history. Patients with these less common thrombophilic defects may merit long-term anticoagulation, particularly if other first-degree family members have sustained VTE. Long-term anticoagulation is frequently recommended for patients with antithrombin deficiency, which is generally considered to be the most penetrant of the hereditary thrombophilias. Although patients with more than a single one of the common thrombophilic defects (eg, homozygous patients with factor V Leiden, double heterozygous carriers of factor V Leiden and prothrombin G20210A) have also been considered to be at higher recurrence risk, a recent case-control study did not find this to be the case.17 The likelihood of identifying more than a single hereditary defect in patients presenting with an unprovoked episode of VTE with a negative family history is in the range of 1% to 3%.
In patients with a first episode of VTE without a convincing positive family history (eg, one or more first-degree relatives with VTE prior to age 50), the utility of screening for the established hereditary thrombophilias has been called into question. It is, therefore, recommended that counseling be conducted prior to undertaking testing for the hereditary thrombophilias and that patients be given the option to decline testing. In patients without a strong family history of VTE, it is my practice to inform patients that current evidence indicates that the presence of a thrombophilic defect should not influence the duration of anticoagulation. Should they carry a heterozygous defect, approximately half of their first-degree relatives will be similarly affected. However the management implications for these individuals are modest because anticoagulation is not advised outside of high-risk situations, such as major surgery; in such instances, thromboprophylaxis should be routinely administered without regard to thrombophilia status. For the propositus with female siblings or children considering the use of oral contraceptives or pregnancy, the case for thrombophilia testing is somewhat stronger. However, alternatives (eg, intrauterine devices) to estrogen-containing contraceptives are available, and routine thromboprophylaxis is generally not warranted during pregnancy unless other thrombotic risk factors are present.
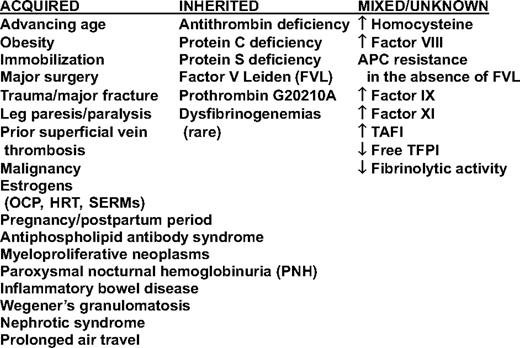
A number of other hemostatic abnormalities, including homocysteine, have been shown to be risk factors for a first unprovoked episode of VTE (Table 1). Several of these are elevations in the levels of procoagulant proteins; the relative risk (RR) increase associated with an elevated level of factor VIII coagulant activity is similar to that among heterozygotes with the factor V Leiden mutation. However, the assay has not been standardized for this purpose, and the cutoff for identifying high-risk patients varies considerably between populations; in the Leiden Thrombophilia Study, the top decile of the population was found to be greater than 150% of normal,18 whereas it was greater than 234% in an Austrian cohort study.19 The molecular basis for these abnormalities is uncertain, and they have not been shown to be hereditary. Because the results of these tests should not affect patient management, testing for these analytes is discouraged. Testing for the presence of hyperhomocysteinemia should no longer be performed because treatment with vitamin B supplements did not reduce the risk of VTE in two clinical trials, even though it did reduce homocysteine levels.20,21 Furthermore, homocysteine levels have decreased in the US population following folate fortification of the wheat supply over a decade ago.
Elevated D-Dimer Levels as a Determinant of Recurrence Risk
Clinical Vignette: A 52-year-old male presented with an unprovoked right leg DVT manifested by pain and swelling. Ultrasound showed noncompressibility of the superficial femoral vein, extending from the midthigh distally, as well as the popliteal and posterior tibial veins. Prior to the initiation of anticoagulation, CBC (complete blood count), serum creatinine, prothrombin time, and partial thromboplastin time were normal, and testing for a lupus anticoagulant returned negative. He was treated with LMWH and warfarin. There was no family history of VTE, and the patient's only clinical risk factor was obesity. He is treated with 6 months of anticoagulation and wears a below-knee compression stocking on his right leg. Physical examination showed minimal residual edema of the right foot; follow-up right leg ultrasound showed normal compressibility with no residual thrombus. Laboratory evaluation while on warfarin (INR 2–3) yielded a quantitative D-dimer level that was < 500 ng/mL. He discontinues warfarin, and his D-dimer is repeated 1 month later and is 812 ng/mL. You place him in a higher risk group for recurrent VTE and advise that he resume warfarin on a long-term basis with a target INR of 2 to 3.
In patients with an initial unprovoked episode of VTE, it would be desirable to be able to identify patients with either a substantially higher or lower than average risk of recurrence. Current evidence suggests that the results of quantitative D-dimer assays, measured at the end of warfarin therapy and then 1 month after its discontinuation can help stratify such patients with respect to recurrence risk.22–28 A meta-analysis, including seven studies of 1,888 patients who completed at least 3 months of anticoagulation, found that a D-dimer level of less than approximately 500 ng/mL (“a negative test”) was associated with a 3.5% (95% CI, 2.7–4.3) annual risk of recurrence, whereas a D-dimer over this level (“a positive test”) was associated with an 8.9% (95% CI, 5.8–11.9) risk in each of the first 2 years (Table 2).29 Potential difficulties of this approach, however, are lack of agreement and different cutpoints for the various D-dimer assays (there is no D-dimer “standard”). Clinicians must, therefore, rely on an assay manufacturer's cutpoint for a positive or negative D-dimer test based on individual studies using D-dimer to assess recurrence risk.
Annualized risk of recurrent VTE in patients with a first unprovoked VTE according to whether D-dimer levels were higher or lower after stopping treatment 29

D-dimer, therefore, appears to be a useful biomarker in evaluating recurrence risk following a first unprovoked episode of VTE and can be used in practice. It is, however, important to understand the patient's preference because there is no point in testing an individual who states a clear preference for either continuing or discontinuing anticoagulation. If one uses one of the validated assays, it is recommended that D-dimer first be checked after a minimum of 3 months of anticoagulation or when discontinuation of anticoagulation is being contemplated. Warfarin reduces thrombin generation in vivo, resulting in decreased D-dimer levels; a positive D-dimer level on warfarin in the setting of an INR > 2 obviates the need for further testing becasue the D-dimer level will be elevated to an even greater extent after its discontinuation. In patients with a negative D-dimer on warfarin, the measurement of D-dimer approximately 1 month following termination of warfarin is used to identify patients with a risk of recurrent VTE in the first year of < 5%. Approximately two-thirds of patients with an unprovoked VTE will have a normal D-dimer level 1 month after discontinuation of anticoagulation; thus, a considerable number of patients can potentially be spared long-term anticoagulation. It should be emphasized that use of the D-dimer test in this way should not be used as a stand-alone strategy to make such decisions; it should be used in context along with consideration of individual clinical risk factors for recurrence, as well as bleeding and individual patient preferences.
The randomized trial of Palareti et al24 (PROLONG) provides the strongest evidence in support of using the D-dimer to identify lower risk patients who do not require long-term anticoagulation. This group performed a follow-up study in which they repeated D-dimer testing every 2 months for 1 year in patients with a normal D-dimer 1 month after stopping anticoagulation.30 Among patients in whom D-dimer became positive at the third month and remained abnormal thereafter, the recurrence risk was 27% per year (7 events in 31 patients). Although data suggest that repeated D-dimer testing could further tailor decisions regarding the risk of recurrence and the need for resuming anticoagulation, these findings require confirmation. In clinical practice, serial measurements of D-dimer should not be performed following the discontinuation of anticoagulation.
Finally, measurement of D-dimer in conjunction with clinical variables shows promise in being able to identify individuals at particularly low risk of recurrence; in the PROLONG trial, women younger than age 65 with a normal D-dimer 1 month after stopping anticoagulation had a very low risk of recurrence (0.4% per year).31
Residual Venous Thrombus on Compression Ultrasound
An association between the presence of residual thrombus on ultrasound and the risk of recurrent VTE has been reported.32,33 Other studies have not found residual DVT to be an independent predictor of recurrence34,35 ; it is also difficult to standardize ultrasound protocols and criteria for assessing residual thrombus. It is, therefore, recommended that residual venous thrombosis not be used to determine the need for extending the duration of anticoagulant therapy. The major utility of obtaining an ultrasound at the completion of anticoagulation is to establish a baseline for distinguishing new from old thrombus in case the patient develops symptoms of a recurrent DVT.
Other Anticoagulation Strategies
Randomized clinical trials have evaluated the efficacy of extended low-intensity warfarin at a target INR of 1.5 to 2 in preventing recurrent events in patients with unprovoked VTE. Low-intensity warfarin reduced the rate of recurrent VTE by 64%, compared with placebo after 3 to 6 months of treatment at an INR of 2 to 336 ; INR monitoring in this trial was done every other month. Low-intensity warfarin, however, was significantly less effective than standard anticoagulation (target INR 2.0–3.0) and did not reduce the risk of major bleeding complications.37 Low-intensity warfarin is recommended as an option for patients with a strong preference for less frequent INR monitoring. Oral anticoagulants targeting either factor Xa or thrombin, which do not require laboratory monitoring, are currently being evaluated for the secondary prevention of VTE.38 Antiplatelet agents (eg, aspirin, thienopyridines) are not efficacious for this indication.
Anticoagulation in Patients With Nonremovable Inferior Vena Caval Filters
Clinical Vignette: A 35-year-old female in seen in consultation regarding the need for long-term anticoagulation following 1 year of warfarin therapy. A year ago, she developed proximal DVT of the right leg following 1 week of near-complete bed rest after tearing her anterior cruciate ligament. She was started on anticoagulation with LMWH twice daily and warfarin. While on LMWH administered twice daily, with an INR that had not yet become therapeutic, she developed the acute onset of shortness of breath. Computed tomography angiography demonstrated PE in multiple segmental and subsegmental vessels of both lower lobes bilaterally. A retrievable inferior vena cava (IVC) filter was placed. She continued on enoxaparin until her INR reached 2 to 3. A limited hypercoagulable workup (testing for the presence of APLAS, factor V Leiden, and prothrombin G20210A mutations) was negative. She had taken oral contraceptives several years ago and had two uncomplicated pregnancies. After 3 months of anticoagulation, an unsuccessful attempt was made to remove the IVC filter.
The 8th Edition of the ACCP Guidelines1 recommends against the routine use of IVC filters in patients with PE (grade 1A). It is recommended for those in whom anticoagulation therapy is not possible because of the risk of bleeding (grade 1C indicating low-quality evidence). Once the risk of bleeding resolves, it is recommended that a “conventional” course of anticoagulation be administered (grade 1C). The guidelines do not provide recommendations on IVC filter placement in patients with recurrent PE or DVT extension on anticoagulant therapy.
IVC filters, whether permanent or retrievable, are widely used in the United States, despite a paucity of evidence that their use leads to improved clinical outcomes. There has been only one large randomized trial that evaluated permanent IVC filter insertion in addition to anticoagulant therapy in patients with proximal DVT considered to be at high risk for PE. The conclusions of this study, which were published at 2 years39 and 8 years40 are as follows:
Routine insertion of IVC filters in patients who are also anticoagulated does not alter the frequency of recurrent VTE (RR of 1.34 at 2 years and 1.03 at 8 years).
IVC filters reduced, but did not eliminate, the risk of PE at 12 days (RR 0.41), 2 years (RR 0.54), and 8 years (RR 0.41).
IVC filters increased the risk of DVT at 2 years (RR 1.8) and 8 years (RR 1.3).
Despite a higher frequency of thrombosis at the filter site in patients with recurrent VTE, which occurred in 43% of these cases, filters were not associated with a higher risk of postthrombotic syndrome (RR 0.87).
At 8 years follow-up, deaths occurred in 2.5% (five patients) in the nonfilter group and 1.0% of the filter group (two patients).
A strong case can be made for indefinite anticoagulation for patients with a first or recurrent unprovoked episode of VTE. However, this patient's VTE was provoked, so she would generally not require more than 3 to 6 months of anticoagulation. Complicating factors in this case include the development of PE during the initiation of anticoagulation and the now permanent IVC filter. Recurrences during the initial 3 months of anticoagulation generally occur in 2% to 4% of patients without cancer who are treated appropriately. In this patient, with a filter that cannot be removed, she would likely have an increased DVT risk, with the potential for filter occlusion if anticoagulation were discontinued. Unfortunately, no data are available regarding the relative or absolute risks in such a patient. These uncertainties would need to be discussed with the patient in deciding whether to discontinue anticoagulation. If anticoagulation were to be discontinued, the patient would certainly need to be administered prophylactic anticoagulation in high-risk situations (eg, major surgery).
Conclusions
Although prolonged anticoagulation is highly effective in preventing recurrent VTE, the benefit is offset by major bleeding that occurs at a rate of 1% to 3% per year. Intracerebral bleeds, in particular, can lead to substantial mortality and morbidity. In patients taking a vitamin K antagonist, factors associated with an increased risk of hemorrhagic complications include recent major bleeding, uncontrolled hypertension, serum creatinine > 1.2 mg/dL, anemia, or age > 75.41 In patients with a first unprovoked episode of VTE and one or more of these risk factors, it is appropriate to discontinue anticoagulation after 3 months of therapy.
Disclosures
Conflict-of-interest disclosure: The author is a consultant for Johnson & Johnson, GlaxoSmithKline, and Pfizer, Inc.
Off-label drug use: None disclosed.
Correspondence
Kenneth A. Bauer, MD, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215; Phone: (617) 667-2174; Fax: (617) 738-1450; e-mail: kbauer@bidmc.harvard.edu