Abstract
The use of nanometer-sized iron oxide nanoparticles and micron-sized iron oxide particles as magnetic resonance (MR) contrast agents has garnered a high degree of interest in diverse areas of biology and medicine. Applications such as cell tracking, molecular imaging, gene detection, and lymphography are being explored to provide insight into disease mechanisms, monitor therapeutic efficacy, and facilitate diagnostic imaging. What makes iron oxide so appealing is a number of favorable properties including high detectability by MR, biodegradability and low toxicity. Here we describe the recent progress on the use of magnetic nanoparticles in imaging circulating cells and lymphoid tissues. The study of the lymph system and the biodistribution of various circulating immune cells is important in the diagnosis, prognosis, and treatment of a wide range of diseases and is expected to have a profound effect on patient outcome.
The field of molecular imaging has experienced rapid growth over the last several years, due to the expected benefits of non-invasively and quantitatively detecting biomolecules and enzymatic activity in vivo. It is envisioned that insight into the molecular underpinnings of disease will allow for the earlier detection of disease, improved diagnosis, and a means to monitor therapeutic efficacy. Magnetic resonance (MR) has been considered a particularly attractive platform for molecular imaging applications due to its ability to non-invasively acquire three-dimensional images with exquisite soft tissue contrast in conjunction with molecular markers of disease. Generally essential to this task of molecular imaging is the use of contrast agents. Iron oxide particles have garnered significant interest as MR contrast agents due to their biocompatibility, biodegradability and strong contrast enhancement. In this review we provide a brief overview on the composition and characteristics of iron oxide–based contrast agents and then provide a more focused discussion on their use in the imaging of circulating cells and lymphoid tissues.
Composition and Magnetic Characteristics of Iron Oxide Particles
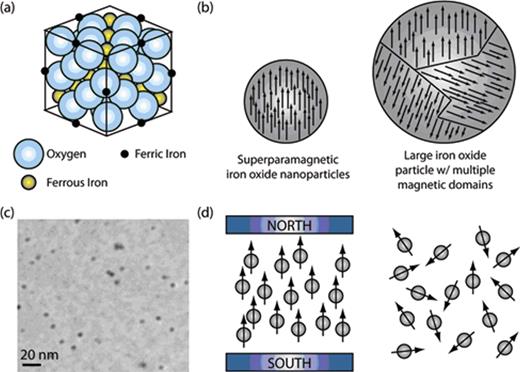
Iron oxide particles typically consist of two basic components, a crystalline magnetic iron oxide core and a hydrophilic surface coating. Most of the iron oxide particles that are used as MR contrast agents are less than 200 nm in diameter and exhibit superparamagnetic properties. This means that they have a high, positive magnetic susceptibility, but in the absence of a magnetic field exhibit essentially no magnetic remanence (no permanent magnetic moment). Superparamagnetic iron oxide (SPIO) nanoparticles exhibit a high magnetic susceptibility because at the nanometer size scale single crystals can be formed with all of the magnetic domains aligned (Figure 1 ). In contrast, large magnetic nanoparticles typically consist of multiple magnetic domains that are not well aligned and thus interfere with each other. Despite their large magnetic susceptibility, superparamagnetic nanoparticles do not exhibit a magnetic remanence in the absence of a magnetic field. This is because under these conditions, Brownian fluctuations are strong enough to randomly orient the single magnetic moments so that the bulk solution has no net magnetization.
In general, SPIO nanoparticles that are to be used for biomedical applications must be coated with surface complexing agents. Surface modification is necessary to prevent nanoparticle agglomeration, reduce toxicity, and control pharmacokinetics and biodistribution. It is also often desirable to select a surface coating that will impart additional functionality by allowing the attachment of targeting ligands and/or therapeutics.
Polymeric coatings represent the most common class of surface coatings used to improve the biocompatibility and stability of iron oxide nanoparticles. Some examples include dextran, carboxymethylated dextran, polyvinyl alcohol (PVA), starches, chitosan, poly(methyl methacrylate) (PMMA), poly(ethylene glycol) (PEG), poly(lactic-coglycolic acid) (PLGA), polyvinylpyrrolidone (PVP), and poly(acrylic acid) (PAA).1,2 Interestingly, it is not only the polymer utilized that effect the pharmacokinetics and biodistribution of the iron oxide nanoparticle, but also surface density of the polymer and its charge.
Mechanism of SPIO Contrast
In contrast to other imaging modalities (x-ray, nuclear, and ultrasound), the effect of MR contrast agents is not seen directly on the image, but rather it is their effect on proton relaxation, normally surrounding water protons, that is observed. In other words, it is the change in relaxation rate of water protons in the presence of SPIO that is detectable by MR and is responsible for enhancing image contrast. SPIO interactions are modulated by the diffusion of water protons near the magnetic field gradients created by the SPIO particles. The dipolar interaction between the proton spins and the magnetic moment of SPIO increases the rate at which protons become out of phase with each other, following removal of the radiofrequency pulse (ie, the T2 relaxation time becomes shorter). Shorter T2 (transverse) relaxation times tend to reduce the signal intensity, that is, darken the image. As a result, iron oxide nanoparticles are often referred to as “negative” contrast agents.
Although the majority of imaging studies take advantage of the T2-weighted contrast generated by SPIO, several examinations have demonstrated that SPIO may also be used as T1-contrast agents.3 In these cases, the SPIO are used to shorten the T1 relaxation time of surrounding water protons by increasing the rate at which they realign with the fixed magnetic field. This leads to an increase in signal intensity creating a “positive” contrast.
SPIO Classifications
There are many forms of SPIO in academic and clinical use, which has made it necessary to create broad classifications to dispel any confusion. Accordingly, SPIO are typically defined by their overall hydrated diameter (including their biocompatible coating) and divided into three categories: oral SPIO at 300 nm- 3.5 um; standard SPIO (SSPIO) at 50–150 nm; and ultrasmall SPIO (USPIO) at < 50 nm. Oral SPIO will not be discussed in this article. A subcategory of USPIO known as MION is also often identified and refers to USPIO that possess an iron oxide core that is monocrystalline in nature (ie, monocrystaline iron oxide nanoparticles). A prevalent derivatization of MION, consisting of a chemically cross-linked and aminated polysaccharide shell, is referred to as CLIO (cross-linked iron oxide).
Aside from the general classifications described above, SPIO are also commonly referred to by their trade names as well as other generic names. Further, there can be multiple trade names associated with any given SPIO, due to marketing in different parts of the world. Table 1 contains a concise listing of various SPIO, their alternate names, surface coating, and size.
Toxicity and Metabolism of SPIO
Previously, when dextran-coated USPIO was examined as a contrast agent in living subjects, its adverse effects were noted as not serious, mild in severity, and short in duration.4 This is not surprising considering that only a small iron dose is administered (~50–200 mg/person), in comparison with total body iron stores (~4000 mg in a normal male adult).
When administered by intravenous injection, both SSPIO and USPIO are predominantly cleared from circulation by macrophages that reside in organs rich in reticuloendothelial cells.5 In general, it has been determined that SSPIO and USPIO are internalized by receptor-mediated endocytosis and are metabolized via the lysosomal pathway.6 Once internalized by macrophages the dextran coating undergoes a progressive degradation and is eliminated almost exclusively in urine (89%). The rest is excreted in feces. The iron contained in SPIO is incorporated into the body’s iron store and is progressively found in red blood cells (hemoglobin). Like endogenous iron, it is eliminated very slowly and predominantly via the feces.5
Imaging Lymphoid Tissues with SSPIO
SSPIO agents are typically administered intravenously and are small enough to traverse the capillary beds in the lung, brain, heart and kidney. The circulation of SSPIO, however, is short-lived as the particles are rapidly removed by phagocytic cells of the reticuloendothelial system (RES). Consequently, the T2-signal of the spleen is rapidly reduced after intravenous injection. Because malignant neoplasms in the spleen are often devoid of phagocytic cells, they show up as areas of bright signal intensity creating a sharp contrast between normal and diseased tissue. As a consequence, SSPIO has been commonly used to improve the detection of splenic tumors.7 However, it should be noted that there are some malignant lesions that may contain phagocytes, in which case there may be no differential uptake compared with normal liver parenchyma.
Imaging Lymphoid Tissue and Macrophages with USPIO
USPIO particles are usually defined as having a hydrated particle diameter of less than 50 nm and a plasma half-life that is significantly longer than SSPIO. For example, the plasma half-life for USPIO has been reported to range anywhere from 80 minutes to more than 24 hours, compared with only ~2 to 4 hours for SSPIO.8 The large variability in USPIO circulation time largely has to do with the composition of the surface coating that is used to stabilize the particle.2 In general, the extended circulation time for USPIO is a consequence of the particles not immediately being recognized by phagocytic cells of the RES. The small size and prolongation of the plasma half-life also enables this agent to cross the capillary wall and have more widespread tissue distribution. Twenty-fours hours after administration, USPIO particles can be found in the lymph nodes, bone marrow, liver, and spleen. This is in contrast to SSPIO, which are found almost exclusively in the liver and spleen. USPIO particles transmigrate the capillary wall by means of vesicular transport and through interendothelial junctions. Upon gaining access to the interstitium, USPIO are cleared by draining lymphatic vessels and are transported to lymph nodes (Figure 2 ), which show up as areas of reduced signal intensity on T2/T2*-weighted images.9 If metastases cause disturbances in node flow or architecture, USPIO lose accessibility to the node, and the node appears hyperintense. The differential uptake of USPIO has made them quite suitable for T2/T2*-weighted MR lymphography. With the help of USPIO, the sensitivity of detecting lymph node metastases increased from 45.4% to 100% with a specificity of 95.7%.10
Aside from MR lymphography, the small size and long circulation time of USPIO also enables improved accessibility to bone marrow compared with SSPIO. Twenty-fours hours after the administration of USPIO, 2.91% of injected dose per gram of tissue is found in bone marrow.11 This is sufficient to reduce signal intensity of bone marrow on T2/T2*-weighted images. Retention of USPIO in bone marrow is a consequence of phagocytosis by macrophages. Since USPIO are not taken up in neoplastic marrow infiltrates, which are devoid of macrophages, these lesions remain hyperintense. Therefore, USPIO can be used to differentiate between normal and neoplastic marrow. This mechanism for contrast enhancement is similar to imaging splenic tumors with SSPIO particles as discussed above. Also, USPIO are capable of differentiating between hypercellular growths and tumor infiltratates in marrow.12 Although USPIO exhibit some unique features that allow them to generate contrast enhancement within the lymph nodes and bone marrow, it should be noted that they can still be used for RES-specific imaging of the spleen, with similar effectiveness as SSPIO.13
The observation that USPIO particles are efficiently internalized by macrophages and other phagocytic cells has recently led to their evaluation as an MR contrast agent for the diagnosis of inflammatory and degenerative disorders associated with high macrophage phagocytic acitivity (Figure 2 ). One particularly promising application is the non-invasive and early detection of atherosclerotic plaques. Atherosclerosis is an inflammatory disease that can be characterized by increased endothelial permeability and high macrophage content. Imaging of atherosclerotic plaques in hyperlipidemic rabbits via the macrophage uptake of USPIO revealed marked susceptibility effects within the aortic wall, whereas the aortic wall of the control rabbit remained smooth and bright.14 Similar results were also observed in humans.15 In addition to atherosclerosis, other pathologic conditions that are associated with high macrophage content and USPIO accumulation include stroke,16 multiple sclerosis (MS),17 arthritis,18 transplant rejection, back pain, kidney disease, infection, and cancer. Currently, it remains unclear whether USPIO are phagocytosed while macrophages/monocytes are in circulation or after tissue infiltration.
Tracking Circulating Cells with SPIO
A promising new direction for SPIO is their use in tracking the migration and biodistribution of cells in vivo (Figure 3 ). The ability to track cells is expected to have a significant impact in many clinical applications, including circulating cell–based therapies. Cell tracking typically involves labeling cells ex vivo and subsequently implanting or systemically administering them into living subjects. It has been well documented that many cell types can be labeled by simple incubation with SPIO including monocytes and macrophages.19,20 SPIO can be internalized by phagocytosis, receptor-mediated endocytosis, or fluid phase–mediated endocytosis. Labeling efficiency is dependent on both SPIO size and surface coating. In general, it has been found that the larger SSPIO nanoparticles and charged surface coatings (anionic or cationic) are preferable to the smaller USPIO particles and neutral surface coatings (eg, dextran), respectively.19,21
Interestingly, it has recently been shown that a number of cell types can even take up micron-sized particles, including hematopoietic and mesenchymal stem cells and macrophages.22–24 This is potentially advantageous because cells labeled with just one or more micron-size particles can be detected by MR due to the large iron content of each particle. Further, it has been demonstrated that uptake of micron-size particles does not alter cell viability, proliferation, colony-forming ability or differentiation capacity.22
Although simple incubation with SPIO can lead to cell labeling, numerous studies have now shown that the extent and rate of uptake can be profoundly improved if SPIO is complexed with transfections agents, cell-penetrating peptides (eg, HIV-tat and polyarginine), or protamine sulfate.25 In general, these agents also allow many cells that do not naturally endocytose SPIO, such as lymphocytes, to be labeled; however, there is some evidence that they could interfere with normal cell functioning.26
Cell surface receptor–mediated endocytosis of SPIO is another avenue through which cells can be labeled.
Recently, the transferrin receptor has been exploited as an efficient intracellular delivery device for oligodencrocytes.27 This approach offers the unique possibility of being able to replenish the supply of internalized SPIO through active targeting of the transferrin receptor using transferrin- or anti-transferrin receptor-bound SPIO. Further, cells that do not naturally express high levels of transferrin can easily be engineered to constitutively overexpress an unregulated form of the transferrin receptor. It has already been demonstrated that transferrin receptor transgene expression can be visualized by MR in vivo using transferrin-labeled USPIO.28
The major application for magnetically labeled cells will likely be to aid in the design and administration of cellular-based repair, replacement, and treatment strategies.25 Numerous pre-clinical studies have already been conducted to illustrate the value of cellular tracking via MR, including applications involving dendritic cells, lymphocytes, natural killer (NK) cells, macrophages, and stem cells.
Dendritic cells are able to stimulate or inhibit immune responses. The important role these cells play in marshaling the body’s defenses has led to their use as a tool to augment the native immune response against malignant cells. A reputed problem with this therapeutic approach has been that the cells do not always localize or migrate to target organs as desired. Therefore, it is envisioned that in vivo imaging of dendritic cell migration may substantially aid in the design and administration of dendritic cell–based therapy. Initial studies have already shown that when SSPIO-loaded dendritic cells, intended as a cancer vaccine for melanoma, were injected intranodally, MR imaging allowed assessment and accuracy of delivery and of inter-and intra-nodal cell migration patterns.29
Similar to dendritic cells, T lymphocytes have been used to treat and image several classes of malignancies. The ability to track T cells via MR has recently revealed that serial administered T cells home to different intratumeral locations.30 This suggests that a repeated-dose delivery strategy may provide a more effective treatment regiment than a single bolus injection. Importantly, it has also been found that T lymphocytes labeled with USPIO elicited normal activation and activation-induced cell death responses.31
NK cells also hold promise for cell-based therapies as they exhibit high cytotoxic activity on multiple malignancy types, while sparing normal cells. A novel molecular imaging study used SPIO carried in a genetically modified NK-92 cell line, NK-92-scFv(FRP5)-zeta, which was directed against malignant cells over-expressing HER2/neu (a receptor found in many human cancers, notably breast cancer). In this study, the MR monitoring of lipofected NK cells with SSPIO led to distinct and long-lasting recognition of HER2/neu-positive NIH 3T3 mammary tumors in mice.32 Persistent labeling and viability was evident for at least 5 days.
Another application for cellular tracking involves the non-invasive imaging of graft rejection after solid organ transplantation. In one study, after the in vivo labeling of macrophages with micron-sized particles, a distinct punctate hypointense pattern could be observed in the myocardium of allograft hearts, which is likely indicative of myocardial rejection.33
Finally, stem cells have received enormous attention for their potential use as therapeutics. Stem cells possess the ability to both maintain themselves in an undifferentiated state as well as differentiate into several mature cell types. It is expected that tracking of these cells using MR could help refine administration of this therapy, as it would be possible to monitor cellular distribution to specific organs and disease sites. SPIO labeling has already aided in the detection of intravascularly administered SPIO-labeled hematopoietic progenitor cells in the spleen and bone marrow.34 In addition, it has recently been found that magnetically labeled stem cells could be recovered from tissues by magnetic separation columns.35 This technique may enable detailed analysis of specific stem cell and organ interactions.
Significant work has also been done on tracking the cerebral distribution of stem cells administered for ischemic therapy, the long-term monitoring of stem cells in developmental and injured brains, and the incorporation of stem cells into the neovasculature in glioma models.36 Similarly, endomyocardial mesenchymal stem cells (MSCs) have been imaged following transplantation into the beating heart.37 This approach may permit in vivo study of stem cell retention, engraftment, and migration. Despite these promising results, one concern that has been documented is that the MR signal from infarcted tissue after transplantation of SPIO-labeled MSCs can arise from resident cardiac macrophages that have engulfed the nanoparticle.38 This can certainly lead to ambiguous results. Furthermore, it should be noted that the effect of the presence of SPIO on stem cell function and their ability to differentiate remains under contention.39 These findings point to the need for continued research in this area.
Conclusion
The unique physical and chemical properties and strong MR enhancement of SPIO make them excellent contrast agents for applications involving the imaging of circulating cells and lymphoid tissues. Therefore, it is expected that SPIO-enhanced MR imaging will have considerable future use in the clinical imaging of hematological diseases and will lead to more personalized patient care and improved patient outcome.
Schematic representations of superparamagnetic iron oxide (SPIO). (a) Spinal crystal structure for SPIO. (b) Comparison of a SPIO nanoparticle, which possesses a single magnetic domain (ie, all of the individual moments of the atoms are aligned) to a larger iron oxide particle that has multiple magnetic domains. In general, the presence of multiple magnetic domains will result in a reduced net magnetization because the domains will interfere with each other. (c) Transmission electron micrograph of SPIO nanoparticles. (d) Schematic illustrating the behavior of SPIO in the presence and absence of an external magnetic field. In the presence of a magnetic field the magnetic moment of SPIO aligns in the direction the magnetic field; however, in the absence of a magnetic field SPIO become randomly oriented due to Brownian fluctuations. Brownian motions are also sufficient to prevent aggregation of the nanometer-sized SPIO, when prepared appropriately.
Schematic representations of superparamagnetic iron oxide (SPIO). (a) Spinal crystal structure for SPIO. (b) Comparison of a SPIO nanoparticle, which possesses a single magnetic domain (ie, all of the individual moments of the atoms are aligned) to a larger iron oxide particle that has multiple magnetic domains. In general, the presence of multiple magnetic domains will result in a reduced net magnetization because the domains will interfere with each other. (c) Transmission electron micrograph of SPIO nanoparticles. (d) Schematic illustrating the behavior of SPIO in the presence and absence of an external magnetic field. In the presence of a magnetic field the magnetic moment of SPIO aligns in the direction the magnetic field; however, in the absence of a magnetic field SPIO become randomly oriented due to Brownian fluctuations. Brownian motions are also sufficient to prevent aggregation of the nanometer-sized SPIO, when prepared appropriately.
Schematic illustrating the transmigration of ultrasmall superparamagnetic iron oxide (USPIO) into the lymphatics and the uptake of USPIO by macrophages at a site of inflammation. (a) Intravenously injected USPIO are able to gain access to the interstitium and the lymphatic vessels as a consequence of their small size and long circulation times. This allows for imaging of the nodal architecture and detection of disturbances in nodal flow as a result of metastases. (b) At sites of inflammation, SPIO accumulate within the interstitium, due to an increase in the porosity of the endothelial wall, and are engulfed by macrophages.
Schematic illustrating the transmigration of ultrasmall superparamagnetic iron oxide (USPIO) into the lymphatics and the uptake of USPIO by macrophages at a site of inflammation. (a) Intravenously injected USPIO are able to gain access to the interstitium and the lymphatic vessels as a consequence of their small size and long circulation times. This allows for imaging of the nodal architecture and detection of disturbances in nodal flow as a result of metastases. (b) At sites of inflammation, SPIO accumulate within the interstitium, due to an increase in the porosity of the endothelial wall, and are engulfed by macrophages.
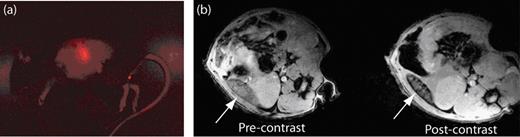
Fluorescent and magnetic resonance (MR) images revealing the biodistribution of B cells in mice. (a) Fluorescent image of a mouse that has been injected with B cells that have been labeled with ultrasmall superparamagnetic iron oxide (USPIO) and the fluorescent dye CellVue® NIR815. The fluorescent image clearly shows the localization of B cells within the spleen. (b) MR images of the spleen (white arrow) before and after the injection of the USPIO-loaded B cells. The migration of USPIO-loaded B cells to the spleen results in a loss in signal intensity on T2-weighted images.
Fluorescent and magnetic resonance (MR) images revealing the biodistribution of B cells in mice. (a) Fluorescent image of a mouse that has been injected with B cells that have been labeled with ultrasmall superparamagnetic iron oxide (USPIO) and the fluorescent dye CellVue® NIR815. The fluorescent image clearly shows the localization of B cells within the spleen. (b) MR images of the spleen (white arrow) before and after the injection of the USPIO-loaded B cells. The migration of USPIO-loaded B cells to the spleen results in a loss in signal intensity on T2-weighted images.
Disclosures Conflict-of-interest disclosures: The authors declare no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Department of Bioengineering, University of Pennsylvania, Philadelphia, PA