Abstract
The association between cancer and thrombosis is well recognized. What is not known, however, is the exact relationship between these two common medical conditions. Although the development of venous thromboembolism (VTE) in a patient with known cancer is the most common presentation, in some patients, VTE may precede the diagnosis of malignancy by many months. The variation in clinical presentation is likely due to the heterogeneous biology of different tumor types and also reflects the limitations of detection or available diagnostic methods. Accumulating evidence now suggests that critical oncogenic events may also trigger activation of the coagulation cascade, leading to a prothrombotic environment that not only manifests as venous thromboembolic disease but also promotes the growth and progression of the malignancy. This chapter will review the evidence for screening for occult malignancy in patients presenting with unprovoked or idiopathic thrombosis, briefly outline the known biological relationships between malignancy and thrombosis, and summarize the clinical data on the potential anticancer effects of low molecular weight heparins (LMWHs).
Screening for Occult Malignancy
Venous thromboembolism (VTE) is a common complication in patients with cancer and may be the first manifestation of malignancy.1–4 Evidence from cohort series and population-based registries has shown that approximately 10% of patients who present with unprovoked or idiopathic thrombosis are diagnosed with cancer within a few years after their thrombotic event.5–7 During the first year of follow-up, the standardized incidence ratio for cancer in these patients is 2.1 to 4.6.1,2,7,8 This risk is 3- to 4-fold higher than in patients diagnosed with secondary VTE or in patients with suspected thrombosis in whom the diagnosis was excluded.9,10 The incidence of cancer is highest within the first 6 months, and approximately 40% of the cases already have metastatic disease at the time of diagnosis.1,2,11 Furthermore, observational data indicate that patients with cancer and VTE have a worse prognosis than those with cancer alone.12,13 According to a study that examined the Danish National Registries of Patients, Death and Cancer, patients who received a diagnosis of cancer at the same time as or within 1 year following an episode of VTE had a shorter life expectancy than patients with cancer who were matched for age, sex, type of cancer, and year of cancer diagnosis but who did not have thrombosis.13 The 1-year survival was 12% in patients diagnosed with cancer and VTE at the same time, as compared with 36% in those with cancer alone.
Cohort studies: the value of routine examination
Given these observations, some clinicians have advocated aggressive search strategies to look for occult malignancies in patients with unprovoked VTE. Moreover, advances in diagnostic methods for various tumor types and new therapeutic options have fueled increasing enthusiasm for routine screening for malignancy in this unique population.
Nevertheless, direct evidence to support routine screening is lacking. To date, only a few studies have assessed the value of screening for occult cancer in patients with unprovoked VTE.
Cornuz and colleagues performed a retrospective chart review to determine the prevalence of clinical abnormalities on routine evaluation in 142 patients with newly diagnosed idiopathic DVT.14 They found that all 16 patients who were also found to have cancer at presentation had other abnormalities. During a median follow-up of 34 months, 3 patients were diagnosed with cancer, of whom 2 did not have any clinical abnormalities at initial evaluation. Only 3.6% of patients who did not have any abnormal findings subsequently developed cancer. This study suggested that routine evaluation is appropriate and that additional testing should be guided by clinical or laboratory abnormalities. Similarly, in a retrospective series of 1383 patients with thrombosis and no known malignancy, Nordström and colleagues found that 38 of the 66 patients who were diagnosed with cancer within 6 months after a diagnosis of deep vein thrombosis were detected by history, physical examination and basic laboratory testing.11 They concluded that most cancers are easily detected by routine methods and that extensive screening does not seem cost effective.
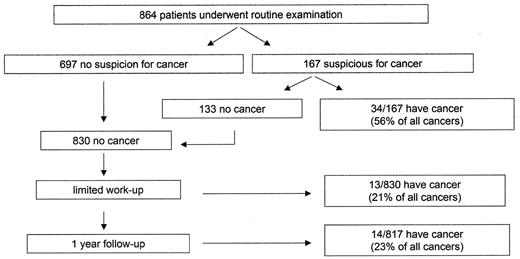
A more recent study prospectively followed 864 patients with a first episode of idiopathic VTE for occult malignancy.15 All of the patients underwent a routine assessment followed by a limited work-up for malignancy if no abnormalities were detected (Figure 1 ). The routine assessment consisted of a clinical history and physical examination, which included a rectal examination and pelvic and breast examinations in women, plus laboratory investigations (a complete blood count [CBC], liver and renal function tests, serum protein electrophoresis, urinalysis) and a chest x-ray. The limited work-up involved abdominopelvic ultrasonography and testing for selected tumor markers (prostate specific antigen [PSA], carcinoma embryonic antigen [CEA], and cancer antigen [CA]-125). Further testing was done to confirm a diagnosis of cancer if the results of these screening tests are abnormal. Of the 864 patients, 697 did not have any abnormalities while 167 had findings suspicious for cancer on routine examination. Cancer was confirmed in 34 of the 167 patients (20%). The remaining 133 patients who did not have cancer confirmed, along with the 697 patients who had a normal routine assessment, then underwent the limited work-up and were followed for 1 year. In this group of 830 patients, limited work-up detected cancer in 13/830 (1.6%) patients and follow-up revealed cancer in another 14/817 (1.7%) patients. Therefore, routine examination identified 34 of the 61 (56%) patients diagnosed with occult malignancy within the first year after a diagnosis of VTE. The important findings in this study are the high yield of the initial standardized routine examination and that 23% of occult cancers remain hidden despite a battery of tests including tumor markers. What this study does not address is whether screening or additional unselected testing in all patients with idiopathic VTE will improve the survival of patients with occult malignancy or simply increase the lead-time bias in cancer detection. Overall, cohort studies suggest that an initial assessment consisting of a comprehensive medical history, physical examination and basic laboratory testing will detect a large proportion of occult malignancies in patients with idiopathic VTE.
Randomized trials: extensive screening versus routine examination
Only one randomized controlled trial has examined whether extensive screening for occult cancer can reduce cancer-related mortality in patients with idiopathic VTE.16 In the SOMIT study, patients with a first episode of symptomatic deep vein thrombosis or pulmonary embolism who had no known risk factors for VTE were evaluated. All patients underwent routine testing for malignant disease, including a clinical history, a thorough physical examination, laboratory testing (CBC, aspartate aminotransferase [AST], ala-nine aminotransferase [ALT], alkaline phosphatase [ALP], calcium, and urinalysis) and a chest x-ray. Those who had no evidence of malignancy were then randomized using the Zelen design to be followed or undergo extensive screening. Using a very comprehensive battery of tests (Table 1 ), 13 of 99 patients allocated to extensive screening were found to have underlying cancer initially compared with none of 102 patients in the control group. Computed tomography (CT) scanning of the abdomen and pelvis yielded the majority of the cancers. During 2 years of follow-up, an additional patient in the screening group and 10 in the control group were diagnosed with cancer. The cancer-related mortality was 2% in the screened group and 4% in the control group. Although this difference was not statistically significant (1.9%; 95% confidence interval [CI] −5.5% to 10.9%), the investigators concluded “earlier [cancer] detection is likely to be associated with improved treatment possibilities and thus prognosis.”16
The results, however, do not support this conclusion. In fact, they lend evidence that early detection of occult cancer does not lead to improved prognosis in patients with idiopathic VTE. The study clearly demonstrated that 93% (13/14; 95% CI 66% to 100%) of the underlying malignancies were indeed detected by screening, yet a favorable influence on survival was not observed. Furthermore, even if the 2% reduction in mortality is real and a true improvement in survival was simply not detected due to insufficient power (type II error), the reduction in mortality was achieved at significant financial and emotional costs because of the invasiveness of some of the testing. The negative findings may also be a consequence of the considerable degree of contamination of the control group because investigators were reluctant to adhere to the protocol and not perform further investigations in these patients.17
Although early detection represents one of the most promising strategies to reduce cancer burden and improve survival, there is a lack of conclusive evidence to support screening for most cancers. In patients with idiopathic VTE, screening tests are not available for the malignancies that are strongly associated with thrombosis, such as cancer of the pancreas, ovary, liver, and brain.1,2,11,12 Furthermore, curative or effective treatments that can alter the natural history of the cancer must be available in order to provide a survival advantage following earlier detection of disease. Otherwise, screening will simply introduce lead-time bias.
In summary, the limited evidence available indicates that the most appropriate strategy in patients with unprovoked VTE is a thorough history and physical examination, followed by patient-specific laboratory testing and imaging. Routine screening for cancer using extensive investigations in these patients does not appear to provide a survival advantage.
Biological Links between Cancer and Thrombosis
In the past, research exploring the biological links between cancer and thrombosis has focused primarily on the mechanisms of thrombosis in patients with cancer. Increasingly, however, studies are examining the two-way biological relationship between activation of coagulation and the growth and metastatic potential of tumor cells.18,19 This interest has been fueled partly by the observation that treatment with low-molecular-weight heparin (LMWH) is associated with a survival advantage in patients with cancer, thereby suggesting that inhibition of the clotting cascade activation or thrombin activity may have antineoplastic effects.20–23
Pathogenesis of cancer-associated thrombosis
In patients with cancer, the capability of tumor cells and their procoagulant products to interact with platelets, clotting and fibrinolytic proteins contributes to the development of VTE. In addition, cytokine release, acute phase reaction and other host responses stimulated by tumor cell interactions with endothelial cells and tumor-associated macrophages further promote clotting activation.24 Some solid tumors, such as renal cell carcinoma, can directly invade the vessel wall and initiate the coagulation cascade. Consequently, the risk of VTE differs widely among patients with different tumor types and burden of disease. For example, adenocarcinoma, particularly of the pancreas, ovary and lung, has the strongest association with VTE compared with other histologies, and patients with metastatic malignancies have a higher incidence of VTE than patients with early stage or limited disease.25–27
The best-characterized cancer procoagulant is tissue factor (TF). This is a transmembrane protein that initiates coagulation by binding to activated factor VII. Sequential downstream activation of clotting protease complexes then leads to thrombin generation and, ultimately, fibrin formation. Normally expressed only on fibroblasts of the vascular adventitia and other stromal cells, TF is found almost constitutively on the surfaces of solid tumor cells and acute myelogenous leukemia cells.28 Its expression on endothelial cells and monocytes can be induced by cytokines such as tumor necrosis factor-α and interleukin-1β that are released by tumor cells and by immune regulatory cells under other pathological conditions. These cytokines in turn will upregulate the expression of TF, leukocyte and cellular adhesion molecules, platelet-activating factor, and plasminogen activator inhibitor type-1, as well as downregulate expression of thrombomodulin and the endothelial cell protein C receptor. Induction of nitric oxide synthase, resulting in enhanced formation of nitric oxide free radicals, may also contribute to thrombogenesis in patients with cancer.29 Therefore, a hypercoagulable state is established through the activation of the clotting cascade and platelets, enhanced endothelial adhesion, suppression of fibrinolysis, and inhibition of the anticoagulant protein C pathway.
Other risk factors, such as chemotherapy, surgery, and immobility, further increase the risk of thrombosis in patients with cancer (Table 2 ). Cytotoxic agents can alter coagulation protease levels and may directly injure the endothelium. Hormonal agents, such as tamoxifen, likely promote thrombogenesis by reducing plasma levels of natural anticoagulants. Surgery and catheterization cause direct trauma to vessels and initiate clotting via TF exposure or contact pathway activation. Lastly, venous stasis as a result of bed rest or extrinsic vessel compression from tumor or nodal masses can also contribute to thrombosis in patients with cancer.
Tissue factor-mediated tumor angiogenesis
Current evidence suggests that TF is likely also an important mediator between activation of coagulation and tumor growth. Studies using TF-knockout mice and other preclinical experiments have now firmly established that TF is a critical promoter of angiogenesis. Phosphorylation of the TF receptor triggers intracellular signaling pathways that result in the transcriptional activation or inactivation of several genes important for angiogenesis. Through the upregulation of potent angiogenic factors such as vascular endothelial growth factor (VEGF) and interleukin 8, and the downregulation of the antiangiogenic factor thrombospondin-1, TF is capable of tipping the angiogenic balance toward a more pro-angiogenic phenotype.30–32 VEGF, in turn, upregulates TF and thereby contributes to a positive cycle of tumor growth and thrombus formation. VEGF also increases vascular permeability, which leads to plasma protein leakage and the deposition of fibrin-rich proangiogenic matrix around tumor cells and vascular endothelial cells. The induction of angiogenesis by TF may occur via a clot-dependent pathway, in which thrombin generated from TF/FVIIa activation leads to subsequent interaction with protease-activated receptors (PAR) 1, 3, and 4, or via a clot-independent pathway initiated by the interaction of cytoplasmic domain of TF and PAR-2.33 Proteolytic cleavage of these G-protein-coupled receptors converts extracellular events into a transmembrane signal that leads to intracellular activities involved in cell growth and differentiation. The importance of PARs in vascular biology is now being recognized and explored.
Tissue factor and oncogenic transformation
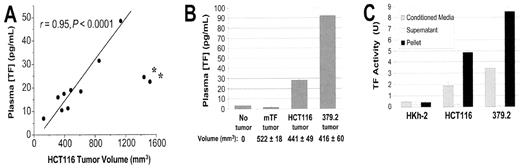
Evidence that further strengthens the relationship between TF and tumor biology comes from studies that have demonstrated a direct correlation between TF expression and the malignant phenotype in various tumors.34–37 Recently, this link has been further explored in a series of experiments by Yu and colleagues, who demonstrate that TF expression is coupled with oncogenic transformation in colorectal cancer.38 Using colorectal tumor cell cultures, TF activity was found to correlate positively with oncogenic transformations due to K-ras mutation and p53 inactivation that mimics the natural progression of this tumor type. These investigators also showed that TF plasma levels correlated with increasing tumor volume of the same tumor type as well as increasing oncogenicity of the same tumor volume (Figure 2 ). Increased TF activity was isolated in the microvesicle fraction of the cell-free culture supernatant and in the plasma of mice harboring human tumor xenografts, suggesting that this is one of the mechanisms for systemic coagulopathy in patients with malignancies.
LMWH as an antineoplastic agent
Given the above preclinical data, it is possible that anticoagulants may exert a negative impact on tumor angiogenesis by interfering with thrombin activity or TF/FVIIa activation.18 The most promising of the anticoagulants are LMWHs. The potential antineoplastic effect of LMWHs was reported in meta-analyses of clinical trials that compared LMWHs with UFH for the initial treatment of acute VTE.39,40 Although the 3-month overall mortality benefit associated with LMWH was not explained by a reduction in fatal PE or bleeding, none of these trials were designed with survival as the primary outcome and potential biases or imbalances in prognostic factors in the patients with cancer could not be ruled out. To date, several randomized trials specifically assessing the effect of LMWHs on cancer patient survival have now been completed and several are ongoing.
The FAMOUS study was the first randomized, placebo-controlled trial to examine the effect of a LMWH on survival in 385 patients with cancer.21 Patients with advanced solid tumors were randomized to dalteparin 5000 IU once daily or placebo for up to 1 year. According to an intention-to-treat analysis, the survival estimates for patients receiving placebo at 1, 2 and 3 years after randomization were 41%, 18%, and 12%, respectively, while the corresponding estimates for patients in the dalteparin group were 46%, 27%, and 21%. The trend for survival benefit, however, was not statistically significant (P = 0.19).
Subsequently, two randomized trials have reported positive results. The MALT trial randomized 302 patients with locally advanced or metastatic cancer to 6 weeks of nadroparin or placebo.22 Patients in the nadroparin group received therapeutic doses for 2 weeks followed by half the therapeutic dose for 4 weeks. The overall hazard ratio of mortality was 0.75 (95% CI 0.59 to 0.96; P = 0.02) with a median survival of 8 months in the nadroparin group versus 6.6 months in the placebo group. The treatment effect remained statistically significant after adjustment of potential confounders. Although this trial provided significant evidence that LMWH has an effect on cancer patient survival, a major criticism was the comparability of the different tumor types between the treatment groups.
To eliminate this confounding factor, randomized trials in specific tumor types have been conducted and are ongoing. The first completed and published trial assessed the effect of dalteparin in patients with newly diagnosed small cell lung cancer.20 A total of 84 patients were randomized to chemotherapy plus dalteparin 5000 IU once daily for 18 weeks or chemotherapy alone. The median progression-free survival was 10.0 months in the combined therapy group versus 6.0 months in the chemotherapy alone group (P = 0.01) and the median overall survival was 13.0 months and 8.0 months (P = 0.01), respectively. The overall tumor response was also better in the dalteparin group but the difference was not statistically significant. In contrast to the previous studies, LMWH was used as an adjuvant agent. Although the results are encouraging, clear conclusions about the potential benefits of LMWH and long-term survival remain premature because the study was small. Studies are underway to confirm these results and to evaluate the effect of LMWH in patients with limited disease, who may experience a better treatment benefit than those with metastatic disease.
Investigation for occult malignancy in patients with idiopathic venous thromboembolism (VTE).15
All patients presented with a first episode of idiopathic venous thromboembolism. The routine assessment consisted of a history and physical, rectal examination, pelvic and breast examinations in women, plus laboratory investigations (a CBC, liver and renal function tests, serum protein electrophoresis, urinalysis) and a chest x-ray. The limited work-up involved abdominopelvic ultrasonography and testing for selected tumor markers (PSA, CEA, and CA-125). Further testing was done if the results of these tests are abnormal.
Investigation for occult malignancy in patients with idiopathic venous thromboembolism (VTE).15
All patients presented with a first episode of idiopathic venous thromboembolism. The routine assessment consisted of a history and physical, rectal examination, pelvic and breast examinations in women, plus laboratory investigations (a CBC, liver and renal function tests, serum protein electrophoresis, urinalysis) and a chest x-ray. The limited work-up involved abdominopelvic ultrasonography and testing for selected tumor markers (PSA, CEA, and CA-125). Further testing was done if the results of these tests are abnormal.
Tissue factor (TF) and oncogenic transformation.38
A. TF levels correlated with increasing tumor volume of same tumor type.
B. TF levels correlated with increasing oncogenicity of same tumor volume.
C. TF activity is concentrated in microparticles.
Modified from Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741.
Tissue factor (TF) and oncogenic transformation.38
A. TF levels correlated with increasing tumor volume of same tumor type.
B. TF levels correlated with increasing oncogenicity of same tumor volume.
C. TF activity is concentrated in microparticles.
Modified from Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741.
Associate Professor, Department of Medicine, McMaster University, Hamilton, Ontario, Canada.