Abstract
For adult patients who present with chronic myeloid leukemia (CML) in chronic phase it is now generally agreed that initial treatment should start with the tyrosine kinase inhibitor (TKI) imatinib at 400 mg daily. Five years after starting imatinib about 60% of these patients will be in complete cytogenetic response (CCyR), still taking imatinib; an appreciable proportion of these will have achieved a major molecular response, defined as a 3-log reduction in the level of BCR-ABL1 transcripts in their blood. The patients in CCyR seem to have a very low risk of relapse to chronic phase or of progression to advanced phase. Other patients may be resistant to imatinib or may experience significant side effects that require change of therapy. The best method of monitoring responding patients is to enumerate Philadelphia chromosome–positive marrow metaphases at 3-month intervals until CCyR and to perform RQ-PCR for BCR-ABL1 transcripts at 3-month intervals after starting imatinib. The recommendations for defining “failure” and “sub-optimal response” proposed by the European LeukemiaNet in 2006 have proved to be a major contribution to assessing responses in individual patients and are now being updated. Patients who fail imatinib may respond to second-generation TKIs, but allogeneic stem cell transplantation still plays an important role for eligible patients who fare badly with TKIs. Patients who present in advanced phases of CML should be treated initially with TKI alone or with TKI in conjunction with cytotoxic drugs, but their overall prognosis is likely to be much inferior to that of those presenting in early chronic phase.
The disease now known as chronic myeloid or chronic myelogenous leukemia (CML) was probably described in the 1840s, first in France and subsequently in Edinburgh and Berlin (reviewed in Geary1 and Deininger2). Treatment in the last century was based predominantly on radiotherapy, busulfan, and hydroxyurea and more recently interferon-alfa and allogeneic stem cell transplantation (reviewed in Pavlovsky3). Important landmarks in our understanding of the biological basis of the disease were the discovery of the Philadelphia (Ph) chromosome in 1960, the characterization of the breakpoint cluster region on chromosome 22 in 1984 and the demonstration of the BCR-ABL (now renamed BCR-ABL1) fusion gene in 1986. These crucial steps laid the foundation for the preclinical work that led to the development of a 2-phenylaminopyrimidine compound, now known as imatinib mesylate or just imatinib, that inhibits the kinase activity of the BCR-ABL1 oncoprotein and was first used in the clinic to treat patients with CML resistant to interferon-alfa in 1998.4 The success of this initial study in so-called late chronic phase CML (LCP-CML) led to the design and very rapid implementation of the IRIS (International Randomized Study of Interferon and STI571) trial for previously untreated chronic phase CML (often referred to as “early chronic phase” or ECP-CML), and the results of this study have fundamentally altered the management of CML during the last decade5,6 and even raised the possibility that some patients with CML could be cured by use of imatinib as a single agent. Conversely, about 35% of patients with CML-CP become resistant to imatinib or cannot tolerate the drug, and for these improved initial therapy is obviously required.5–7 Thus, one challenge is to recognize as early as possible the patient destined to fail imatinib and so to revise the therapeutic strategy. Another is to devise better ways of using tyrosine kinases in general, either alone or in combination with other agents. A number of the other areas for clinical debate are listed in Table 1 , some of which are addressed in this paper. It is interesting to reflect that the actual mechanism by which imatinib kills leukemia cells or at least prevents their proliferation, while generally leaving their normal counterparts unaffected, remains largely mysterious.
Starting with Imatinib
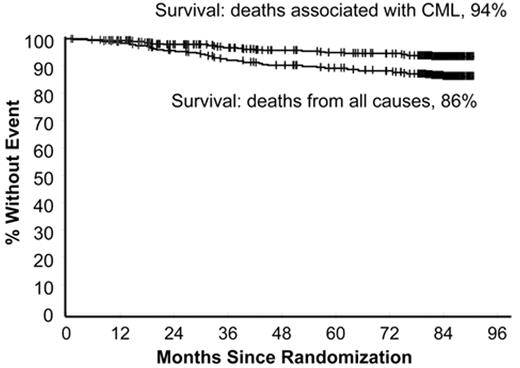
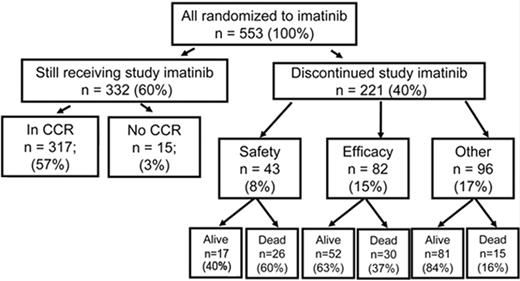
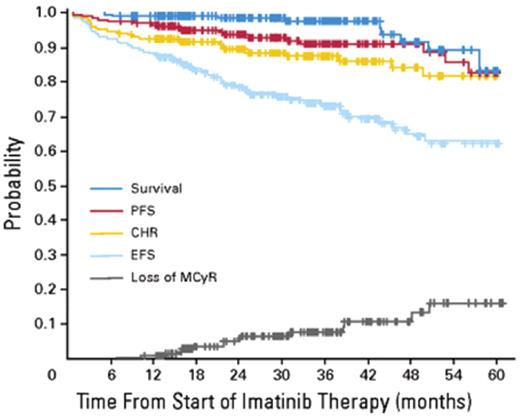
In the 1990s the recommended approach to managing a patient newly diagnosed with CML-CP was allogeneic stem cell transplantation if the patient was relatively young and had a suitable donor; for other patients interferon-alfa with or without cytarabine was recommended. From 2000 onward imatinib at 400 mg daily became the preferred initial treatment8 (despite the fact that the maximal tolerated dose was not clearly established in the early clinical studies), and this practice received substantial support from the interim results of the IRIS study published in 20039; the report showed that with a median follow-up time of 19 months an estimated 95% of patients randomized to start treatment with imatinib had achieved a complete hematologic response and 74% had achieved a complete cytogenetic response (CCyR). The 7-year update for patients who received imatinib as first-line treatment confirmed these initially impressive results10; it showed an actuarial overall survival of 86% (Figure 1 ), which was very substantially better than historical results achievable with interferon-alfa or interferon plus cytarabine,11,12 and also showed that responding patients whose disease had not progressed in any way in their first 3 years of the study were extremely unlikely to relapse at a later stage and also unlikely to suffer from any late onset side effects. However, the 7-year update also showed that only 57% of the original patient cohort were still in continuing CCyR taking imatinib on study according to the original protocol (Figure 2 ), a figure very similar to that reported in an independent single center study in the United Kingdom7 (Figure 3 ).
A case can be made for offering initial treatment by transplantation for the rare patient with a genetically identical twin since the risk of transplant-related mortality is extremely low with syngeneic donors. Until recently some investigators recommended an initial allograft for younger patients with a high probability of surviving the transplant based on the European scoring system,13 but even this approach has now fallen from favor.
Dosage
Investigators at the MD Anderson Cancer Center in Houston reasoned that clinical results might be improved by starting treatment with a higher dose of imatinib and therefore designed a study in which newly diagnosed patients started treatment with imatinib at 800 mg daily.14 Some patients were unable to tolerate this higher dosage, but comparison with results of treating patients with the standard 400 mg daily showed that CCyRs and major molecular responses (MMRs) were achieved more rapidly with the higher dose of imatinib. This experience led other investigators to design studies to compare prospectively 400 mg daily with 600 or 800 mg daily, some of which are still ongoing.15,16 It does, however, appear that whereas response to the higher dose is clearly more rapid, the longer-term results (ie, at 18 months) show less convincing superiority for the higher dose and no survival benefit has (yet) been demonstrated. Thus for the present there seems no good reason to alter the “standard” 400 mg initial dosage for adults. Parenthetically, most pediatricians have opted to adapt imatinib dosage based on the child’s body weight or body surface area,17 though in children prolonged use of imatinib may cause skeletal decalcification and growth retardation.18
Side Effects
Though patients taking imatinib are spared the more unpleasant side effects associated with conventional cytotoxic drugs, imatinib can still cause a variety of unwanted adverse reactions and some are severe enough to necessitate reducing dosage or discontinuing the drug.19,20 Among the most prominent non-hematologic side effects are nausea, fluid retention, weight gain, diarrhea, bone pains, rashes and disturbances of liver function. Imatinib can also cause significant cytopenias. Patients with anemia may benefit from administration of erythropoietin and those with neutropenia may be able to tolerate imatinib at full dosage if supported with granulocyte colony-stimulating factor (G-CSF)19,20; severe thrombocytopenia may, however, necessitate dose reduction or a change to another agent.
Monitoring Responses
It is generally believed that CML starts with acquisition of a BCR-ABL1 fusion gene in a single hematopoietic stem cell, which then proliferates to produce a large population of leukemia cells in the bone marrow and blood. This sequence of events is presumably gradually reversed in a patient with CML responding to therapy. This would mean that the first evidence of response should be reduction of the excessive leukocyte count, followed then by normalization of a more sensitive measure of residual leukemia, namely the number of Ph-positive metaphases in the bone marrow. The most sensitive available test for low levels of leukemia is to measure BCR-ABL1 transcript numbers in the blood or marrow using a real time quantitative reverse transcriptase PCR (RQ-PCR).21–23 The use of blood gives results equivalent to those derived from bone marrow, but the specimen needs to be processed within 48 hours of collection. The use of fluorescence in situ hybridization (FISH) to identify a BCR-ABL1 fusion gene in interphase cells is more sensitive than metaphase cytogenetics but less sensitive than a RQ-PCR.24 Thus, for the present the best approach to monitoring the reduction in leukemia cell numbers as a given patient responds to treatment, or indeed to recognize incipient relapse as early as possible, is to assess marrow metaphase cytogenetics until a patient achieves CCyR and then to carry out RQ-PCR routinely for BCR-ABL1 transcripts. FISH may be used if RQ-PCR for BCR-ABL1 is not available (Table 2 ).
Though in practice the use of RQ-PCR for BCR-ABL1 transcript numbers is now fairly widely available, it must be remembered that the technique is demanding and can give both false-positive results (for example, if the specimen is accidentally contaminated with material from another patient) and false-negative results (for example, if the patient’s specimen is degraded as a consequence of too much time spent between collection and processing). Moreover, the methods by which results are expressed in different laboratories are not yet standardized, although considerable progress has been made towards the production of an international scale based on internationally validated reference materials.25,26 Finally the clinician must bear in mind that the failure in a given laboratory to detect any BCR-ABL1 transcripts, often referred to as a complete molecular response (CMR), is still consistent with the survival in a patient’s body of perhaps 1 × 107 leukemia cells,21,27 some of which may be totally resistant to all currently available tyrosine kinase inhibitors (TKIs).
Predicting Response to Imatinib
It would be extremely valuable if the clinician could predict with reasonable accuracy whether a given patient would or would not respond to imatinib. It is interesting that the Sokal index, developed 25 years ago based on the survival of patients treated predominantly with busulfan or hydroxyurea,28 was valuable in predicting response to interferon-alfa in the 1990s and also differentiates patients destined to do less well on imatinib from those destined to respond better.5 This observation is best interpreted as evidence that there is intrinsic heterogeneity in the leukemia that may reflect on the one hand still unknown differences in the leukemia cells in different patients at the time of diagnosis and, on the other hand, differences in a given patient’s genetic make-up (eg, differential pharmacogenomics or differing immunological responses to leukemia cells).
Efforts have been made to measure the concentration of imatinib that will inhibit proliferation of a patient’s cells collected at the time of diagnosis using a cell-based in vitro assay and thereby to establish an imatinib concentration that will inhibit phosphorylation of a given substrate by 50% (IC50).29 Other investigators have measured membrane transporters that regulate influx (eg, human organic cationic transporter 1 [hOCT1]) and those that regulate efflux (eg, MDR1),30,31 but neither approach can yet be recommended for routine clinical use. The same applies to gene expression profiling performed on cells collected from patients at diagnosis; one study was able to differentiate patients in chronic phase responding well to imatinib from those who responded less well,32 but this interesting report still requires confirmation.
Two recent studies suggest that low plasma levels of imatinib, based on “trough” levels defined as the lowest level before the next scheduled dose of imatinib is due, can correlate with a lower probability of achieving a CCyR.33,34 Such low trough levels might be an indication for increasing the prescribed dose of imatinib to improve responses in individual patients, but the value of this approach has not yet been demonstrated. Indeed, it must be appreciated that one major cause of an inadequate response or loss of initial response to imatinib may be the simple fact that some patients may not take the prescribed dose on a regular basis, for which there may be a variety of reasons, including forgetfulness, desire to lessen side effects or desire to save money in countries where the patient has to pay for the drug from his or her own financial resources. Moreover, direct questioning of the patient about his/her adherence to the prescribed dosage may not always yield reliable answers.
European LeukemiaNet Recommendations
When the success of imatinib in managing CP disease was first recognized, some investigators reasoned that most or all of the patients who responded well would lose their responses over the ensuing years. Happily, the reverse seems to be true—for those who respond well responses have proved to be extremely durable.10 It therefore became important to establish criteria for response, and in 2005 the European LeukemiaNet brought together a panel of experts who agreed on a series of criteria to define “failure” and “sub-optimal response” for ECP patients who started imatinib at 400 mg daily.27 A patient was defined as having failed the standard dose of imatinib if he/she had not achieved any of the following: some level of hematologic response after 3 months, a complete hematologic response with some level of cytogenetic response at 6 months, less than 35% of Ph-positive marrow metaphases at 12 months or a CCyR at 18 months (Table 3 ). It was recommended that such patients should have their treatment changed. Failure to achieve somewhat more stringent criteria at the same timepoints was defined as “sub-optimal response,” which was not automatically an indication to change therapy. Exactly how treatment should best be continued for patients deemed to have failed imatinib at 400 mg/day is not entirely clear. One relatively easy option is to increase the dosage to 600 or 800 mg/day. These higher dosages seem to be effective in some patients in the short term,35 but the majority seem to lose any benefit they may initially achieve. A better alternative may be to opt as early as possible for a second-generation TKI; it may be possible to assess the benefit of such change of strategy within 3 to 6 months of starting nilotinib or dasatinib36 or even to predict the likelihood of failure before starting the second-generation TKI using the Sokal score and the degree of resistance to imatinib (D Milojkovic et al, Haematologica, in press).
Though these European LeukemiaNet recommendations were not formally based on published evidence, many clinicians found them useful. A subsequent analysis in which a cohort of patients treated at a single institution were classified as “failure” or “non-failure” at the four time-points showed that their probability of subsequent progression-free survival and achievement of CCyR was very significantly related to the category to which they had been allocated; this provided impressive confirmation that the recommendations were in fact valid.37 Broadly analogous recommendations that have proved equally useful have been prepared by the National Comprehensive Cancer Network in the USA (www.NCCN.org). The LeukemiaNet recommendations were recently updated (M Baccarani et al, J Clin Oncol, in press); they now include a definition of “optimal response” and also emphasize the importance of obtaining a major molecular response (Table 3 ). There is now a clearer distinction between those BCR-ABL1 kinase domain mutations thought to be associated with resistance to imatinib and those that are probably bystander effects. Because the efficacy of the second-generation TKIs, notably dasatinib, nilotinib and bosutinib, is now reasonably well defined, a major objective must now be to recognize imatinib failure at the earliest opportunity to enable to clinician to implement alternative strategies, such as stem cell transplantation, if possible.
Stopping Imatinib
A small number of patients treated with interferon-alfa in the 1990s achieved durable CCyR, which has continued for many years after the interferon was stopped.38 In 2007 Rousselot and colleagues reported details of 12 patients with CML in France who had received imatinib as primary treatment or after prior treatment with interferon and achieved CMR.39 For different reasons these patients had stopped their imatinib after 2 or more years in CMR; 6 had relapsed at the molecular level and 6 were still in CMR at the time of the report. Fortunately it seems that patients who do relapse at molecular level may respond to the reintroduction of imatinib just as well as they did originally.40 The French study has now been extended and equivalent data have been acquired in 70 patients who stopped imatinib after 2 or more years in CMR.41 It seems probable that a small number of patients who take imatinib for prolonged periods may be able to stop the drug without evidence of relapse over a number of ensuing years. Whether such patients can be really be regarded as “cured” is debatable, but undoubtedly one major target today must be to find strategies that will enable us to increase the proportion of patients who may safely stop treatment.
Imatinib and Pregnancy
Preclinical studies based on animal data suggested that imatinib could be teratogenic in certain circumstances, so women taking the drug have been routinely advised to take steps to avoid conception. Nonetheless, some women have conceived while taking imatinib and in most cases where the pregnancy went to term the baby appears to have been normal. However, certain specific developmental abnormalities including hypospadias, exomphalos and defective skeletal formation have been seen more often than would have been expected in women not taking imatinib,42 and the advice to avoid pregnancy while being treated with the drug must be upheld.
A problem that currently defies a standardized approach is how to advise a woman already on imatinib who wants to start or to enlarge her family. Assuming the patient has achieved a CCyR or, even better, a MMR, it might be reasonable to stop the imatinib for a finite period to allow the patient to conceive and to carry the child without exposure to imatinib, but this approach almost certainly puts the patient at increased risk of disease progression. Whether the use of interferon-alfa, a drug that appears not to be teratogenic, during the pregnancy is a useful compromise is unknown. One might argue that a patient who originally responded very rapidly to imatinib might relapse off imatinib only very slowly, but this is quite untested. Such evidence as is available suggests that patients who stop imatinib and then relapse respond as well to reintroduction of imatinib as they did originally, as mentioned above.40,41 Little is known about the possible harmful effect of imatinib on male spermatogenesis, so again no firm recommendations can be offered to the potential father treated with imatinib.
Other Approaches to Initial Treatment of ECP-CML
A number of clinical studies have been designed to test the possibility that combining imatinib at standard dosage with other agents could increase the speed of response, the “depth” of response and, by extrapolation, the event-free survival or overall survival. Preliminary results of multi-center studies show that the incidence of CCyR at various timepoints is increased when imatinib is combined with interferon-alfa16 or with cytarabine,43 but it is too early to assess the possible benefits on overall survival. It may be logical to combine imatinib with another molecule that inhibits an intermediate molecule in signal transduction, such as mTOR, STAT 5 or PI3K. Another approach of interest is use a specific farnesyl transferase inhibitor that seems on the basis of preclinical evidence to target quiescent stem cells.44 One of the most attractive approaches to increasing the efficacy of imatinib would be to combine its use, either simultaneously or sequentially, with a second-generation TKI45; such trials are now in progress.
The question also arises as to whether one of the new TKIs, namely dasatinib, nilotinib or bosutinib, should be used instead of imatinib as initial therapy for patients in ECP. It seems clear that cytogenetic and molecular responses are achieved more rapidly with second-generation TKIs than with imatinib at 400 mg daily, but whether such apparent short-term benefit will translate into long-term benefit and improved survival is still unknown. For the present it seems that up-front use of these second-generation TKIs is best confined to prospective studies in which imatinib is a comparator.
Patients Who Present in Advanced Phases of CML
It is conventional now to start treating patients who present in advanced phases (a term including accelerated and blastic phases) with a starting dose of imatinib at 600 mg daily, but in general such patients respond in the longer term much less well to imatinib than those who start treatment in chronic phase. Patients who satisfy criteria for acceleration are in fact heterogeneous; at one end of the spectrum the term covers patients whose leukemia is only slightly more advanced than late chronic phase, while at the other end the leukemia may be verging on blastic phase. Patients with “early” accelerated phase may obtain long-term responses to imatinib as a single agent, while others may have much shorter responses. Patients presenting in blastic transformation (BT) do, however, need a much more aggressive initial strategy. Thus they may start treatment with imatinib, but dasatinib with its wider spectrum of activity against SRC and SRC family kinases may be preferable. For patients in lymphoid BT extrapolation from results obtained with treatment of Ph-positive acute lymphoblastic leukemia (ALL)46,47 suggest that combining imatinib with standard ALL treatment may be the best initial approach. Once remission is achieved maintenance treatment with cytotoxic drugs together with a TKI can then be continued. Neuroprophylaxis is also advisable. For patients presenting in myeloid BT the combined use of a TKI with therapy appropriate to AML may be the best approach. In both lymphoid and myeloid BT treated as suggested above, the probability of relapse is high, and this risk may be reduced by allogeneic stem cell transplantation (allo-SCT) carried out while the patient is in apparent remission. It is logical to continue the use of a TKI after allo-SCT but no controlled series have been reported. It should be remembered that patients treated for BT of CML, unlike patients treated with imatinib in CP, may proceed from CMR to overt relapse very rapidly; to be useful, molecular monitoring must be carried out at much more frequent intervals than for patients treated in ECP.
Estimated survival at 7 years for 553 patients treated with imatinib as initial therapy in the IRIS study. The upper curve shows survival if only CML-related deaths are considered (94%) and the lower curve shows survival taking account of deaths from all causes (86%). Reprinted with permission from O’Brien SG et al. Blood. 2008;112:76a.10
Estimated survival at 7 years for 553 patients treated with imatinib as initial therapy in the IRIS study. The upper curve shows survival if only CML-related deaths are considered (94%) and the lower curve shows survival taking account of deaths from all causes (86%). Reprinted with permission from O’Brien SG et al. Blood. 2008;112:76a.10
Outcome at 7 years for 553 patients randomized to receive imatinib as initial therapy in the IRIS study. At 7 years 60% of patients were still taking imatinib in accordance with the study protocol. Reprinted with permission from O’Brien SG et al. Blood. 2008;112:76a.10
Outcome at 7 years for 553 patients randomized to receive imatinib as initial therapy in the IRIS study. At 7 years 60% of patients were still taking imatinib in accordance with the study protocol. Reprinted with permission from O’Brien SG et al. Blood. 2008;112:76a.10
Intention to treat analysis of results for 204 patients with CML-CP treated with imatinib at the Hammersmith Hospital in London. Reprinted with permission from de Lavallade et al.7 PFS indicates progression-free survival; CHR, complete hematologic response; MCyR, major cytogenetic response; EFS, patients alive at 5 years in stable complete cytogenetic response (CCyR) and still on imatinib.
Intention to treat analysis of results for 204 patients with CML-CP treated with imatinib at the Hammersmith Hospital in London. Reprinted with permission from de Lavallade et al.7 PFS indicates progression-free survival; CHR, complete hematologic response; MCyR, major cytogenetic response; EFS, patients alive at 5 years in stable complete cytogenetic response (CCyR) and still on imatinib.
Disclosures Conflict-of-interest disclosure: The author received honoraria and serves on the speakers bureaus of Novartis Pharmaceuticals and Bristol-Myers Squibb. Off-label drug use: None disclosed
Acknowledgments
I am very grateful to Drs David Marin, Jane Apperley and Alex Bazeos who kindly read and unquestionably improved this manuscript.
References
Author notes
Imperial College London, Hammersmith Hospital, London, United Kingdom