Abstract
The association between thrombosis and cancer has been extensively studied since first pointed out by Trousseau in 1895. It is, however, not commonly appreciated that the incidence of thrombosis in malignant hematologic disorders is as high or even higher than in solid tumors. Thrombotic complications in acute leukemia are often overlooked because bleeding complications generally dominate the clinical picture. Yet, the patient is at risk for both. While there are many thrombogenic factors shared by both solid tumors and leukemia, many additional prothrombotic features are present in leukemia. The prothrombotic factors include hyperleukocytosis, increased expression of tissue factor and its activation in leukemic cells, and the prothrombotic adverse effects of therapeutic agents and vascular access catheters. In addition, comorbid conditions including hereditary thrombophilia, infection, endothelial cell activation by cytokines, antiphospholipid syndrome and acquired activated protein C resistance are major contributory factors. Factors that increase the bleeding risk include thrombocytopenia, disseminated intravascular coagulation, and excessive fibrinolysis, which is enhanced by increased expression of Annexin II by leukemic cells. Therapeutic approaches to both bleeding and thrombotic conditions require special considerations of these factors.
Incidence of Thrombosis
Among the malignant hematologic disorders, the incidence of thrombosis is higher in patients with lymphoma or with acute leukemia. Significant morbidity and high mortality in acute leukemia due to complications of bleeding and infection frequently overshadow thromboembolic events. Case-controlled studies of patients with cancer revealed a fourfold increase in thromboembolic occurrence in acute leukemia, with about the same rate in acute myelogenous leukemia (AML) and in acute lymphocytic leukemia (ALL).1 Among patients with acute leukemia, thrombosis has the highest incidence in acute promyelocytic leukemia (APL)2–5 (Table 1 ). It is also high in those patients with central venous catheters, especially in children with ALL.6 Of interest, increased thromboembolic events take place even prior to the diagnosis of acute leukemia, similar to the situation seen in solid tumors, indicating that a prothrombotic state is present even at the earliest phase of leukemia. Furthermore, the risk of thrombosis persists after remission, as the cumulative incidence is higher with a longer period of observation.2 Also, there are many thrombogenic factors in leukemia that are not seen in solid tumors. This is especially evident in APL, which carries a higher incidence of thrombosis than other types of acute leukemia. While most thromboembolic events are venous, arterial thromboembolism is also seen in the FAB subtypes M3, M4, and M5 of AML. In children with ALL, the incidence varies from 1.1% to 36.7%, with 50% of these being life-threatening thrombosis in the central nervous system (CNS).5 The use of central venous catheter and treatment protocol involving corticosteroids and L-asparaginase may play an important thrombogenic role.3,5
Pathogenesis of Thrombosis
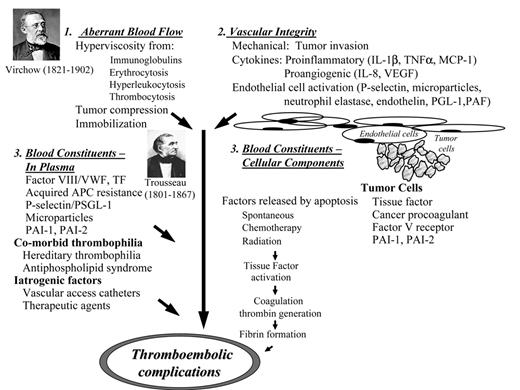
Virchow’s classical triad of abnormalities in blood flow, vessel integrity and blood components have now evolved into a complex picture with multiple prothrombotic factors. These various factors interact with each other, thereby enhancing their combined effects (Figure 1 ).
Aberrent blood flow
Whole blood viscosity is determined by both the plasma and cellular components. In the hyperleukocytosis syndrome in acute leukemia, abnormalities in the rheologic properties of leukemic blasts lead to sluggish blood flow in the microcirculation, causing impaired function of the brain, eyes, myocardium, lungs and kidneys. In this syndrome, the patient may experience headache, dizziness, visual changes, chest pain and dyspnea. The microthrombi can extend to larger vessels, causing either or both venous and arterial thromboembolism. A major causative factor is hyper-leukocytosis, when the peripheral blood blast counts at presentation are higher than 100,000/μL.7 This complication is associated with an increase in morbidity and mortality as there is often multi-organ dysfunction. It is more frequently seen in AML than in ALL, and rare in CML or CLL. It occurs in 5% to 13% of adult AML, and in 12% to 25% of pediatric AML, with mortality as high as 40%. In AML, it is more common in young age; in FAB subtypes M4, M5, microvariant of M3; and in those with cytogenetic abnormalities of 11q23 rearrangement, inv(16)(p13q22) and chromosome 6 abnormalities, and expression of lung resistance protein. In AML especially with the M4 and M5 subtypes, there is often extramedullary involvement with organo-megaly. A high early mortality is seen, with CNS bleeding being a major cause. Leukostasis is believed to cause the microcirculatory blockade with organ dysfunction. Myeloblasts, especially in the M4, M5 subtypes, are larger and less deformable, and thus the leukostasis syndrome is more frequently seen. Recent in vitro studies, however, revealed that a more significant determinant for the leukostasis syndrome may be an increased expression of adhesion molecules by endothelial cells and of the corresponding receptors in the myeloblasts. Myeloblasts, through their production of cytokines including TNFα and IL-1β, were shown to upregulate the expression of ICAM-1, VCAM-1, P-selectin and E-selectin by endothelial cells. These cytokines enable the endothelial cells to recruit additional myeloblasts, forming a vicious cycle in which more cells are trapped in the microcirculation.8 It was also found that CD11b, a receptor of ICAM-1, ICAM-2, was highly expressed in myeloblasts in M4 and M5 subtypes of AML and lower in M0, M1, M2 and M3 subtypes, thus offering an explanation for the higher frequency of the leukostasis syndrome in the M4 and M5 subtypes. Such findings cast doubt at the role of whole blood viscosity in this syndrome. Since whole-blood viscosity is affected only when the leukocrit reaches 12 to 15 mL/dL, corresponding to 300,000 to 450,000/dL in AML and 600,000 to 800,000/dL in ALL, levels which are rarely reached, leukostasis is more likely related to the above-mentioned changes in the blast–endothelial cell interaction, rather than an increase in circulating whole blood viscosity.
In ALL, the leukostasis syndrome is more often seen in younger patients, males, and those with T-cell phenotype, hypoploidy, 11q23 rearrangements, loss of p16 expression, and positive Ph1 chromosome. The peripheral white cell count is a prognostic factor, with those higher than 50,000/ dL having the worst outcome. In addition, white cell counts higher than 250,000/dL or with neurologic impairment are associated with a higher risk for early mortality, caused almost always by CNS bleeding. CNS leukemic involvement is common in this setting. The other major cause of early death is pulmonary failure.
The treatment for the leukostasis syndrome is commonly thought to be cytoreduction by both leukopheresis and chemotherapy. This is based on the belief that by lowering the peripheral blast count, the leukostasis may resolve. However, a recent study on 48 patients showed that reducing the white cell count had no significant effect on early death or survival.9 Thus, prompt initiation of chemotherapy is essential, and additional therapeutic measures should be instituted. The use of high doses of corticosteroids had been the standard treatment for hyperleukocytosis in the retinoic acid syndrome associated with all-trans retinoic acid (ATRA) therapy in APL. Corticosteroids are known to block in vitro the upregulation of the adhesion glycoprotein CD18, L-selectin and IL-8 receptors in myeloid cells. This beneficial effect of corticosteroids may thus benefit the leukostasis syndrome as well. More clinical trials are needed to evaluate the currently available therapeutic measures of leukopheresis, chemotherapy and corticosteroids.
Thrombocytosis occurring in AML with chromosome 3 aberration may also trigger arterial thrombosis.10 The mechanism for dysregulation of thrombopoiesis associated with this abnormality is not well understood.
Role of Leukemic Cells
Prothrombotic factors produced by leukemic cells include tissue factor (TF) and cancer procoagulant (CP).11 Analysis of leukemic cells isolated from peripheral blood and bone marrow showed that TF antigen and procoagulant activity were greatly increased in AML subtypes M3 and M4–5.12 TF is located mostly on the outer surface of the cell membrane and normally remains dormant. When cell death or apoptosis occurs, the inner membrane phospholipid, phosphatidylserine, is exteriorized, resulting in the assembly of the earliest coagulation complex, TF–factor VIIa, thus initiating the coagulation cascade. In vitro measurements revealed that the amount of thrombin produced is directly proportional to the degree of apoptosis.13 Since the rate of cell death is highest during rapid turnover of leukemic cells, chemotherapy or radiation therapy, thrombin generation and the thrombotic risk are correspondingly increased.
CP is a cysteine proteinase that activates factor X directly and has been found in both ALL and AML subtypes M1 to M4, with the highest activity in M311. Its role in thrombogenesis in acute leukemia has not been verified clinically.
Iatrogenic Factors
Vascular access catheters
Results from several studies on thromboembolic complications in leukemia show a higher incidence in patients with central venous catheters,14 with the risk highest in children with ALL.6 The overall incidence of catheter-related deep vein thrombosis (DVT) in patients with cancer was formerly estimated to be 30% to 74%, but is now found to be much lower (around 4% to 16%). A fibrin sheath almost always forms around the intravascular portion of the catheter. Though this may convert to an intraluminal thrombus, causing difficulty in withdrawing blood or infusing fluids, DVT does not always occur. DVT should be diagnosed only with color flow Doppler imaging or venography. In children, ultrasound is relatively insensitive, and a combination of ultrasound and venography is recommended. The increased risk in ALL may be related more to the use of L-asparaginase and corticosteorids than to the catheter itself. Recent studies failed to show the benefit of thromboprophylaxis with warfarin15 or low-molecular-weight heparin.16,17 Thus, prophylaxis should be limited only to those patients with other comorbid risk factors for thrombosis.
Therapeutic agents
Among those agents used to treat acute leukemia, L-asparaginase has adverse effects that cause both bleeding and thrombosis. This drug has a profound effect on hepatic synthesis of coagulation and fibrinolytic factors. This results in a decrease in plasma levels of fibrinogen, factors VII, IX, X and XI, histidine-rich glycoprotein, α-2 macroglobulin, and α-2 antiplasmin, producing an increased bleeding risk. The incidence of bleeding complications is low. One reason for this low frequency is the concurrent impaired synthesis of naturally occurring anticoagulant proteins, including antithrombin, protein C and protein S, and plasminogen. Immediately following the cessation of L-asparaginase therapy, the hemostatic balance is shifted towards a prothrombotic tendency when recovery of the coagulant proteins (fibrinogen, factors VII, IX, X and XI) takes place sooner than the recovery of anticoagulant proteins (anti-thrombin, protein C and protein S, and plasminogen). L-asparaginase is used in many treatment protocols for acute lymphoblastic leukemia, with various combinations of prednisone, daunorubicin, vincristine, cytarabine, cyclophosphamide, methotrexate and thioguanine.18 In protocols that include giving prednisone for the first 3 to 5 days, the fibrinogen level is reduced by the steroid action. Thus, the combined hypofibrinogenemic effects of both prednisone and L-asparaginase can be quite pronounced. The bleeding diathesis is further increased by concurrent thrombocytopenia. Laboratory monitoring of plasma fibrinogen, prothrombin time and partial thromboplastin time should be done. Cryoprecipitate is recommended when severe hypofibrinogenemia, with levels of 100 mg/dL or less, develops, although there are no clinical trials that show beneficial effects. Following the cessation of L-asparaginase therapy, the incidence of thromboembolic complications is around 2% to 10% in adults. However, in a recent prospective study on 60 children treated for ALL with L-asparaginase, a much higher rate of 36.7% was found.18 Most thrombosis was asymptomatic and occurred in the upper extremities, suggesting that the use of central venous access catheters may be a contributory factor. Others reported intracranial thrombotic and hemorrhagic complications;19 the former were sinus venous thrombosis, and the latter were cerebral hemorrhages. When thrombotic complications occur and require thrombolytic therapy with plasminogen activators, it should be recognized that the patient’s plasminogen level may be low, resulting in reduced therapeutic plasmin activity.20 Supplementation of plasminogen with fresh frozen plasma is recommended.
Glucocorticoids
Prednisone treatment results in increased plasma levels of prothrombin, von Willebrand factor and antithrombin, with decreased fibrinogen and plasminogen.
Vascular endothelial growth factor inhibitors
These compounds, by blocking vascular endothelial growth factor (VEGF) activity, may interfere with endothelial cell function. Most cases of thrombotic complications involve the use of bevacizumab (Avastin), a monoclonal antibody against VEGF. VEGF modulates endothelial cell function including increased permeability, proliferation and migration,21 and has been shown to induce rapid release of von Willebrand factor. It also increases the expression of tissue factor, thrombomodulin, plasminogen activators (tPA and uPA), PAI-1 and uPAR, as well as promotes adhesion and activation of platelets.22 Thus, effective inhibition of VEGF activity would theoretically cause both bleeding and thrombosis,23 both of which have been observed in the treatment of solid tumors with bevacizumab. Since its use in acute leukemia is limited, no reports of these adverse effects have yet appeared in the literature.
Gemtuzumab ozogamicin
Gemtuzumab ozogamicin (Mylotarg™, GO), and immuno-conjugate of a humanized monoclonal antibody against CD-33 conjugated with calicheamicin, is used in AML as the myeloblasts are positive for the CD33 marker. Hepatic veno-occlusive disease were reported in 0.9% of patients treated with GO alone for AML, but the incidence increased to 5% when given with other chemotherapeutic agents and up to 19% of those treated after hematopoietic stem cell transplantation.24 Postulated mechanisms include injury by free radicals due to glutathione deficiency and endothelial cell activation associated with inflammatory cytokines.
ATRA
Treatment with ATRA in APL results in rapid resolution of the coagulopathy and bleeding, but paradoxically induces thrombosis in a small number of patients. The prothrombotic complications should be distinguished from the retinoic acid syndrome found in 4% to 26% of patients treated with ATRA. ATRA-associated thrombosis occurs 1 to 3 weeks following treatment, at a time when the coagulopathy has been corrected, and can involve multiple organs, including the heart, brain, lungs and spleen.25 The incidence was 8.8% in one study, in which the thrombotic risk was found to be associated with phenotypic and molecular features of a higher median white cell count of 17,000/dL, prevalence of the bcr3 transcript, isoform subtype of PML-RARα, presence of FLT-3 ITD (fms-like tyrosine kinase 3 internal tandem duplication) mutation, and expression of CD2 and CD15.26 Antifibrinolytic agents, such as tranexamic acid, have also been shown to add to this potentially fatal risk27,28 and thus are contraindicated. Various thrombogenic factors have been proposed, including the induction of apoptosis by ATRA,29 upregulation of adhesive molecules and increased production of cytokines.30 It is interesting that arsenic trioxide, which also causes apoptosis and cell differentiation in APL by degradation of the PML-RARα fusion protein, is not associated with significant thromboembolic events, though hyperleukocytosis does occur.31 Retinoic acid syndrome, seen in as many as 26% of patients with APL, is characterized by fever, weight gain, pneumonitis, pleural and pericardial effusions, hyperleukocytosis, and extravasation of leukocytes, especially into pulmonary alveoli.32
Hematopoietic growth factors
Recombinant human erythropoietin available as epoetin alfa or darbopoetin alfa, as well as G-CSF and GM-CSF have all been found to increase the risk of thrombotic complications.33 The relative risk with erythropoietin was 1.67, prompting recent FDA warning and guidelines. The incidence with granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) was 2.8% and includes both arterial and venous events. Both have been shown in normal donors to cause increased markers of coagulation activation in their blood. The risk in patients with leukemia has not been studied.
Drug-associated thrombotic microangiopathy
The syndrome of thrombotic microangiopathy hemolytic anemia and renal failure has recently been reported in a patient with APL treated with ATRA.34 The syndrome is more often seen after hematopoietic stem cell transplantation. The drugs implicated are cyclosporine and tacrolimus. It is distinguished from other forms of thrombotic microangiopathy, especially thrombotic thrombocytopenic purpura, by having a normal plasma ADAMTS-13 level. The mortality rate is high and is not altered by therapeutic plasma exchange. The removal of the calcineurin inhibitor and replacement with other immunosuppressive drugs are recommended.
Co-morbid thrombophilia
Co-morbid conditions including hereditary thrombophilia, infection, endothelial cell activation by cytokines, antiphospholipid syndrome and acquired activated protein C resistance are major contributory factors. Among these, factor V Leiden mutation has been well studied and shows a 12- to 17-fold increase in venous thromboembolism among patients with cancer, a rate likely to be similar in patients with leukemia. Antiphospholipid antibodies are found more frequently in patients with cancer than in controls, and their titer may decrease after remission. The incidence is higher in lymphoma and in AML, in which 26% to 68% of patients have the antibodies. However, thrombotic manifestations of the antiphospholid syndrome are estimated to be present in only 20% of those carrying the anti-phospholipid antibodies.
Bleeding in Leukemia
Most patients with leukemia have some bleeding manifestations during the course of their illness. The factors contributing to an increased risk of bleeding are shown in Table 2 . These are well recognized and will not be discussed here.
Acute Promyelocytic Leukemia (AML-M3)
Both bleeding and thrombotic complications are major causes of morbidity and mortality in AML-M3. Despite the reversal of the coagulopathy by prompt ATRA therapy, hemorrhagic complications remain the major cause of early deaths, accounting for more than 50% of patients with and without ATRA therapy (Table 3 ).35–39 Intracranial bleeding accounts for most of the fatal hemorrhages, with other sites including diffuse pulmonary alveolar and gastrointestinal hemorrhage. Risk factors for bleeding include a high white count greater than 20,000/μL at presentation and severe thrombocytopenia. The pathogenesis of the bleeding is multifactorial, with a combined effect of disseminated intra-vascular coagulation (DIC), excessive fibrinolysis and thrombocytopenia. Almost all patients with APL manifest DIC at the time of diagnosis. Their blood shows markers of activated coagulation such as decreased fibrinogen, increased fibrin degradation products, D-dimer, fibrinopeptide A, prothrombin fragment 1+2, thrombin-antithrombin complex and decreased antithrombin, as well as thrombocytopenia. However, the severity of bleeding is not proportional to the degree of DIC but more to the level of white count. This can be explained by a high expression of TF and CP in the APL cells. ATRA treatment in vitro downregulates both TF and CP expression in APL cells, reversing the coagulopathy.11
The other major contributory factor to bleeding risk is excessive fibrinolysis. In vitro studies showed that APL cells express both uPA and tPA. More significantly, a receptor of tPA, Annexin II, is highly expressed in both APL cells and in the human APL cell line NB4 cells. Annexin II is a cell membrane surface protein found in endothelial cells, macrophages and several malignant cell lines. It is a protein with a molecular weight of 40 kDa. In addition to binding tPA, it is a co-receptor for plasminogen, with tPA binding at the amino-terminal of the core 1 domain, while plasminogen binding occurs at the lysine-binding site at the core 4 domain. The close proximity of the two ligands on the cell surface facilitates their interaction enhancing plasmin generation as much as 60-fold in vitro. Thus, an increased expression of Annexin II in APL cells, in conjunction with the expression of tPA, contributes to excessive fibrinolysis.40 NB4 cells treated in vitro with ATRA for 5 to 7 days downregulate their production of Annexin II. In addition to APL, the expression of Annexin II has also been found to be increased in a small number of patients with AML M4-5 or ALL. Furthermore, in a comparative study of the expression of Annexin II in human microvascular endothelial cells, it was found that the highest expression is seen in those endothelial cells derived from the brain, up to three fold more than Annexin II expressed in other organs.41 Larger amounts of plasmin were also found to be generated in vitro by the brain endothelial cells. This may provide an explanation for the relatively higher incidence of intracranial hemorrhage in APL. In addition to plasmin, elastase and chymotrypsin released by leukemic blasts may also contribute to the impaired hemostasis by proteolysis of von Willebrand factor, producing an acquired von Willebrand factor deficiency.42
Therapeutic Implications in the Management of Bleeding Complications
Management of DIC by aggressive platelet transfusions to keep the platelet count higher than 30,000/μL, maintenance of plasma fibrinogen level above 150 mg/dL with cryoprecipitate, and replacement of coagulation factors with fresh frozen plasma remains the mainstay of treatment options.43 Since ATRA downregulates the expression of TF, CP and Annexin II, it should be started as soon as the diagnosis of APL is suspected even before cytogenetic or molecular genetic confirmation, especially in those patients presenting with a high white cell count. Despite anecdotal reports in earlier literature, heparin has not been shown to be of benefit for DIC. Likewise, earlier reports of reduction of fibrinolytic bleeding by tranexamic acid have not been verified. In recent clinical trials, the prophylactic use of tranexamic acid did not change the incidence of early fatal hemorrhage.44 In fact, two reports suggested that anti-fibrinolytic agents may lead to fatal thromboembolism.27,28 A recent report suggested that recombinant factor VII may provide some hemostatic benefit and is recommended in severe bleeding and in intracranial hemorrhage.39
Conclusion
Despite strides made in the understanding of the patho-physiology of thrombophilia and bleeding diathesis in leukemia, attempts to improve outcome due to thrombosis and bleeding have made less progress. An awareness of the risk factors discussed in this article should lead to an earlier application of prophylactic measures. This may produce more benefits than when therapeutic measures are given after these complications have occurred. A limited number of recent clinical trials have verified the validity of this approach. However, more clinical trials are needed to clarify some questionable anecdotal recommendations.
Division of Hematology-Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL
Acknowledgments
The author would like to thank Dr. Brian Vicuna for his assistance in preparing the manuscript.