Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening condition characterized by uncontrolled hyperinflammation on the basis of various inherited or acquired immune deficiencies. Cardinal symptoms are prolonged fever, hepatosplenomegaly and cytopenias. Central nervous system (CNS) symptoms are common. Biochemical markers include elevated triglyceride and ferritin, high levels of the α chain of the soluble interleukin-2 receptor and low fibrinogen. Impaired function of natural killer (NK) cells and cytotoxic T-cells (CTL) is a characteristic of all forms of HLH.
Genetic HLH occurs in familial forms (FHLH), in which HLH is the primary and only manifestation, and in association with the immune deficiencies Chédiak-Higashi syndrome (CHS), Griscelli syndrome (GS) and X-linked lymphoproliferative syndrome (XLP), in which secondary HLH occurs sporadically. Most patients with acquired HLH have no known underlying immune deficiency. Both acquired and genetic forms are triggered by infections, mostly viruses, or other stimuli. HLH also occurs as a complication of rheumatic diseases (macrophage activation syndrome) and of malignancies. The recent discovery of several genetic defects causing FHLH as well as the identification of the genes responsible for CHS, GS and XLP have underscored the role of granule (perforin/granzymes)-mediated cytotoxicity in both the killing of infected cells and the termination of the immune response. The immediate aim of therapy is suppression of the increased inflammatory response by immunosuppressive/immunomodulatory agents and cytotoxic drugs. Genetic cases can only be cured with stem cell transplantation. Awareness of the clinical symptoms and of diagnostic criteria for HLH is crucial to starting life-saving therapy in time.
The hemophagocytic lymphohistiocytoses (HLH), whether familial or acquired, share one common feature, namely a highly stimulated but ineffective immune response that threatens the life of the patient and may lead to death unless arrested by appropriate treatment.
The Clinical Symptoms of HLH Are Characteristic but Non-specific
The cardinal symptoms of HLH are prolonged high fever, hepatosplenomegaly and cytopenias. Lymphadenopathy, icterus, or neurological symptoms such as cranial nerve palsies or seizures may also be present. Characteristic laboratory findings include high triglycerides, ferritin, transaminases and bilirubin and decreased fibrinogen. Hemophagocytosis, although it has given the disease its name, is found at presentation in only the minority of cases, but usually develops as the disease progresses. A hallmark of HLH is impaired or absent function of natural killer (NK) cells and cytotoxic T-cells (CTL). For more detailed information the reader is referred to recent reviews.1–3
While many of these cardinal symptoms are found in immune-competent patients in response to an infectious organism, they are more pronounced in patients with HLH. In particular, the progression of organomegaly, blood count changes and biochemical parameters should alert the physician that this could be an unusual response to an infectious agent. Without treatment, the uncontrolled inflammatory response leads to sustained neutropenia and death from bacterial or fungal infections as well as from cerebral dysfunction.
Underlying Conditions Associated with or Predisposing to HLH
HLH occurs in all age groups. It is not a single disease but can be encountered in association with a variety of underlying conditions leading to the same hyperinflammatory phenotype (Table 1 ).
Genetic HLH is inherited in an autosomal recessive fashion and can be divided into 2 subgroups: familial HLH (FHLH) and the immune deficiencies Chédiak-Higashi syndrome (CHS), Griscelli syndrome (GS), and X-linked lymphoproliferative syndrome (XLP). Both genetic subgroups are associated with impaired NK cell function. In FHLH, originally described by Farquhar and Claireaux in 1952,4 the clinical syndrome of HLH is the primary and only manifestation. FHLH is estimated to occur with a frequency of 1 in 50,000 births. The onset of the disease is during the first year of life in 70% of the children. A symptom-free interval after birth is typical. CHS, GS, and XLP are immune deficiencies with distinctive clinical features in which the development of HLH is sporadic, though frequent. HLH is often the presenting symptom but may also occur later during the course of disease. Patients with CHS show albinism and frequent pyogenic infections. Their white blood cells exhibit decreased chemotaxis and characteristic giant inclusion bodies (lysosomes). Patients with GS also have hypopigmentation and various degrees of neutrophil dysfunction but lack the giant granules. XLP is mainly characterized by a predisposition for Epstein-Barr virus (EBV)-associated HLH. XLP patients may develop lymphomas and dysgammaglobulinemia.
There are no data about the incidence of acquired HLH in children or in adults. From numerous case reports and from own experience, however, it appears that acquired HLH may be more common than previously believed.
The clinical picture of HLH can be induced by a variety of infectious organisms, mostly viruses, but also bacteria, protozoa and fungi. The patients in the original report by Risdall and colleagues were mostly adults with a viral infection following organ transplantation.5 Subsequently it became clear that nonviral agents could trigger HLH, and the term virus-associated hemophagocytic syndrome (VAHS) was redesignated infection-associated hemophagocytic syndrome (IAHS). Whereas in the first report by Risdall et al the majority of cases had an acquired iatrogenic immune deficiency, most patients in subsequent reports had no known genetic or acquired immune defect. A review of the published cases in children diagnosed with IAHS before 1996 reported that more than half of them were from the Far East. EBV was the triggering virus in 74% of the children in whom an infectious agent could be identified.2 In this series, most patients received only supportive care and the mortality was 50%.
The identification of an infectious organism does not help to differentiate FHLH from acquired HLH since the former is also usually triggered by infectious agents. This cannot be emphasized enough, since appropriate therapy should not be withheld when an infectious agent has been found.
HLH in association with malignant diseases, especially lymphomas (lymphoma-associated hemophagocytic syndrome; LAHS), is a well-known entity in adults but is rare in children. Cases formerly diagnosed as histiocytic medullary reticulosis or malignant histiocytosis included patients with LAHS, but also IAHS. Interestingly, in a recent review of patients with LAHS from Japan, the EBV genome was detected only rarely in patients with B-cell lymphoma but was demonstrated in more than 80% of patients with T/NK cell lymphoma.6 EBV-infected T/NK cells appear to play a major role in the development of LAHS as well as EBV-associated HLH without lymphoma,7 and in both LAHS and EBV-HLH the infected T/NK cells show a monoclonal or oligoclonal proliferation.
Hemophagocytosis and symptoms of HLH have also been described in association with inborn errors of metabolism, including lysinuric protein intolerance and multiple sulfatase deficiency. In such cases, it is not clear what role metabolic products may play as triggers to the immune response.
Genetic Defects in FHLH Elucidate the Pathophysiology of the Spectrum of HLH
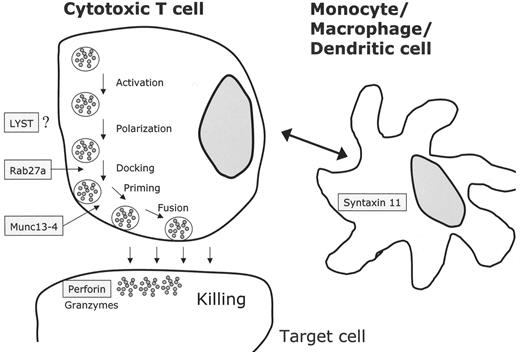
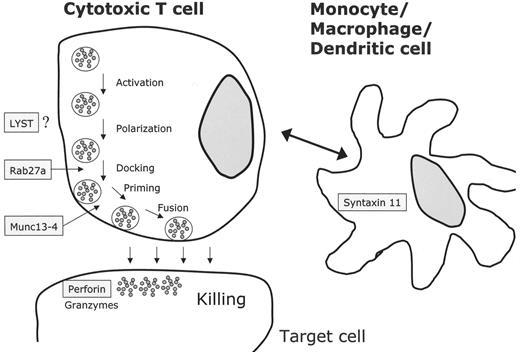
The cytotoxic activity of NK cells and CTLs is mediated by the release of cytolytic granules (containing large amounts of perforin, granzymes and other serine like proteases) via the immunological synapse to the target cell. Several independent genetic loci related to this activity have been implicated in the pathophysiology of genetic HLH (Table 2 ). In 1999 mutations in the perforin gene at locus 10q24 were described in 8 patients with FHLH.8 The overall frequency of perforin mutations in FHLH is between 15% and 50% and depends on the geographical and ethnic origin of the patients (reviewed in 9). UNC13D, at locus 17q25, was the second gene associated with FHLH. The encoded protein (Munc13-4) is important for cytolytic granule exocytosis.10UNC13D mutations do not affect docking of secretory vesicles (also known as lytic granules or secretory lysosomes) at the plasma membrane, but they impair priming of these vesicles and the subsequent release of cytolytic enzymes. The frequency of UNC13D mutations in FHLH was estimated to be around 30%.11 Our group recently identified a third FHLH associated gene on chromosome 6q24 with mutations in STX11.12 The encoded protein, t-SNARE syntaxin 11, also plays a role in intracellular trafficking, but its precise role is not known. The protein is strongly associated with intracellular membrane fractions and is detectable only in monocytes and not in lymphocytes. So far, mutations in the coding exon of syntaxin 11 seem to be restricted to patients from Turkey. Linkage analysis in 4 HLH families of Pakistani origin revealed a fourth putative disease gene for familial HLH on chromosome 9q21.3-22 but the gene has not yet been identified.13
In patients with GS type 2 (GS-2), immune dysfunction results from mutations in RAB27A on chromosome 15q21 (Table 2 ).14 Activated T cells of Rab27α-deficient patients are unable to dock their secretory granules at the plasma membrane, thereby impairing secretion via the immunological synapse.11 A direct interaction between Munc13-4 and Rab27α has recently been demonstrated, and the complex seems to be an essential regulator of secretory granule fusion with the plasma membrane.15 CHS is caused by mutations in the LYST gene, located on chromosome 1q42.1-q42.2, which encodes a 3801 amino acid protein required, by an unknown mechanism, for the final steps of granule secretion.16,17 XLP results from mutations in the SH2D1A gene.18SH2D1A(SAP) seems to be required for the signaling lymphocytic activating molecule (SLAM), which itself is important for signal transduction in immune cells and for activation of granule-mediated cytotoxicity of NK cells and CTLs.19 Mutation screening in male patients with HLH may be useful as some of them harbor mutations in SH2D1A.20
Defective Cytotoxic Function is Crucial in the Pathophysiology of HLH
The clinical picture of HLH is due to an increased inflammatory response caused by hypersecretion of pro-inflammatory cytokines such as interferon γ (IFNγ), tumor necrosis factor α (TNFα), interleukin (IL)-6, IL-10 and macrophage-colony-stimulating factor (M-CSF). These mediators are secreted by activated T-lymphocytes and histiocytes that infiltrate all tissues, and lead to tissue necrosis and organ failure. The pivotal role of IFNγ together with CD8+ T cells for the development of HLH was demonstrated in a perforin knock-out mouse model.21 Inflammatory cytokines are responsible for the characteristic disease markers such as cytopenias, coagulopathy and high triglycerides.
In spite of the excessive expansion and activation of cytotoxic cells, patients with HLH have severe impairment of the cytotoxic function of NK cells and CTLs. Impaired NK cell activity is seen in FHLH, GS, CHS and XLP as well as in acquired HLH. In the latter, impaired NK cell activity may be due in part to reduced numbers of NK cells and is usually transient. Defective cytolytic activity thus seems to be the common denominator that predisposes to HLH.
NK cells and cytotoxic T lymphocytes kill their targets through cytolytic granules containing perforin and granzyme. Upon contact between the effector killer cell and the target cell, an immunological synapse is formed and cytolytic granules have to traffic to the contact site, dock and fuse with the plasma membrane and release their contents.17 All known defects in HLH seem to be involved in this process (Figure 1 ): LYST mutations impair granule secretion, RAB27α deficiency leads to impaired docking at the membrane, mutations in UNC13D do not impair docking but cause defective granule priming at the immunological synapse. Finally, lack of PRF1 leads to loss of cytolytic activity. In XLP, granule-mediated cytotoxicity is defective through impaired lymphocyte activation. Syntaxin 11 mutations might theoretically impair cytolytic activity involving the interaction of dendritic and killer cells.22
The mechanisms leading to cytolytic defects in immune competent patients with acquired HLH are less clear. Viruses may interfere with CTL function by specific proteins,23 and high levels of cytokines may have the same effect.24 The prevalence of EBV-associated HLH in Asia suggests that a specific genetic susceptibility may result in an abnormal immune response to the virus. HLH cases associated with genetic defects in the granule exocytosis pathway demonstrate a critical role of the granule-dependent cytotoxic activity in T lymphocyte homeostasis.11 Activated NK cells and CTLs can kill infected cells and antigen-presenting cells, thus reducing the antigen load; however, more complex mechanisms seem to be involved in the downregulation of the immune response. Studies in mice indicate that NK cells and perforin-based systems play an important role in this process.25
Macrophage Activation Syndrome Is Closely Related to HLH
The macrophage activation syndrome (MAS) occurs in children and adults with autoimmune diseases. It is most commonly seen in association with systemic onset juvenile arthritis (sJRA) or adult-onset Still’s disease, but also occurs rarely with systemic lupus erythematosus or other entities.26–28 The clinical picture has all of the characteristic features of HLH including clinical symptoms, laboratory findings (especially very high ferritin levels), and hemophagocytosis. Pronounced coagulopathy and severe cardiac impairment are common and potentially life-threatening.27 Patients with MAS exhibit the defective NK cell function common to other patients with HLH. They may also have decreased expression of perforin or SAP, mimicking the defects associated with FHLH and XLP, respectively.29–31 Furthermore, low NK cell function and perforin expression was found to distinguish patients with sJRA from those with other clinical forms of JRA.32 The overall incidence of MAS is not known, but about 100 cases have been published to date. It generally develops in the earlier and active phases of the underlying disease. Viruses have been identified as triggering factors, but other inciting factors that have been implicated include nonsteroidal anti-inflammatory drugs, methotrexate, and gold-salt injections. Mortality of patients with MAS is between 10% and 20%. It has been suggested by some rheumatologists that MAS be classified as a form of secondary HLH.33,34
Diagnostic Problems in HLH
Many patients with HLH present with mild signs of an upper respiratory or gastrointestinal infection and a high fever. The fever often subsides spontaneously and recurs after some days to weeks. Transient improvements in cytopenias, especially thrombocytopenia, with unspecific measures such as antibiotics and transfusions are seen frequently. Organomegaly, anemia or other changes commonly persist. In babies, the initial suspicion often focuses on a metabolic disorder due to organomegaly, disturbed liver function or excessively high triglycerides.
Severe, fulminant liver failure with coagulopathy or neurological symptoms may dominate the presentation and thereby delay the diagnosis of HLH. Several patients, including 2 with a novel perforin mutation, have been described with isolated CNS involvement mimicking chronic encephalitits and preceding systemic HLH by several months.35–37
The characteristic laboratory findings of high triglycerides and ferritin and a low fibrinogen may be absent; therefore, repeat studies are indicated when HLH is suspected.38 A high lactate dehydrogenase may suggest a hemolytic anemia, but most patients with HLH characteristically have only moderately elevated reticulocytes in the presence of severe anemia, compatible with ineffective erythropoiesis. Highly activated immature-looking lymphocytes in lymph node biopsies may suggest a malignant process, and periportal infiltrates in a liver biopsy are often interpreted as viral hepatitis.
Laboratory Investigations
When a patient presents with prolonged fever, hepatosplenomegaly and cytopenias, the diagnosis of HLH should be considered. Minimal diagnostic requirements are a complete blood count, liver enzymes, bilirubin, triglycerides, ferritin and a coagulation profile including fibrinogen. All patients should have a bone marrow aspirate; however, this test is insensitive. In the majority of cases, hemophagocytosis is not observed in the initial bone marrow aspirate and only increased monocytes and monohistiocytic cells may be present. A myelodysplastic syndrome is sometimes suspected due to marked dysplastic changes in the red cell precursors. Lumbar puncture is also indicated and more than half of the patients will have an elevated cell count or protein or both, even in the absence of clinical symptoms. Two highly diagnostic disease parameters are an increased plasma concentration of the α chain of the soluble IL-2 receptor (sCD25) and impaired NK cell activity.39 The latter is usually not available in time but should be measured in all patients. It is persistently decreased to absent in nearly all genetic cases. In acquired cases it usually reverts to normal, but some patients show prolonged impairment. In these patients genetic testing is recommended.
The patients should be screened for an underlying disease like GS, XLP, CHS, autoimmune diseases or malignancy by detailed clinical history, physical examination and other appropriate studies. The search for a triggering infectious agent like EBV, cytomegalovirus (CMV), herpes simplex virus (HSV), adenovirus, varicella zoster virus (VZV) and leishmania is important since most of these organisms are treatable. However, it should be emphasized that, with the possible exception of leishmaniasis, anti-infectious therapy alone is not sufficient to control HLH. Viral PCRs are more helpful than serology. Positive results are useful to follow during antiviral treatment. Expression of perforin by flow cytometry can identify patients with perforin defects and is also desirable in patients with MAS or other acquired forms of HLH in which data on perforin expression are still scant. In proven familial cases or in presumed familial cases with persistent or relapsing disease material for genetic analysis should be obtained.
Diagnostic Criteria
The revised criteria of the Histiocyte Society for the diagnosis of HLH are shown in Table 3 . They are based primarily on the findings in familial cases but also apply to the diagnosis of infection-associated acquired HLH. For patients with systemic onset juvenile arthritis who develop MAS some criteria may not be relevant. For example pre-existing inflammation is associated with a higher white blood count and platelets as well as fibrinogen. Accordingly, for MAS, other diagnostic criteria have recently been advocated that take the specific characteristics of these patients into account.40
Therapeutic Options and Problems
The immediate aim in the treatment of any patient with HLH is to suppress the severe hyperinflammation that is responsible for the life-threatening symptoms. Another aim is to kill pathogen-infected antigen-presenting cells and thus remove the stimulus for the ongoing but ineffective activation of T cells. It has to be specifically emphasized that pathogen-directed therapy is usually not sufficient to control hyperinflammation with the possible exception of leishmaniasis, in which most patients promptly respond to liposomal amphotericin B.
In genetic HLH the ultimate aim must be stem cell transplantation to exchange the congenitally defective immune system with normal functioning immune effector cells. In our experience treatment should be guided mainly by the severity of clinical symptoms, but age of the patient, presence of familial disease, underlying secondary disease, and the identification of an infectious organism also should be taken into account.
Hyperinflammation can be treated with corticosteroids, which are cytotoxic for lymphocytes and inhibit expression of cytokines and differentiation of dendritic cells. Since dexamethasone crosses the blood brain barrier better than prednisolone it is the preferred corticosteroid. Cyclosporin A (CSA) prevents T-lymphocyte activation and immunoglobulin infusions probably act by providing cytokine-and pathogen-specific antibodies. The cytostatic drug etoposide has high activity in monocytic and histiocytic diseases and it inhibits EBNA synthesis in EBV-infected cells.
In patients with less severe symptoms, corticosteroids and immunoglobulin infusions are sometimes sufficient to control hyperinflammation and to reverse the clinical and laboratory symptoms. However, if symptoms progress, the treating physician should not hesitate to use therapy with dexamethasone, etoposide and CSA. The risk of etoposide, even if given for 8 weeks, is by far exceeded by the risk of losing a patient through inadequate treatment. Etoposide may be life-saving, especially in patients with HLH due to EBV infection. Mortality was 14 times higher for patients with EBV-associated HLH who did not receive etoposide within the first 4 weeks.41 Since initially mild cases may progress rapidly it is our opinion that the indication for the use of etoposide should be determined liberally.
Several problems may arise during the initial treatment of patients with HLH. Although symptoms and laboratory abnormalities usually improve within 2–3 weeks of treatment, persistent cytopenias could indicate either non-response to therapy or myelosuppression by etoposide. A repeat bone marrow examination may be helpful. However, since uncontrolled hypercytokinemia also leads to bone marrow aplasia, at least 3–4 doses of etoposide should be given before a decision is made to interrupt the drug, while continuing with CSA and dexamethasone. The close monitoring of other parameters associated with disease activity is important in this situation.
If there is no response to treatment after 4 weeks it is unlikely that the patient will have a benefit from continuing the same combination. There is no established salvage regimen; in single patients there has been anecdotal evidence of transient responses to daclizumab or alemtuzumab. Antithymocyte globulin, which may be effective in initial treatment, rarely produces a response in patients with no response to an etoposide-containing combination. If a donor is available and the clinical condition is not prohibitive, stem cell transplant could be tried, although there is little experience proving its benefit in this situation.
Reactivation during treatment is a frequent problem, either systemically or in the CNS. Immunosuppressive treatment should be reinforced in this situation. Intrathecal therapy with methotrexate +/− corticosteroids has been beneficial for some patients with recurrent CNS involvement and is recommended in view of the deleterious late effects of uncontrolled CNS disease.
Study HLH 94, which enrolled children up to the age of 15 years, used a combination of dexamethasone, CSA and etoposide for the initial treatment of HLH. Familial cases or patients with recurrences and therefore presumed genetic disease, subsequently received a bone marrow transplant from related or unrelated donors. Probability of survival at 3 years in 113 children was 55% for all cases and 51% for proven familial cases.42 Based on these data, patients with known familial disease, suspected genetic disease due to age below 1 year, or those with a severe clinical picture such as marked cytopenias, coagulation problems and CNS symptoms should receive treatment according to the present HLH protocol (available at www.histio.org/society/protocols). In familial cases or presumed familial cases (no complete resolution of symptoms, reactivations during or after cessation of therapy), active therapy should be continued until stem cell transplantation can be performed. A similar approach should be used for patients with the accelerated phase of CHS, GS or XLP, based on documented responses to HLH therapy. If a treatable infectious agent is identified, appropriate antimicrobial or antiviral therapy should be given.
The management of patients with MAS is usually based on the administration of high doses of corticosteroids. CSA has also proven effective in treating severe or corticosteroid resistant cases. Whereas immunoglobulin infusions failed in children with MAS, responses were observed in adult patients.26 Limited experience exists with etoposide in MAS; however, similarities in pathophysiology between MAS and other forms of HLH43 suggest that it might be beneficial in cases refractory to CSA and corticosteroids.
Summary
HLH is a life-threatening disease characterized by uncontrolled hyperinflammation on the basis of a variety of inherited or acquired immune deficiencies. Awareness of its clinical symptoms and diagnostic criteria is important to starting prompt life-saving therapy. While HLH and its genetic defects have provided insight into the mechanisms of host defense, our understanding of its complex pathophysiology still remains incomplete.
Molecular mechanisms based on the identification of genetic defects associated with the clinical picture of familial hemophagocytic lymphohistiocytosis (FHLH), Griscelli syndrome (GS-2) and Chédiak-Higashi syndrome (CHS). Perforin is secreted via cytotoxic granules and leads to disruption of the target cell. Cytotoxic granule processing occurs by means of a complex that contains at least a Rab27α/Munc13-4 complex and several other unknown proteins. The exact functions of LYST and syntaxin 11 are not known. In case of syntaxin 11, monocytes or macrophages/dendritic cells may interact with cytotoxic cells by an unknown mechanism.
Molecular mechanisms based on the identification of genetic defects associated with the clinical picture of familial hemophagocytic lymphohistiocytosis (FHLH), Griscelli syndrome (GS-2) and Chédiak-Higashi syndrome (CHS). Perforin is secreted via cytotoxic granules and leads to disruption of the target cell. Cytotoxic granule processing occurs by means of a complex that contains at least a Rab27α/Munc13-4 complex and several other unknown proteins. The exact functions of LYST and syntaxin 11 are not known. In case of syntaxin 11, monocytes or macrophages/dendritic cells may interact with cytotoxic cells by an unknown mechanism.